Baseline D-Dimer Levels as a Risk Assessment Biomarker for Recurrent Stroke in Patients with Combined Atrial Fibrillation and Atherosclerosis
Abstract
1. Introduction
2. Materials and Methods
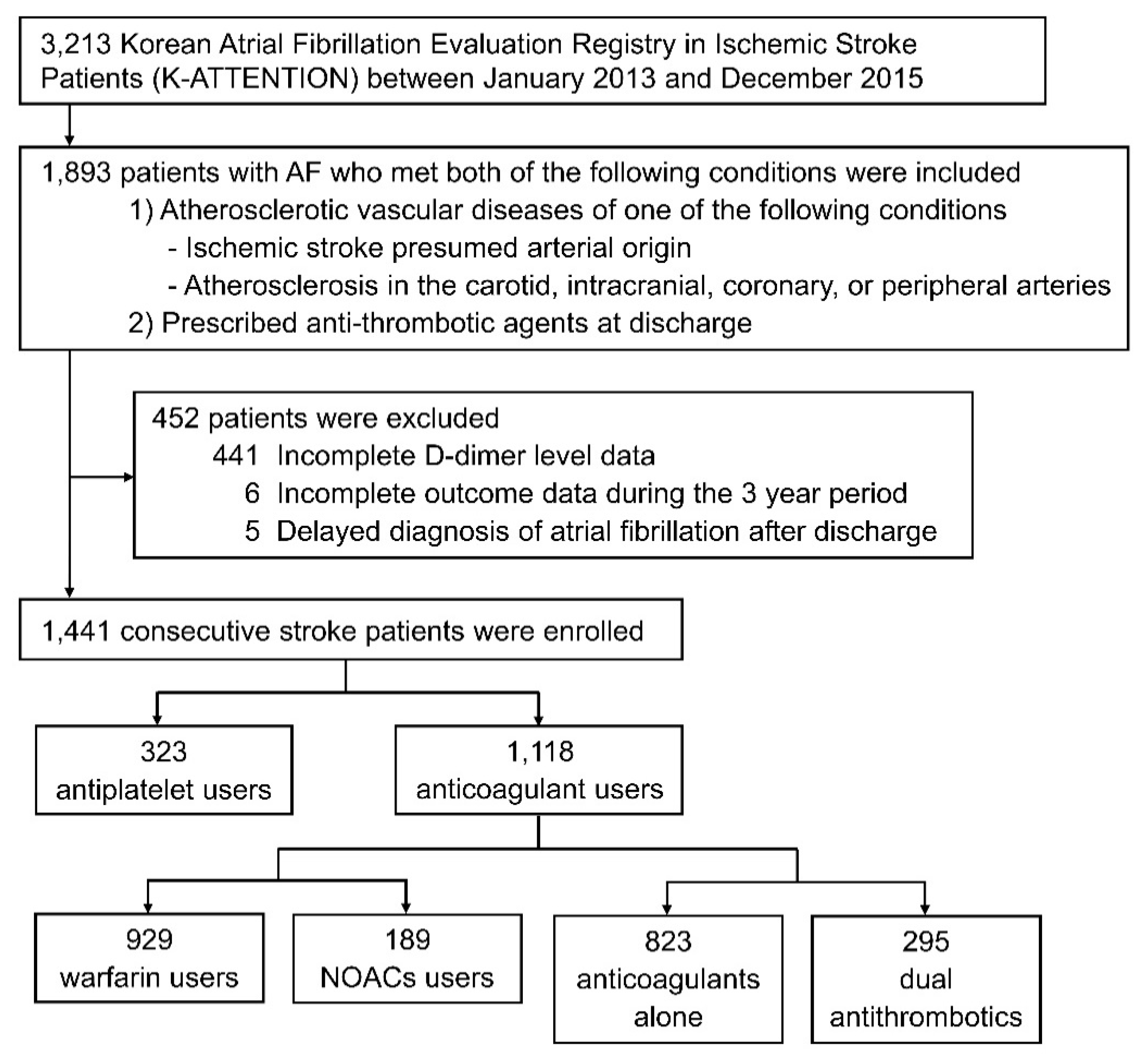
2.1. Subjects
2.2. Ethics Statement
2.3. Protocol and Outcome Measurements
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
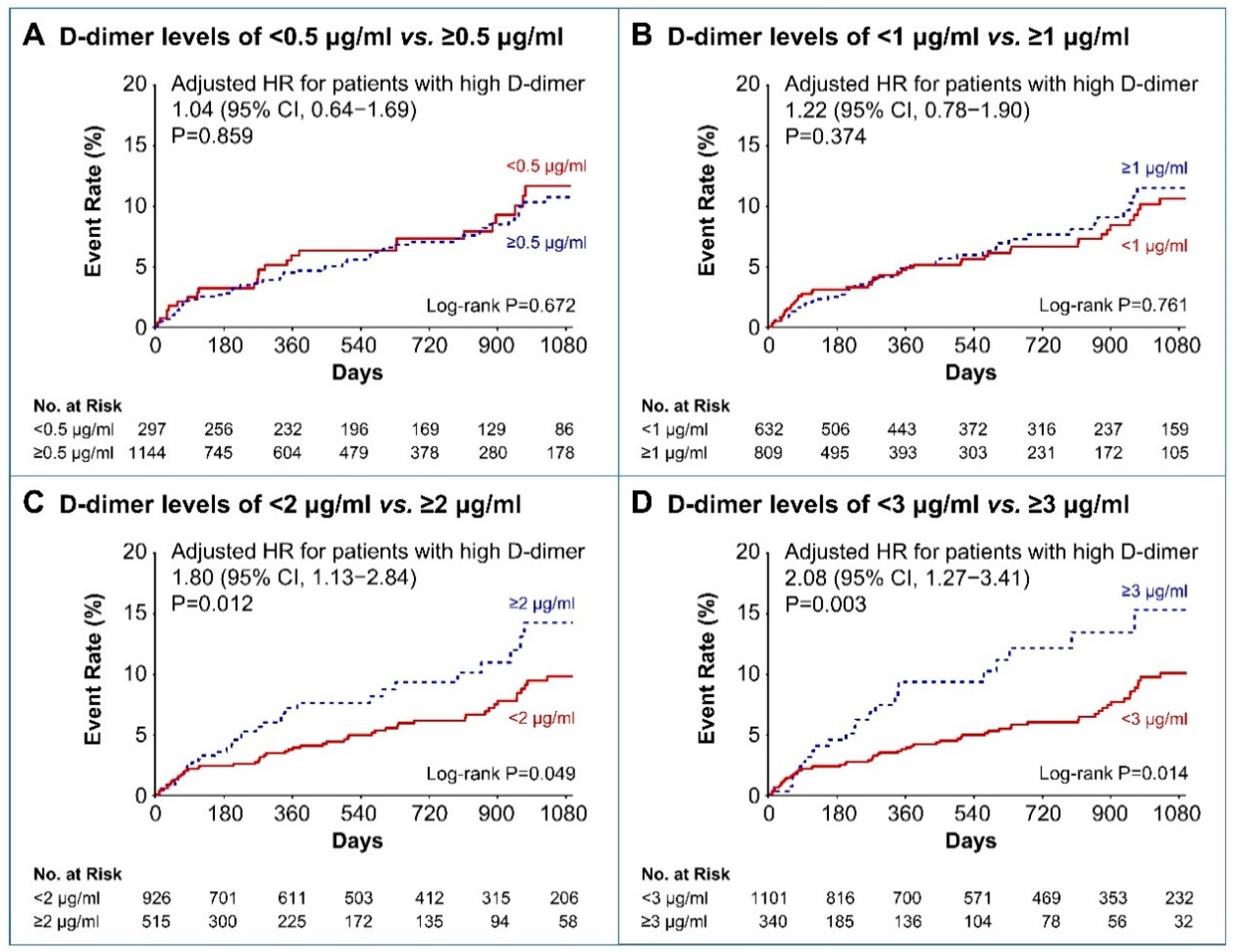
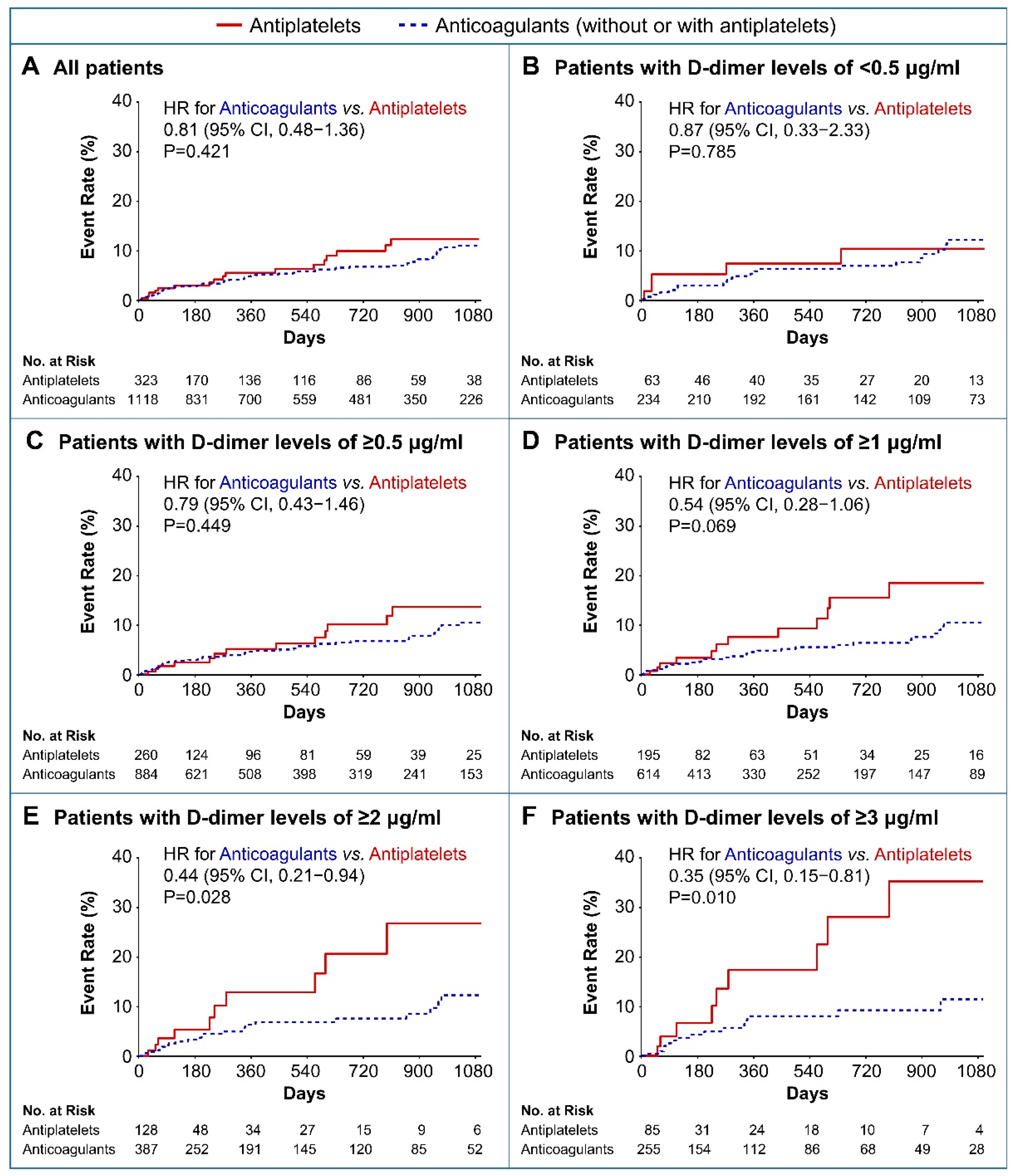
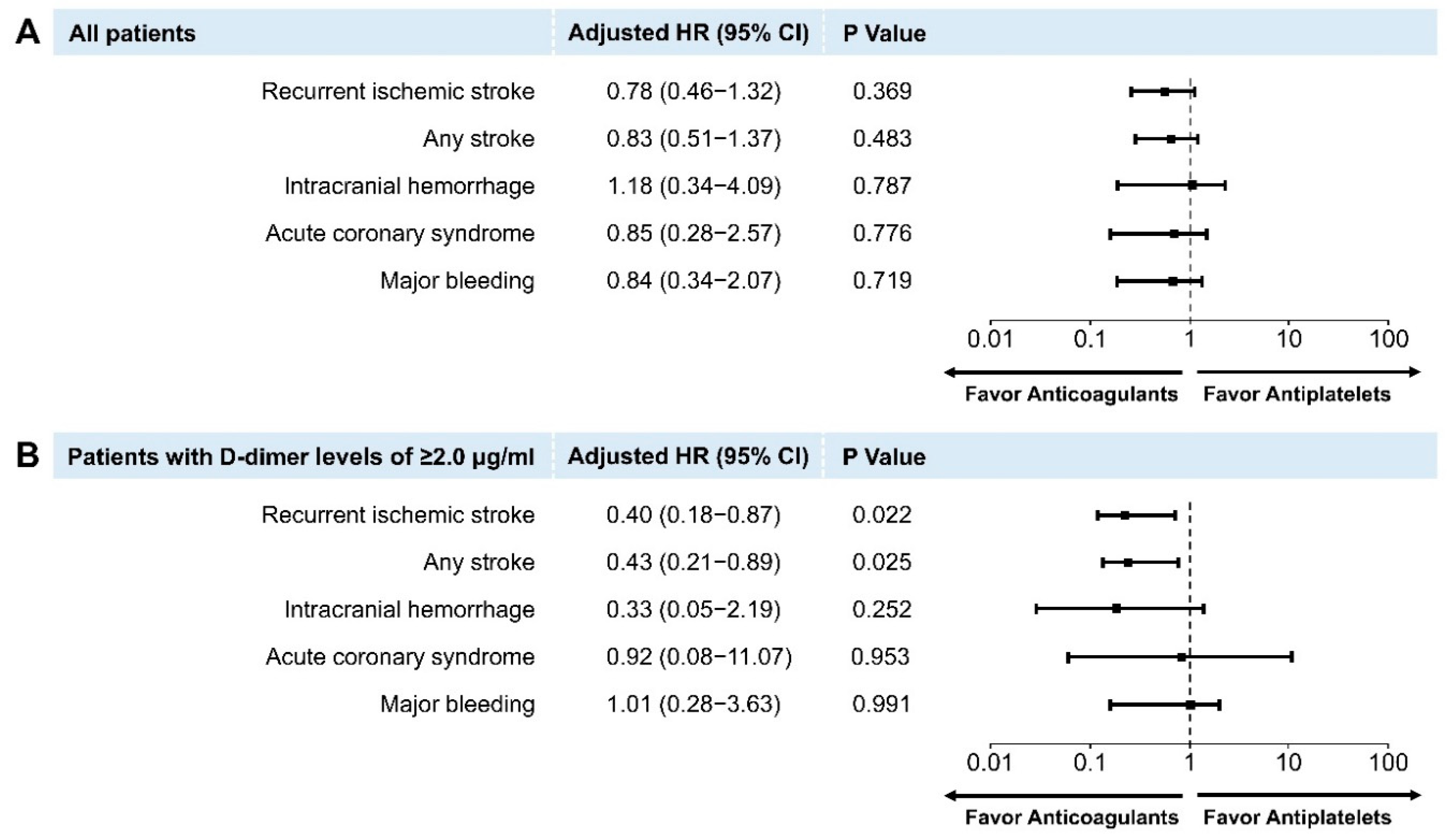
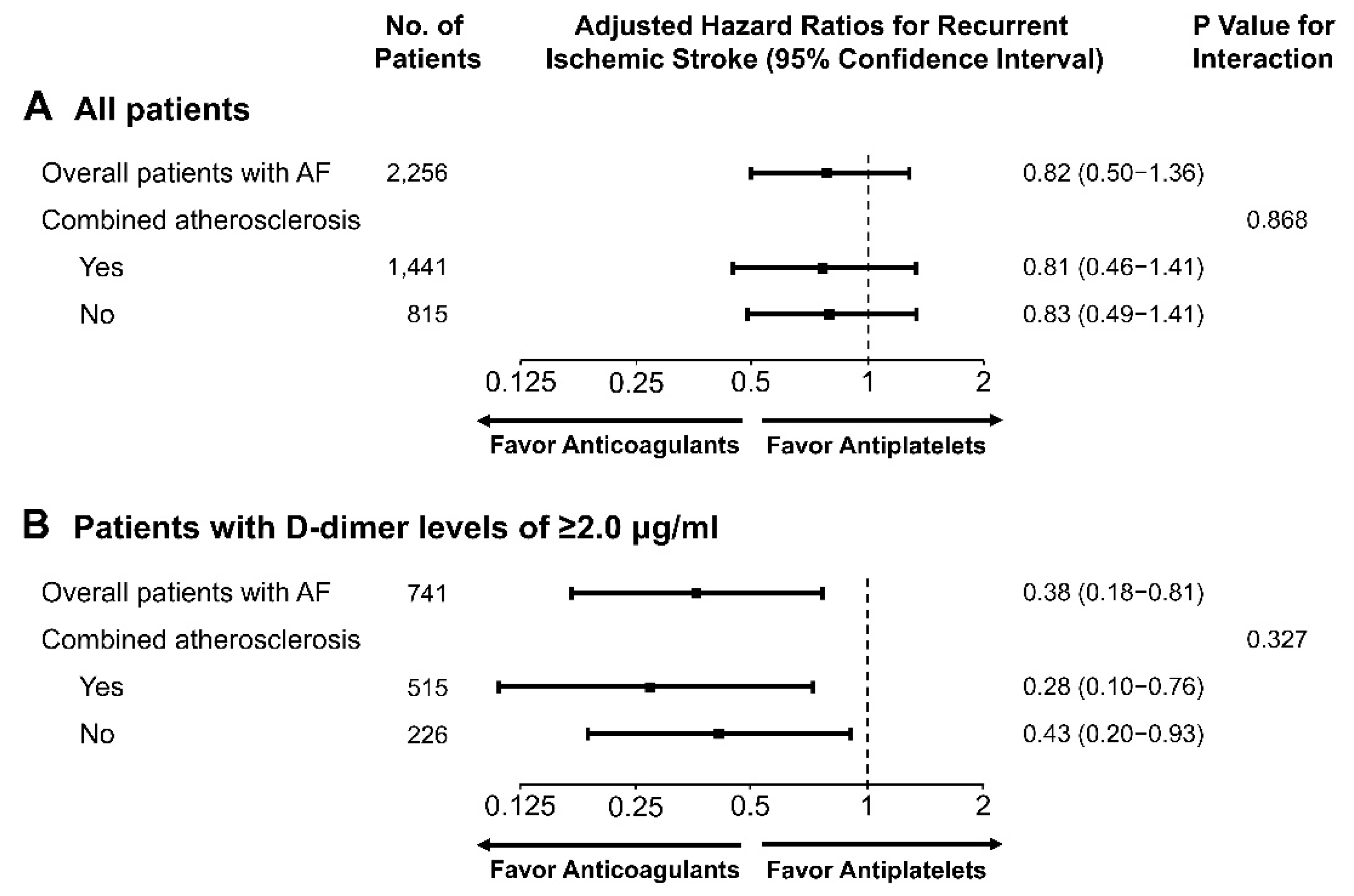
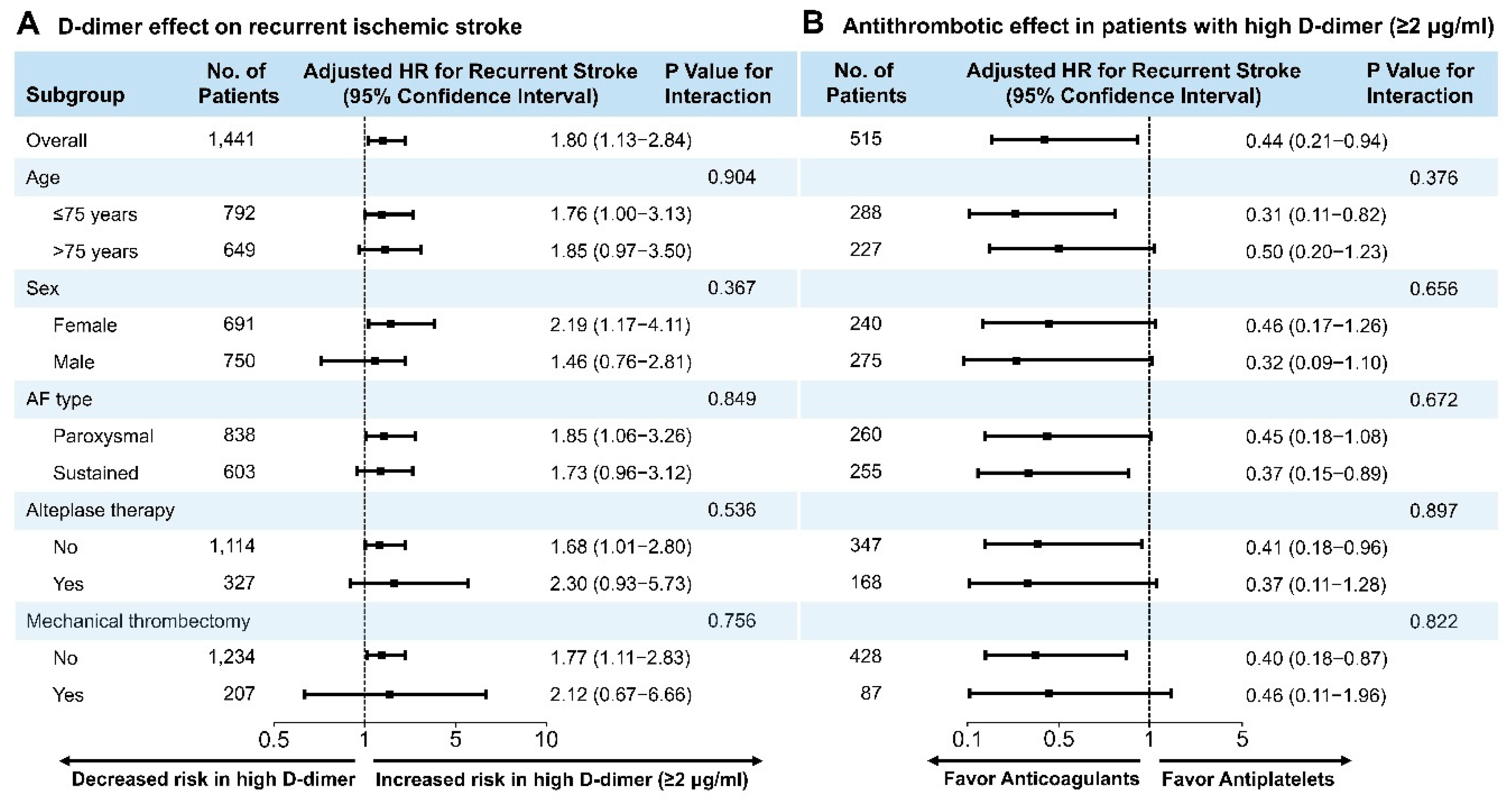
3.2. Clinical Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hankey, G.J. Stroke. Lancet 2017, 389, 641–654. [Google Scholar] [CrossRef]
- Spence, J.D. Secondary stroke prevention. Nat. Rev. Neurol. 2010, 6, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. 2018 Guidelines for the Early Management of Patients with Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2018, 49, e46–e110. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Bhatt, D.L.; Rother, J.; Alberts, M.; Hill, M.D.; Ikeda, Y.; Uchiyama, S.; D’Agostino, R.; Ohman, E.M.; Liau, C.S.; et al. Prevalence, clinical profile, and cardiovascular outcomes of atrial fibrillation patients with atherothrombosis. Am. Heart J. 2008, 156, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, Z.; Oldgren, J.; Siegbahn, A.; Granger, C.B.; Wallentin, L. Biomarkers in atrial fibrillation: A clinical review. Eur. Heart J. 2013, 34, 1475–1480. [Google Scholar] [CrossRef]
- Christersson, C.; Wallentin, L.; Andersson, U.; Alexander, J.H.; Ansell, J.; De Caterina, R.; Gersh, B.J.; Granger, C.B.; Hanna, M.; Horowitz, J.D.; et al. D-dimer and risk of thromboembolic and bleeding events in patients with atrial fibrillation—Observations from the ARISTOTLE trial. J. Thromb. Haemost. 2014, 12, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Siegbahn, A.; Oldgren, J.; Andersson, U.; Ezekowitz, M.D.; Reilly, P.A.; Connolly, S.J.; Yusuf, S.; Wallentin, L.; Eikelboom, J.W. D-dimer and factor VIIa in atrial fibrillation—Prognostic values for cardiovascular events and effects of anticoagulation therapy. A RE-LY substudy. Thromb. Haemost. 2016, 115, 921–930. [Google Scholar] [CrossRef]
