Clinical Infections by Herpesviruses in Patients Treated with Valproic Acid: A Nested Case-Control Study in the Spanish Primary Care Database, BIFAP
Abstract
1. Introduction
2. Experimental Section
2.1. Data Source Description
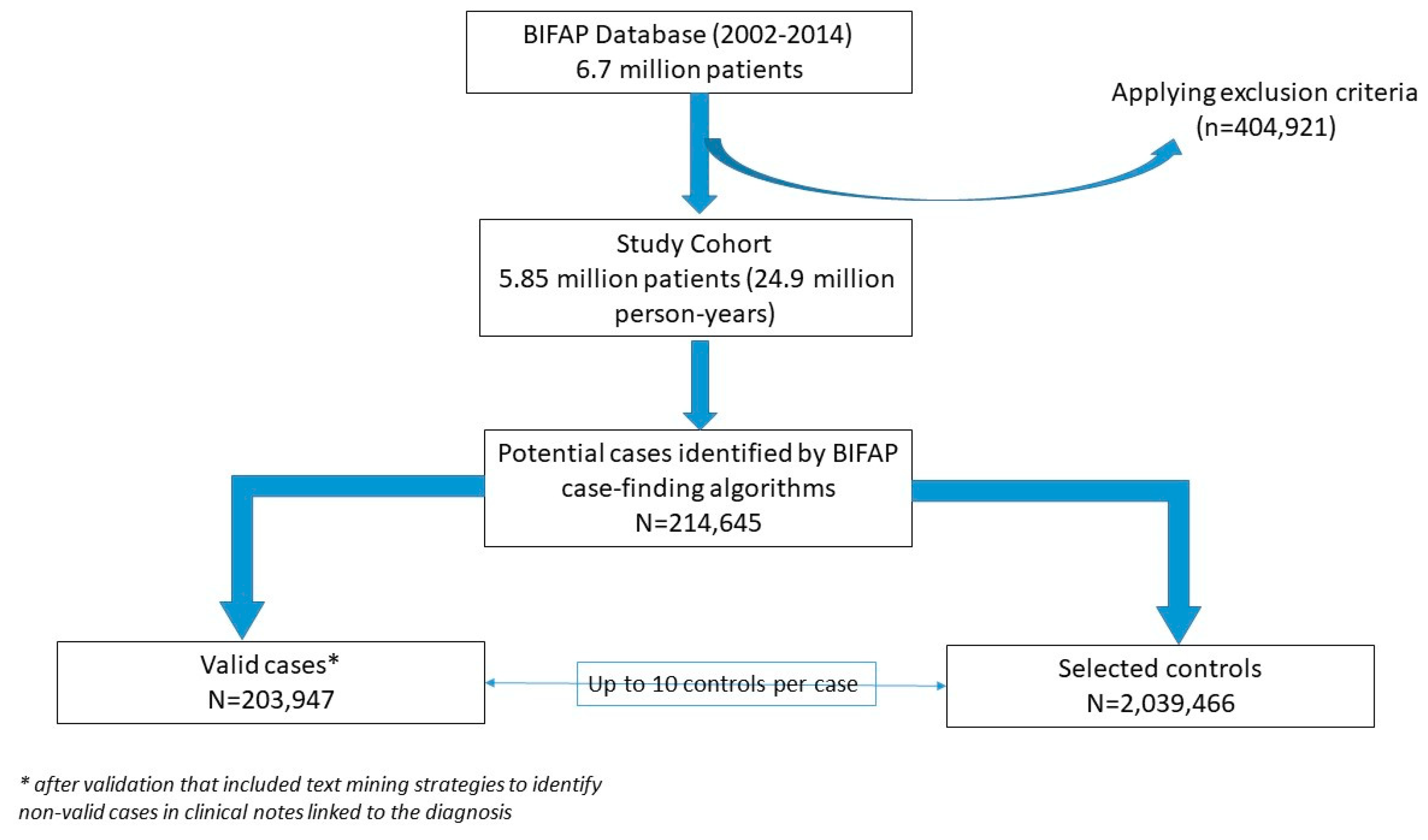
2.2. Study Design
2.3. Case Identification
- Orolabial herpes and genital herpes: caused by HHV-1 and 2 (herpes simplex virus);
- Varicella: caused by HHV-3 (varicella-zoster virus);
- Infectious mononucleosis: caused by HHV-4 (Epstein–Barr virus) or HHV-5 (human cytomegalovirus);
- Roseola infantum: caused by HHV-6;
- Kaposi’s sarcoma: caused by HHV-8 (Kaposi’s sarcoma-associated herpesvirus).