- Christersson, C.; Wallentin, L.; Andersson, U.; Alexander, J.H.; Alings, M.; De Caterina, R.; Gersh, B.J.; Granger, C.B.; Halvorsen, S.; Hanna, M.; et al. Effect of apixaban compared with warfarin on coagulation markers in atrial fibrillation. Heart 2019, 105, 235–242. [Google Scholar] [CrossRef]
- Kelly, M.S.; Moczygemba, L.R.; Gatewood, S.S. Concordance of Pharmacist Assessment of Medication Nonadherence with a Self-Report Medication Adherence Scale. J. Pharm. Pract. 2016, 29, 194–198. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1321–1360. [Google Scholar] [CrossRef]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.D.; Aronis, K.N.; Chrispin, J.; Patil, K.D.; Marine, J.E.; Martin, S.S.; Blaha, M.J.; Blumenthal, R.S.; Calkins, H. Obesity, Exercise, Obstructive Sleep Apnea, and Modifiable Atherosclerotic Cardiovascular Disease Risk Factors in Atrial Fibrillation. J. Am. Coll. Cardiol. 2015, 66, 2899–2906. [Google Scholar] [CrossRef] [PubMed]
- Koracevic, G.P. Pragmatic classification of the causes of high D-dimer. Am. J. Emerg. Med. 2009, 27, 1016-e5. [Google Scholar] [CrossRef] [PubMed]
- Habara, S.; Dote, K.; Kato, M.; Sasaki, S.; Goto, K.; Takemoto, H.; Hasegawa, D.; Matsuda, O. Prediction of left atrial appendage thrombi in non-valvular atrial fibrillation. Eur. Heart J. 2007, 28, 2217–2222. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Krarup, L.H.; Sandset, E.C.; Sandset, P.M.; Berge, E. D-dimer levels and stroke progression in patients with acute ischemic stroke and atrial fibrillation. Acta Neurol. Scand. 2011, 124, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, Z.; Lindback, J.; Alexander, J.H.; Hanna, M.; Held, C.; Hylek, E.M.; Lopes, R.D.; Oldgren, J.; Siegbahn, A.; Stewart, R.A.; et al. The ABC (age, biomarkers, clinical history) stroke risk score: A biomarker-based risk score for predicting stroke in atrial fibrillation. Eur. Heart J. 2016, 37, 1582–1590. [Google Scholar] [CrossRef]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Murphy, S.A.; Brown, K.; Jarolim, P.; Mercuri, M.; Antman, E.M.; Morrow, D.A. Cardiovascular Biomarker Score and Clinical Outcomes in Patients with Atrial Fibrillation: A Subanalysis of the ENGAGE AF-TIMI 48 Randomized Clinical Trial. JAMA Cardiol 2016, 1, 999–1006. [Google Scholar] [CrossRef]