2.4. Selection of Controls
2.5. Exposure Definition
2.6. Potential Confounding Variables
- Diseases affecting the immune system; Human Immunodeficiency Virus (HIV) infection, other immunodeficiencies;
- Chronic diseases impairing health conditions: asthma, chronic obstructive pulmonary disease, ischaemic heart disease, stroke, diabetes mellitus, heart failure, hypertension, hyperlipidemia, chronic hepatitis, chronic renal failure, and Alzheimer disease;
- Lifestyle conditions: alcohol abuse, smoking, body mass index, and obesity;
- Diseases related to the potential use of VPA: depression, neuropathic pain, epilepsy, bipolar disorder, and migraine;
- Comedications: systemic corticosteroids, systemic antiviral drugs, immunosuppressant, antibiotics, anxiolytics, antipsychotic drugs, and antiepileptic drugs other than VPA.
- The number of visits to the PCP (<6, 6–15, 16–24, >24) was ascertained in the two-year period before the index date.
2.7. Statistical Analysis
2.8. Ethics Review
3. Results
3.1. Characteristics of the Study Cohort, Validation, and Incidence of Clinical Infections by Herpesviruses in BIFAP Database
3.2. Characteristics of Cases and Controls Included in the Study
3.3. Risk of Clinical Infection by Herpesviruses Associated to the Use of VPA
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Procedures to Build the ICPC-BIFAP Medical Terms Dictionaries
Appendix B. BIFAP Case-Finding Strategies to Identify Diseases Caused by Herpesvirus Infections
| ICD-9 Code | Description |
|---|---|
| 052 | Chickenpox |
| 052.0 | Postvaricella encephalitis |
| 052.1 | Varicella (hemorrhagic) pneumonitis |
| 052.2 | Postvaricella myelitis |
| 052.7 | Chickenpox with other specified complications |
| 052.8 | Chickenpox with unspecified complication |
| 052.9 | Varicella without mention of complication |
| 054 | Herpex simplex |
| 054.0 | Eczema herpeticum |
| 054.1 | Genital herpes |
| 054.10 | Genital herpes, unspecified |
| 054.11 | Herpetic vulvovaginitis |
| 054.12 | Herpetic ulceration of vulva |
| 054.13 | Herpetic infection of penis |
| 054.19 | Other genital herpes |
| 054.2 | Herpetic gingivostomatitis |
| 054.3 | Herpetic meningoencephalitis |
| 054.4 | Herpex simplex with ophthalmic complications |
| 054.40 | Herpes simplex with unspecified ophthalmic complication |
| 054.41 | Herpes simplex dermatitis of eyelid |
| 054.42 | Dendritic keratitis |
| 054.43 | Herpes simplex disciform keratitis |
| 054.44 | Herpes simplex iridocyclitis |
| 054.49 | Herpes simplex with other ophthalmic complications |
| 054.5 | Herpetic septicemia |
| 054.6 | Herpetic whitlow |
| 054.7 | Herpex simplex with other specified complications |
| 054.71 | Visceral herpes simplex |
| 054.72 | Herpes simplex meningitis |
| 054.73 | Herpes simplex otitis externa |
| 054.74 | Herpes simplex myelitis |
| 054.79 | Herpes simplex with other specified complications |
| 054.8 | Herpes simplex with unspecified complication |
| 054.9 | Herpes simplex without mention of complication |
| 075 | Infectious mononucleosis |
| 078.5 | Cytomegaloviral disease |
| 771.1 | Congenital cytomegalovirus infection |
| 058.1 | Roseola infantum |
| 058.10 | Roseola infantum, unspecified |
| 058.11 | Roseola infantum due to human herpesvirus 6 |