- Choi, J.Y.; Jung, J.M.; Kwon, D.Y.; Park, M.H.; Kim, J.H.; Oh, K.; Koh, S.B.; Seo, W.K. Free fatty acid as an outcome predictor of atrial fibrillation-associated stroke. Ann. Neurol. 2016, 79, 317–325. [Google Scholar] [CrossRef]
- Feinberg, W.M.; Erickson, L.P.; Bruck, D.; Kittelson, J. Hemostatic markers in acute ischemic stroke. Association with stroke type, severity, and outcome. Stroke 1996, 27, 1296–1300. [Google Scholar] [CrossRef]
- Montaner, J.; Perea-Gainza, M.; Delgado, P.; Ribo, M.; Chacon, P.; Rosell, A.; Quintana, M.; Palacios, M.E.; Molina, C.A.; Alvarez-Sabin, J. Etiologic diagnosis of ischemic stroke subtypes with plasma biomarkers. Stroke 2008, 39, 2280–2287. [Google Scholar] [CrossRef]
- Paciaroni, M.; Agnelli, G.; Falocci, N.; Caso, V.; Becattini, C.; Marcheselli, S.; Rueckert, C.; Pezzini, A.; Poli, L.; Padovani, A.; et al. Early Recurrence and Cerebral Bleeding in Patients with Acute Ischemic Stroke and Atrial Fibrillation: Effect of Anticoagulation and Its Timing: The RAF Study. Stroke 2015, 46, 2175–2182. [Google Scholar] [CrossRef] [PubMed]
- Koga, M.; Yoshimura, S.; Hasegawa, Y.; Shibuya, S.; Ito, Y.; Matsuoka, H.; Takamatsu, K.; Nishiyama, K.; Todo, K.; Kimura, K.; et al. Higher Risk of Ischemic Events in Secondary Prevention for Patients with Persistent Than Those with Paroxysmal Atrial Fibrillation. Stroke 2016, 47, 2582–2588. [Google Scholar] [CrossRef] [PubMed]
- Takabayashi, K.; Hamatani, Y.; Yamashita, Y.; Takagi, D.; Unoki, T.; Ishii, M.; Iguchi, M.; Masunaga, N.; Ogawa, H.; Esato, M.; et al. Incidence of Stroke or Systemic Embolism in Paroxysmal Versus Sustained Atrial Fibrillation: The Fushimi Atrial Fibrillation Registry. Stroke 2015, 46, 3354–3361. [Google Scholar] [CrossRef] [PubMed]