| 058.12 | Roseola infantum due to human herpesvirus 7 |
| 058.21 | Human herpesvirus 6 encephalitis |
| 058.81 | Human herpesvirus 6 infection |
| 058.82 | Human herpesvirus 7 infection |
| ICPC Code | BIFAP Subcode | Description |
|---|---|---|
| A77 | 9 | Cytomegalovirus infection |
| A75 | 1 | Infectious mononucleosis |
| A75 | 2 | Epstein–Barr virus infection |
| A72 | 1 | Chickenpox |
| D83 | 19 | Herpetic stomatitis |
| D83 | 20 | Herpetic gingivostomatitis |
| D83 | 26 | Herpetic gingivitis |
| F16 | 10 | Herpes simplex eyelid |
| F73 | 18 | Herpes simplex eye |
| S71 | 1 | Herpex simplex mouth/lip |
| S71 | 2 | Herpetic whitlow |
| S71 | 3 | Herpetic infection skin |
| S71 | 5 | Herpes simplex |
| X90 | 1 | Herpetic infection genital female |
| Y72 | 1 | Herpetic infection genital male |
| Y72 | 999 | Herpetic infection genital & annus male |
| W71 | 6 | Herpes pregnancy |
| F73 | 20 | Herpes keratitis |
| S71 | 4 | Herpes not specified |
| Specific Strings (in Spanish) |
|---|
| HERPES OR HERPET% OR HERPEX OR CITOMEGALOVIRUS OR CMV OR MONONUCLEOSIS OR VARICELA * OR EPSTEIN OR ROSEOLA† OR KAPOSI |
References
- Cowie, M.R.; Blomster, J.I.; Curtis, L.H.; Duclaux, S.; Ford, I.; Fritz, F.; Goldman, S.; Janmohamed, S.; Kreuzer, J.; Leenay, M.; et al. Electronic health records to facilitate clinical research. Clin. Res. Cardiol. 2017, 106, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Peterson, G.M.; Naunton, M. Valproate: A simple chemical with so much to offer. J. Clin. Pharm. Ther. 2005, 30, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Calvo, A.; Saiz, J.C.; Sobrino, F. and Martín-Acebes, M.A. Inhibition of enveloped virus infection of cultured cells by valproic acid. J. Virol. 2011, 85, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Crespillo, A.J.; Praena, B.; Bello-Morales, R.; Lerma, L.; Vázquez-Calvo, A.; Martín-Acebes, M.A.; Tabarés, E.; Sobrino, F.; López-Guerrero, J.A. Inhibition of herpes virus infection in oligodendrocyte cultured cells by valproic acid. Virus. Res. 2016, 214, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Vivithanaporn, P.; Siemieniuk, R.A.; Krentz, H.B.; Maingat, F.; Gill, M.J.; Power, C. Outcomes and immune benefits of anti-epileptic drug therapy in HIV/AIDS. BMC Neurology 2010. [Google Scholar] [CrossRef] [PubMed]
- Fujii, K.; Suzuki, N.; Yamamoto, T.; Suzuki, D.; Iwatsuki, K. Valproic acid inhibits proliferation of EB virus-infected natural killer cells. Hematology 2012, 17, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Ornaghi, S.; Davis, J.N.; Gorres, K.L.; Miller, G.; Paidas, M.J.; van den Pol, A.N. Mood stabilizers inhibit citomegalovirus infection. Virology 2016, 499, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.N.; Arbiser, J.L.; Offermann, M.K. Valproic acid induces human herpes virus-8 lytic gene expression in BCBL-1cells. AIDS 2000, 14, 899–902. [Google Scholar] [CrossRef]
- Vázquez-Calvo, A.; Martín-Acebes, M.A.; Sáiz, J.C.; Sobrino, F.; de la Torre, J.C. Inhibition of multiplication of the prototypic arenavirus LCMV by valproic acid. Antiviral. Res. 2013, 99, 172–179. [Google Scholar]
- BIFAP. Base de datos para la Investigación Farmacoepidemiológica en Atención Primaria. Available online: www.bifap.org (accessed on 4 August 2019).