- Al-Saady, N.M.; Obel, O.A.; Camm, A.J. Left atrial appendage: Structure, function, and role in thromboembolism. Heart 1999, 82, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Stoddard, M.F.; Dawkins, P.R.; Prince, C.R.; Ammash, N.M. Left atrial appendage thrombus is not uncommon in patients with acute atrial fibrillation and a recent embolic event: A transesophageal echocardiographic study. J. Am. Coll. Cardiol. 1995, 25, 452–459. [Google Scholar] [CrossRef]
- Park, H.C.; Shin, J.; Ban, J.E.; Choi, J.I.; Park, S.W.; Kim, Y.H. Left atrial appendage: Morphology and function in patients with paroxysmal and persistent atrial fibrillation. Int. J. Cardiovasc. Imaging 2013, 29, 935–944. [Google Scholar] [CrossRef]
- Manning, W.J.; Weintraub, R.M.; Waksmonski, C.A.; Haering, J.M.; Rooney, P.S.; Maslow, A.D.; Johnson, R.G.; Douglas, P.S. Accuracy of transesophageal echocardiography for identifying left atrial thrombi. A prospective, intraoperative study. Ann. Intern. Med. 1995, 123, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Herzog, C.A.; Bass, D.; Kane, M.; Asinger, R. Two-dimensional echocardiographic imaging of left atrial appendage thrombi. J. Am. Coll. Cardiol. 1984, 3, 1340–1344. [Google Scholar] [CrossRef][Green Version]
- Anaissie, J.; Monlezun, D.; Seelochan, A.; Siegler, J.E.; Chavez-Keatts, M.; Tiu, J.; Pineda, D.; George, A.; Shaban, A.; Abi Rafeh, N.; et al. Left Atrial Enlargement on Transthoracic Echocardiography Predicts Left Atrial Thrombus on Transesophageal Echocardiography in Ischemic Stroke Patients. Biomed. Res. Int. 2016, 2016, 7194676. [Google Scholar] [CrossRef]
- Paciaroni, M.; Agnelli, G.; Falocci, N.; Caso, V.; Becattini, C.; Marcheselli, S.; Rueckert, C.; Pezzini, A.; Poli, L.; Padovani, A.; et al. Prognostic value of trans-thoracic echocardiography in patients with acute stroke and atrial fibrillation: Findings from the RAF study. J. Neurol. 2016, 263, 231–237. [Google Scholar] [CrossRef]
- Seo, W.K.; Jung, J.M.; Kim, J.H.; Koh, S.B.; Bang, O.Y.; Oh, K. Free Fatty Acid Is Associated with Thrombogenicity in Cardioembolic Stroke. Cerebrovasc. Dis. 2017, 44, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Wu, S.; Yang, Y.; Zhu, J.; Zhang, A.; Liang, Y. Plasma fibrin D-dimer and the risk of left atrial thrombus: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0172272. [Google Scholar] [CrossRef] [PubMed]