- Abbing-Karahagopian, V.; Kurz, X.; de Vries, F.P.; van Staa, T.; Alvarez, Y.; Hesse, U.; Hasford, J.; van Dijk, L.; J. de Abajo, F.; G. Weil, J.; et al. Bridging differences in outcomes of pharmacoepidemiological studies: Design and first results of the PROTECT project. Curr. Clin. Pharmacol. 2014, 9, 130–138. [Google Scholar] [CrossRef]
- Huerta, C.; Abbing-Karahagopian, V.; Requena, G.; Oliva, B.; Alvarez, Y.; Gardarsdottir, H.; Miret, M.; Schneider, C.; Gil, M.; Souverein, P.C.; et al. Exposure to benzodiazepines (anxiolytics, hypnotics and related drugs) in seven European electronic healthcare databases: A cross-national descriptive study from the PROTECT-EU Project. Pharmacoepidemiol Drug Saf. 2016, 25, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Requena, G.; Huerta, C.; Gardarsdottir, H.; Logie, J.; González-González, R.; Abbing-Karahagopian, V.; Miret, M.; Schneider, C.; Souverein, P.C.; Webb, D.; et al. Hip/femur fractures associated with the use of benzodiazepines (anxiolytics, hypnotics and related drugs): A methodological approach to assess consistencies across databases from the PROTECT-EU project. Pharmacoepidemiol. Drug Saf. 2016, 25, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.; Rodríguez-Miguel, A.; Montoya-Catalá, H.; González-González, R.; Álvarez-Gutiérrez, A.; Rodríguez-Martín, S.; García-Rodríguez, L.A.; de Abajo, F.J. Validation study of colorectal cancer diagnosis in the Spanish primary care database, BIFAP. Pharmacoepidemiol. Drug Saf. 2019, 28, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Ray, W. Evaluating Medication Effects Outside of Clinical Trials: New-User Designs. Am. J. Epidemiol. 2003, 158, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Routy, J.P. Valproic acid: A potential role in treating latent HIV infection. Lancet 2005, 366, 523–524. [Google Scholar] [CrossRef]
- Daigle, D.; Gradoville, L.; Tuck, D.; Schulz, V.; Wang’ondu, R.; Ye, J.; Gorres, K.; Miller, G. Valproic acid antagonizes the capacity of other histone deacetylase inhibitors to activate the Epstein-Barr virus lytic cycle. J. Virol. 2011, 85, 5628–5643. [Google Scholar] [CrossRef] [PubMed]
- Mardivirin, L.; Descamps, V.; Lacroix, A.; Delebassée, S.; Ranger-Rogez, S. Early effects of drugs responsible for DRESS on HHV-6 replication in vitro. J. Clin Virol 2009, 46, 300–302. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, H.; Kaufmann, J.K.; Wang, P.Y.; Nguyen, T.; Speranza, M.-C.; Kasai, K.; Okemoto, k.; Otsuki, A.; Nakano, I.; Fernandez, S.; et al. Histone deacetylase 6 inhibition enhances oncolytic viral replication in glioma. J. Clin Invest. 2015, 125, 4269–4280. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, A.; Patel, A.; Kasai, K.; Suzuki, M.; Kurozumi, K.; Antonio Chiocca, E.; Saeki, Y. Histone Deacetylase Inhibitors Augment Antitumor Efficacy of Herpes-based Oncolytic Viruses. Mol. Ther. 2008, 16, 1546–1555. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, M.; Suhan, T.; Reinisch, A.; Reisenauer, A.; Fleckenstein, C.; Eikel, D.; Gümbel, H.; Doerr, H.W.; Nau, H.; Cinatl, J. Increased Replication of Human Cytomegalovirus in Retinal Pigment Epithelial Cells by Valproic Acid Depends on Histone Deacetylase Inhibition. Invest. Ophthalmol. Vis. Sci 2005, 46, 3451–3457. [Google Scholar] [CrossRef]
- Falkenberg, K.J.; Johnstone, R.W. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Drug Discov. 2014, 13, 673–691. [Google Scholar] [CrossRef] [PubMed]
- Paglino, J.C.; van den Pol, A.N. Vesicular stomatitis virus has extensive oncolytic activity against human sarcomas: Rare resistance is overcome by blocking interferon pathways. J. Virol. 2011, 85, 9346–9358. [Google Scholar] [CrossRef] [PubMed]
- Peña-Rey, I.; Martínez, M.V.; Cortés, M.; Amela, C. La varicela en España: Incidencia y hospitalización. Rev Pediatr Aten Primaria 2004, 6, 559–571. [Google Scholar]
- Asociacion Española Vacunología website. Available online: https://www.vacunas.org/vacunacion-frente-a-la-varicela/ (accessed on 18 August 2019).