- Pathan, F.; Hecht, H.; Narula, J.; Marwick, T.H. Roles of Transesophageal Echocardiography and Cardiac Computed Tomography for Evaluation of Left Atrial Thrombus and Associated Pathology: A Review and Critical Analysis. JACC Cardiovasc. Imaging 2018, 11, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Manning, W.J.; Silverman, D.I.; Waksmonski, C.A.; Oettgen, P.; Douglas, P.S. Prevalence of residual left atrial thrombi among patients with acute thromboembolism and newly recognized atrial fibrillation. Arch. Intern. Med. 1995, 155, 2193–2198. [Google Scholar] [CrossRef] [PubMed]
- Jaber, W.A.; Prior, D.L.; Thamilarasan, M.; Grimm, R.A.; Thomas, J.D.; Klein, A.L.; Asher, C.R. Efficacy of anticoagulation in resolving left atrial and left atrial appendage thrombi: A transesophageal echocardiographic study. Am. Heart J. 2000, 140, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, K.; Ninomiya, T. Stroke and cerebrovascular diseases in patients with chronic kidney disease. Lancet Neurol. 2014, 13, 823–833. [Google Scholar] [CrossRef]
- Gordge, M.P.; Faint, R.W.; Rylance, P.B.; Ireland, H.; Lane, D.A.; Neild, G.H. Plasma D dimer: A useful marker of fibrin breakdown in renal failure. Thromb. Haemost. 1989, 61, 522–525. [Google Scholar] [CrossRef]
- Heidbuchel, H.; Verhamme, P.; Alings, M.; Antz, M.; Diener, H.C.; Hacke, W.; Oldgren, J.; Sinnaeve, P.; Camm, A.J.; Kirchhof, P.; et al. Updated European Heart Rhythm Association practical guide on the use of non-vitamin-K antagonist anticoagulants in patients with non-valvular atrial fibrillation: Executive summary. Eur. Heart J. 2017, 38, 2137–2149. [Google Scholar] [CrossRef]
- Kim, S.J.; Ryoo, S.; Kwon, S.; Park, Y.K.; Kim, J.P.; Lee, G.Y.; Bang, O.Y. Is atrial fibrillation always a culprit of stroke in patients with atrial fibrillation plus stroke? Cerebrovasc. Dis. 2013, 36, 373–382. [Google Scholar] [CrossRef]