- Pharmacovigilance Risk Assessment Committee (PRAC) Assessment report Referral under Article 31 of Directive 2001/83/EC Medicinal products containing substances related to valproate EMA/198940/2018. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Valproate_2017_31/Position_provided_by_CMDh/WC500250221.pdf (accessed on 4 August 2019).
| Herpes Virus Type | Disease in Humans | Controls (N = 2,039,466) | Cases (N = 203,947) |
|---|---|---|---|
| HHV-1/HHV-2 | Oral/genital herpes | 1,018,000 (49.9%) | 101,800 (49.9%) |
| HHV-3 | Varicella/herpes zoster | 869,906 (42.7%) | 86,991 (42.7%) |
| HHV-4/HHV-5 | Infectious mononucleosis | 146,030 (7.2%) | 14,603 (7.2%) |
| HHV-6 | Roseola infantum | 5390 (0.3%) | 539 (0.3%) |
| HHV-8 | Kaposi’s sarcoma | 140 (0.007%) | 14 (0.007%) |
| Sociodemographic Variable | Categories | Cases (n (%)) N = 203,947 | Controls (n (%)) N = 2,039,466 |
|---|---|---|---|
| Age | 0 year | 122,452 (6%) | 9317 (4.57%) |
| 1–4 years | 529,832 (25.98%) | 56,789 (27.84%) | |
| 5–9 years | 233,747 (11.46%) | 22,660 (11.11%) | |
| 10–17 years | 135,515 (6.64%) | 13,465 (6.6%) | |
| 18–44 years | 557,298 (27.33%) | 55,627 (27.28%) | |
| 45–64 years | 283,505 (13.9%) | 28,318 (13.88%) | |
| 65–74 years | 103,221 (5.06%) | 10,361 (5.08%) | |
| 75+ | 73,896 (3.62%) | 7410 (3.63%) | |
| Gender | Male | 1,190,590 (58.38%) | 119,059 (58.38%) |
| Female | 848,876 (41.62%) | 84,888 (41.62%) |
| Lifestyle/Comorbidities | Categories | Cases (n (%)) N = 203,947 | Controls (n (%)) N = 2,039,466 | Crude OR (95%CI) |
|---|---|---|---|---|
| Number of visits to the PCP (last 3 years) | 1–5 | 686,728 (33.67%) | 26,081 (12.79%) | 1 (ref) |
| 6–15 | 696,274 (34.14%) | 73,880 (36.23%) | 3.14 (3.09–3.18) | |
| 16–24 | 332,186 (16.29%) | 46,920 (23.01%) | 4.85 (4.77–4.93) | |
| 25+ | 324,278 (15.9%) | 57,066 (27.98%) | 6.78 (6.67–6.90) | |
| Smoking | Non smoker | 271,264 (13.3%) | 30,853 (15.13%) | 1 (ref) |
| Ex-smoker | 27,752 (1.36%) | 3673 (1.8%) | 1.19 (1.14–1.23) | |
| Current smoker | 194,223 (9.52%) | 20,395 (10%) | 0.92 (0.90–0.94) | |
| Missing information | 1,546,227 (75.82%) | 149,026 (73.07%) | 0.81 (0.80–0.83) | |
| Alcohol consumption | No | 1,533,808 (75.21%) | 147,434 (72.29%) | 1 (ref) |
| Yes | 505,658 (24.79%) | 56,513 (27.71%) | 1.24 (1.23–1.26) | |
| Chronic obstructive pulmonary disease | No | 2,021,282 (99.11%) | 201,892 (98.99%) | 1 (ref) |
| Yes | 2055 (1.01%) | 18,184 (0.89%) | 1.14 (1.09–1.20) | |
| Asthma | No | 181,273 (88.88%) | 1,874,907 (91.93%) | 1 (ref) |
| Yes | 164,559 (8.07%) | 22,674 (11.12%) | 1.44 (1.42–1.46) | |
| Ischaemic heart disease | No | 2,019,995 (99.05%) | 201,768 (98.93%) | 1 (ref) |
| Yes | 19,471 (0.95%) | 2179 (1.07%) | 1.13 (1.08–1.18) | |
| Cerebrovascular accident | No | 2,022,049 (99.15%) | 202,073 (99.08%) | 1 (ref) |
| TIA | 12,405 (0.61%) | 1233 (0.6%) | 1 (0.94–1.06) | |
| Stroke | 5012 (0.25%) | 641 (0.31%) | 1.29 (1.18–1.40) | |
| Diabetes Mellitus | No | 1,973,842 (96.78%) | 197,431 (96.81%) | 1 (ref) |
| Yes | 65,624 (3.22%) | 6516 (3.19%) | 0.99 (0.96–1.02) | |
| Heart failure | No | 2,032,388 (99.65%) | 203,246 (99.66%) | 1 (ref) |
| Yes | 7078 (0.35%) | 701 (0.34%) | 0.99 (0.91–1.07) | |
| Hypertension | No | 1,856,925 (91.05%) | 184,572 (90.5%) | 1 (ref) |
| Yes | 182,541 (8.95%) | 19,375 (9.5%) | 1.11 (1.09–1.14) | |
| Hyperlipidemia | No | 1,845,499 (90.49%) | 181,358 (88.92%) | 1 (ref) |
| Yes | 193,967 (9.51%) | 22,589 (11.08%) | 1.28 (1.25–1.30) | |
| Chronic renal disease | No | 2,032,754 (99.67%) | 203,196 (99.63%) | 1 (ref) |
| Yes | 6712 (0.33%) | 751 (0.37%) | 1.12 (1.04–1.21) | |
| AIDS/ HIV+ | No | 2,037,298 (99.89%) | 203,427 (99.75%) | 1 (ref) |
| Yes | 2168 (0.11%) | 520 (0.25%) | 2.41 (2.19–2.66) | |
| Chronic hepatitis | No | 2,034,550 (99.76%) | 203,355 (99.71%) | 1 (ref) |
| Yes | 4916 (0.24%) | 592 (0.29%) | 1.21 (1.11–1.31) | |
| Alzheimer disease | No | 2,032,190 (99.64%) | 203,350 (99.71%) | 1 (ref) |
| Yes | 7276 (0.36%) | 597 (0.29%) | 0.81 (0.74–0.88) | |
| Immunodeficiency | No | 2,038,212 (99.94%) | 203,744 (99.9%) | 1 (ref) |
| Yes | 1254 (0.06%) | 203 (0.1%) | 1.62 (1.40–1.88) | |
| Depression | No | 1,854,589 (90.94%) | 179,097 (87.82%) | 1 (ref) |
| Yes | 184,877 (9.06%) | 24,850 (12.18%) | 1.49 (1.47–1.51) | |
| Neuropathic pain | No | 2,030,151 (99.54%) | 202,443 (99.26%) | 1 (ref) |
| Yes | 9315 (0.46%) | 1504 (0.74%) | 1.63 (1.54–1.72) | |
| Epilepsy | No | 2,025,726 (99.33%) | 202,001 (99.05%) | 1 (ref) |
| Yes | 13,740 (0.67%) | 1946 (0.95%) | 1.43 (1.36–1.50) | |
| Bipolar disorder | No | 2,038,214 (99.94%) | 203,782 (99.92%) | 1 (ref) |
| Yes | 165 (0.08%) | 1252 (0.06%) | 1.32 (1.12–1.55) | |
| Migraine | No | 197,068 (96.63%) | 1,990,109 (97.58%) | 1 (ref) |
| Yes | 6879 (3.37%) | 49,357 (2.42%) | 1.43 (1.39–1.46) |
| Previous Medications | Categories | Cases (n (%)) N = 203,947 | Controls (n (%)) N = 2,039,466 | Crude OR (95% CI) |
|---|---|---|---|---|
| Corticoids for systemic use (Glucocorticoids) | Non use | 1,756,946 (86.15%) | 162,552 (79.7%) | 1 (ref) |
| 0–30 days | 23,529 (1.15%) | 3688 (1.81%) | 1.76 (1.69–1.82) | |