- Mohr, J.P.; Thompson, J.L.; Lazar, R.M.; Levin, B.; Sacco, R.L.; Furie, K.L.; Kistler, J.P.; Albers, G.W.; Pettigrew, L.C.; Adams, H.P., Jr.; et al. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N. Engl. J. Med. 2001, 345, 1444–1451. [Google Scholar] [CrossRef]
- McGrath, E.R.; Kapral, M.K.; Fang, J.; Eikelboom, J.W.; Conghaile, A.; Canavan, M.; O’Donnell, M.J.; Investigators of the Ontario Stroke, R. Antithrombotic therapy after acute ischemic stroke in patients with atrial fibrillation. Stroke 2014, 45, 3637–3642. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, K.; Li, Y.G.; Rashed, W.A.; Al Mahmeed, W.; Shehab, A.; Zubaid, M.; Lip, G.Y.H. Secondary stroke prevention and guideline adherent antithrombotic treatment in patients with atrial fibrillation: Insights from the Gulf Survey of atrial fibrillation events (Gulf SAFE). Int. J. Cardiol. 2019, 274, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, Y.; Mizumaki, K.; Nishida, K.; Hirai, T.; Sakabe, M.; Oda, Y.; Joho, S.; Fujiki, A.; Nozawa, T.; Inoue, H. Anticoagulation control quality affects the D-dimer levels of atrial fibrillation patients. Circ. J. 2012, 76, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Chung, J.W.; Ahn, M.J.; Kim, S.; Seok, J.M.; Jang, H.M.; Kim, G.M.; Chung, C.S.; Lee, K.H.; Bang, O.Y. Hypercoagulability and Mortality of Patients with Stroke and Active Cancer: The OASIS-CANCER Study. J. Stroke 2017, 19, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; Mariani, M.V.; Maraone, A.; Piro, A.; Ceccacci, A.; Tarsitani, L.; Maestrini, V.; Mancone, M.; Lavalle, C.; Pasquini, M.; et al. Triggers for Atrial Fibrillation: The Role of Anxiety. Cardiol. Res. Pract. 2019, 2019, 1208505. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.H.; Nattel, S.; Kalman, J.M.; Sanders, P. Modifiable Risk Factors and Atrial Fibrillation. Circulation 2017, 136, 583–596. [Google Scholar] [CrossRef]

| Patients with D-Dimer Levels of < 2.0 μg/mL (n = 926) | Patients with D-Dimer Levels of ≥ 2.0 μg/mL (n = 515) | Total (n = 1441) | p Value | |
|---|---|---|---|---|
| Age, years (mean ± SD) | 73.6 ± 9.6 | 73.2 ± 9.8 | 73.5 ± 9.7 | 0.454 |
| Male, n (%) | 475 (51.3) | 275 (53.4) | 750 (52.0) | 0.477 |
| Valvular AF, n (%) | 23 (2.5) | 8 (1.6) | 31 (2.2) | 0.328 |
| AF type, n (%) | <0.001 | |||
| Paroxysmal AF | 578 (62.4) | 260 (50.5) | 838 (58.2) | |
| Sustained AF | 348 (37.6) | 255 (49.5) | 603 (41.8) | |
| Body mass index | 23.2 ± 3.3 | 23.4 ± 3.4 | 23.3 ± 3.3 | 0.465 |
| History of Risk Factors, n (%) | ||||
| Hypertension | 650 (70.2) | 376 (73.0) | 1026 (71.2) | 0.284 |
| Diabetes mellitus | 268 (28.9) | 142 (27.6) | 410 (28.5) | 0.623 |
| Dyslipidemia | 217 (23.4) | 96 (18.6) | 313 (21.7) | 0.041 |
| Congestive heart failure | 34 (3.7) | 22 (4.3) | 56 (3.9) | 0.673 |
| Current smoking | 142 (15.3) | 62 (12.0) | 204 (14.2) | 0.101 |
| Prior stroke or TIA | 276 (29.8) | 178 (34.6) | 454 (31.5) | 0.071 |
| Biochemical Variables (mean ± SD) | ||||
| D-dimer, μg/mL | 0.8 ± 0.5 | 6.1 ± 6.5 | 2.7 ± 4.6 | <0.001 |
| LDL-C, mg/dL | 98.4 ± 33.9 | 97.6 ± 34.6 | 98.1 ± 34.1 | 0.708 |
| Triglyceride, mg/dL | 99.4 ± 63.1 | 92.4 ± 48.2 | 96.9 ± 58.3 | 0.019 |
| HDL-C, mg/dL | 48.3 ± 19.2 | 47.0 ± 17.1 | 47.8 ± 18.5 | 0.186 |
| Glycated hemoglobin, % | 6.1 ± 2.0 | 5.9 ± 1.0 | 6.0 ± 1.7 | 0.078 |
| Admission glucose, mg/dL | 139.2 ± 81.2 | 137.8 ± 48.5 | 138.7 ± 71.2 | 0.687 |
| Creatinine clearance, mL/min | 65.6 ± 28.0 | 56.3 ± 27.8 | 62.3 ± 28.3 | <0.001 |
| Pre-stroke mRS, median (IQR) | 0 (0;1) | 0 (0;2) | 0 (0; 1) | 0.054 |
| Initial NIHSS, median (IQR) | 6 (2;13) | 13 (6;18) | 9 (2; 15) | <0.001 |
| Intravenous alteplase, n (%) | 159 (17.2) | 168 (32.6) | 327 (22.7) | <0.001 |
| Mechanical thrombectomy, n (%) | 120 (13.0) | 87 (16.9) | 207 (14.4) | 0.050 |
| CHA2DS2-VASc score, median (IQR) | 5 (4; 6) | 5 (4;6) | 5 (4;6) | 0.001 |
| CHA2DS2-VASc ≥ 5, n (%) | 552 (59.6) | 372 (72.2) | 924 (64.1) | <0.001 |
| AIS presumed arterial origin, n (%) | 276 (29.8) | 162 (31.5) | 438 (30.4) | 0.553 |
| Large Artery Atherosclerosis, n (%) | ||||
| Carotid atherosclerosis | 260 (31.7) | 154 (32.7) | 414 (32.1) | 0.772 |
| Intracranial atherosclerosis | 648 (78.0) | 314 (65.4) | 962 (73.4) | <0.001 |
| Coronary atherosclerosis | 144 (15.6) | 69 (13.4) | 213 (14.8) | 0.305 |