| 31–365 | 97,827 (4.8%) | 14,787 (7.25%) | 1.7 (1.67–1.73) | |
| 365+ | 161,164 (7.9%) | 22,920 (11.24%) | 1.6 (1.57–1.62) | |
| Antivirals for systemic use | Non use | 2,025,268 (99.3%) | 200,749 (98.43%) | 1 (ref) |
| 0–30 days | 723 (0.04%) | 291 (0.14%) | 4.09 (3.57–4.68) | |
| 31–365 | 3843 (0.19%) | 888 (0.44%) | 2.35 (2.18–2.53) | |
| 365+ | 9632 (0.47%) | 2019 (0.99%) | 2.14 (2.04–2.25) | |
| Immunosuppressants | Non use | 2,035,287 (99.8%) | 203,157 (99.61%) | 1 (ref) |
| 0–30 days | 2265 (0.11%) | 490 (0.24%) | 2.17 (1.97–2.39) | |
| 31–365 | 719 (0.04%) | 134 (0.07%) | 1.87 (1.56–2.25) | |
| 365+ | 1195 (0.06%) | 166 (0.08%) | 1.4 (1.19–1.64) | |
| Antibacterials for systemic use | Non use | 777,879 (38.14%) | 45,965 (22.54%) | 1 (ref) |
| 0–30 days | 136,679 (6.7%) | 25,353 (12.43%) | 3.37 (3.31–3.43) | |
| 31–365 | 541,039 (26.53%) | 75,211 (36.88%) | 2.52 (2.48–2.55) | |
| 365+ | 583,869 (28.63%) | 57,418 (28.15%) | 1.75 (1.73–1.78) | |
| Anxiolytics | Non use | 1,669,397 (81.85%) | 153,836 (75.43%) | 1 (ref) |
| 0–30 days | 76,617 (3.76%) | 11,381 (5.58%) | 1.84 (1.80–1.88) | |
| 31–365 | 103,121 (5.06%) | 17,975 (7.34%) | 1.70 (1.67–1.73) | |
| 365+ | 190,331 (9.33%) | 23755 (11.65%) | 1.45 (1.43–1.48) | |
| Antipsychotics | Non use | 1,950,135 (95.62%) | 191,695 (93.99%) | 1 (ref) |
| 0–30 days | 11,729 (0.58%) | 1267 (0.62%) | 1.13 (1.07–1.20) | |
| 31–365 | 21,457 (1.05%) | 3094 (1.52%) | 1.51 (1.46–1.57) | |
| 365+ | 56,145 (2.75%) | 7891 (3.87%) | 1.48 (1.44–1.52) | |
| Antiepileptic drugs (other than valproic acid) | Non use | 2,012,359 (98.67%) | 199,751 (97.94%) | 1 (ref) |
| 0–30 days | 8786 (0.43%) | 1307 (0.64%) | 1.52 (1.44–1.62) | |
| 31–365 | 7572 (0.37%) | 1243 (0.61%) | 1.68 (1.58–1.79) | |
| 365+ | 10,749 (0.53%) | 1646 (0.81%) | 1.57 (1.49–1.66) |
| CASES n = 203,947 | CONTROLS n = 2,039,466 | Non-Adjusted OR † (95%CI) | Adjusted OR ‡ (95%CI) | |
|---|---|---|---|---|
| Exposure | ||||
| Non use | 203,644 (99.9%) | 2,037,218 (99.9%) | 1 (ref) | 1 (ref) |
| Current use (0–30 days) | 148 (0.1%) | 1076 (0.1%) | 1.38 (1.16–1.63) | 0.84 (0.70–1.00) |
| Recent use (31–365 days) | 65 (0.0%) | 540 (0.0%) | 1.20 (0.93–1.55) | 0.79 (0.60–1.02) |
| Past use (>365 days) | 90 (0.0%) | 632 (0.0%) | 1.42 (1.14–1.78) | 0.94 (0.75–1.18) |
| Continuous duration * | ||||
| Non use | 203,644 (99.9%) | 2,037,218 (99.9%) | 1 (ref) | 1 (ref) |
| <91 days | 52 (35.1%) | 331 (30.8%) | 1.57 (1.17–2.10) | 1.02 (0.75–1.37) |
| 91–365 days | 57 (38.5%) | 415 (38.6%) | 1.37 (1.04–1.81) | 0.83 (0.63–1.11) |
| >365 days | 39 (26.4%) | 330 (30.7%) | 1.18 (0.85–1.64) | 0.68 (0.48–0.95) |
| HHV-1/HHV-2 OR (95% CI) | HHV-3 OR (95% CI) | HHV-4/HHV-5 OR (95%CI) | |
|---|---|---|---|
| Exposure | |||
| Non use | 1 (ref) | 1 (ref) | 1 (ref) |
| Current use (0–30 days) | 0.89 (0.70–1.15) | 0.92 (0.68–1.24) | 0.97 (0.51–1.83) |
| Recent use (31–365 days) | 0.87 (0.61–1.24) | 0.80 (0.52–1.24) | 0.84 (0.29–2.45) |
| Past use (>365 days) | 1.04 (0.79–1.37) | 0.88 (0.54–1.43) | 0.79 (0.34–1.81) |
| Continuous duration† | |||
| Non use | 1 (ref) | 1 (ref) | 1 (ref) |
| <91 days | 1.16 (0.77–1.74) | 0.92 (0.56–1.52) | 1.40 (0.49–4.02) |
| 91–365 days | 0.85 (0.56–1.30) | 0.87 (0.56–1.36) | 1.87 (0.77–4.53) |
| >365 days | 0.71 (0.45–1.12) | 0.95 (0.55–1.65) | 0.15 (0.02–1.16) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil, M.; González-González, R.; Vázquez-Calvo, A.; Álvarez-Gutiérrez, A.; Martín-Acebes, M.A.; Praena, B.; Bello-Morales, R.; Saiz, J.-C.; López-Guerrero, J.A.; Tabarés, E.; et al. Clinical Infections by Herpesviruses in Patients Treated with Valproic Acid: A Nested Case-Control Study in the Spanish Primary Care Database, BIFAP. J. Clin. Med. 2019, 8, 1442. https://doi.org/10.3390/jcm8091442
Gil M, González-González R, Vázquez-Calvo A, Álvarez-Gutiérrez A, Martín-Acebes MA, Praena B, Bello-Morales R, Saiz J-C, López-Guerrero JA, Tabarés E, et al. Clinical Infections by Herpesviruses in Patients Treated with Valproic Acid: A Nested Case-Control Study in the Spanish Primary Care Database, BIFAP. Journal of Clinical Medicine. 2019; 8(9):1442. https://doi.org/10.3390/jcm8091442
Chicago/Turabian StyleGil, Miguel, Rocío González-González, Angela Vázquez-Calvo, Arturo Álvarez-Gutiérrez, Miguel A. Martín-Acebes, Beatriz Praena, Raquel Bello-Morales, Juan-Carlos Saiz, Jose A. López-Guerrero, Enrique Tabarés, and et al. 2019. "Clinical Infections by Herpesviruses in Patients Treated with Valproic Acid: A Nested Case-Control Study in the Spanish Primary Care Database, BIFAP" Journal of Clinical Medicine 8, no. 9: 1442. https://doi.org/10.3390/jcm8091442
APA StyleGil, M., González-González, R., Vázquez-Calvo, A., Álvarez-Gutiérrez, A., Martín-Acebes, M. A., Praena, B., Bello-Morales, R., Saiz, J.-C., López-Guerrero, J. A., Tabarés, E., & Sobrino, F. (2019). Clinical Infections by Herpesviruses in Patients Treated with Valproic Acid: A Nested Case-Control Study in the Spanish Primary Care Database, BIFAP. Journal of Clinical Medicine, 8(9), 1442. https://doi.org/10.3390/jcm8091442