| Peripheral atherosclerosis | 13 (1.4) | 8 (1.6) | 21 (1.5) | 1.000 |
| Transthoracic Echocardiographic Findings * | ||||
| LA thrombus, n (%) | 11 (1.3) | 11 (2.3) | 22 (1.7) | 0.259 |
| LA volume index, mL/m2 | 66.0 ± 24.8 | 67.0 ± 24.9 | 66.3 ± 24.8 | 0.493 |
| LVEF < 50%, n (%) | 122 (14.5) | 74 (15.6) | 196 (14.9) | 0.677 |
| LV diastolic dysfunction, n (%) | 596 (71.2) | 343 (72.3) | 939 (71.6) | 0.727 |
| Moderate–severe AR, n (%) | 9 (1.1) | 13 (2.7) | 22 (1.7) | 0.042 |
| Moderate–severe MR, n (%) | 26 (3.1) | 23 (4.9) | 49 (3.8) | 0.149 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, K.-H.; Seo, W.-K.; Park, M.-S.; Kim, J.-T.; Chung, J.-W.; Bang, O.Y.; Kim, G.-M.; Song, T.-J.; Kim, B.J.; Heo, S.H.; et al. Baseline D-Dimer Levels as a Risk Assessment Biomarker for Recurrent Stroke in Patients with Combined Atrial Fibrillation and Atherosclerosis. J. Clin. Med. 2019, 8, 1457. https://doi.org/10.3390/jcm8091457
Choi K-H, Seo W-K, Park M-S, Kim J-T, Chung J-W, Bang OY, Kim G-M, Song T-J, Kim BJ, Heo SH, et al. Baseline D-Dimer Levels as a Risk Assessment Biomarker for Recurrent Stroke in Patients with Combined Atrial Fibrillation and Atherosclerosis. Journal of Clinical Medicine. 2019; 8(9):1457. https://doi.org/10.3390/jcm8091457
Chicago/Turabian StyleChoi, Kang-Ho, Woo-Keun Seo, Man-Seok Park, Joon-Tae Kim, Jong-Won Chung, Oh Young Bang, Geong-Moon Kim, Tae-Jin Song, Bum Joon Kim, Sung Hyuk Heo, and et al. 2019. "Baseline D-Dimer Levels as a Risk Assessment Biomarker for Recurrent Stroke in Patients with Combined Atrial Fibrillation and Atherosclerosis" Journal of Clinical Medicine 8, no. 9: 1457. https://doi.org/10.3390/jcm8091457
APA StyleChoi, K.-H., Seo, W.-K., Park, M.-S., Kim, J.-T., Chung, J.-W., Bang, O. Y., Kim, G.-M., Song, T.-J., Kim, B. J., Heo, S. H., Jung, J.-M., Oh, K., Kim, C. K., Yu, S., Park, K. Y., Kim, J.-M., Park, J.-H., Choi, J. C., Hwang, Y.-H., & Kim, Y.-J. (2019). Baseline D-Dimer Levels as a Risk Assessment Biomarker for Recurrent Stroke in Patients with Combined Atrial Fibrillation and Atherosclerosis. Journal of Clinical Medicine, 8(9), 1457. https://doi.org/10.3390/jcm8091457





