Deficits in Conditional Discrimination Learning in Children with ADHD Are Independent of Delay Aversion and Working Memory
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
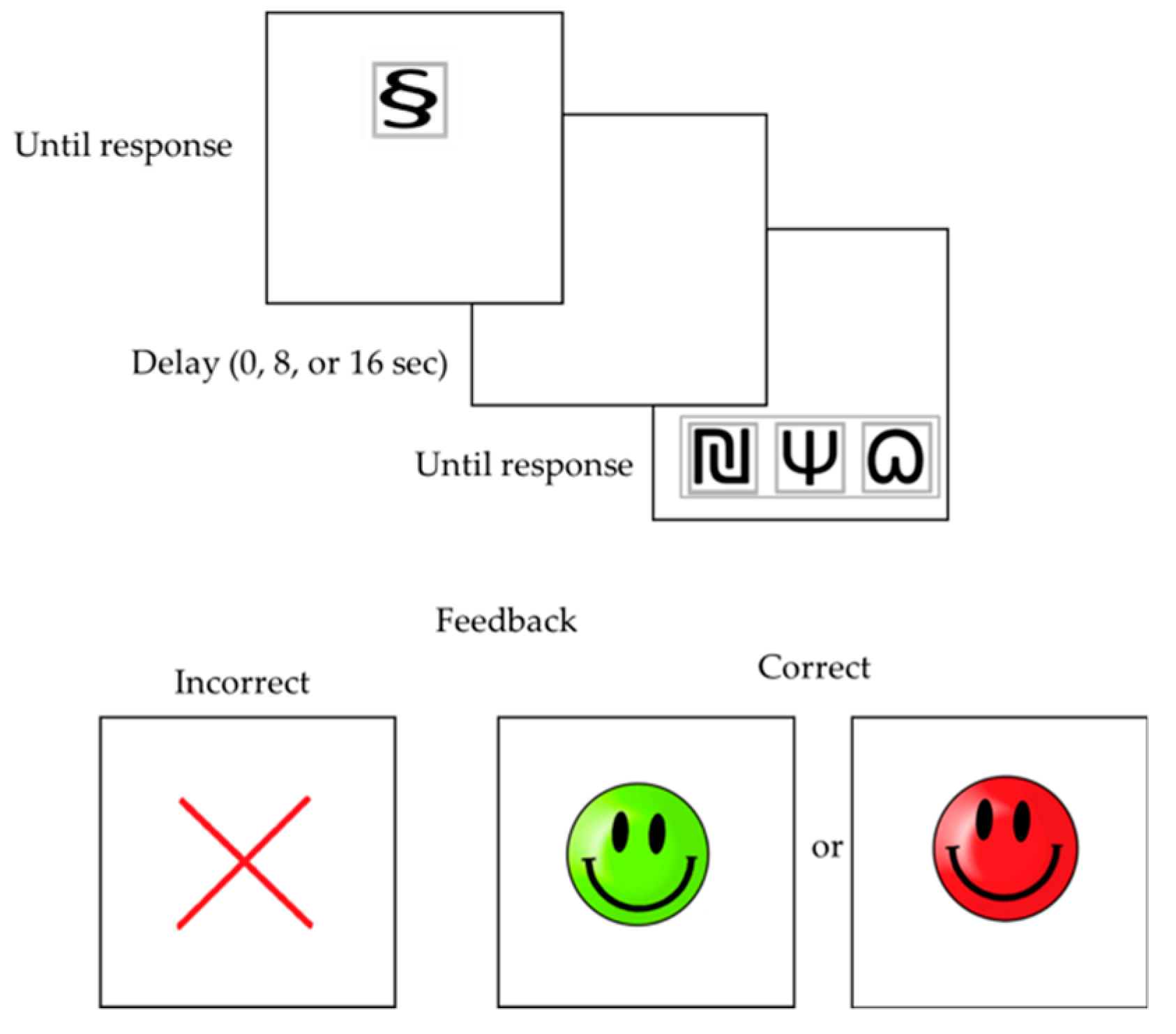
2.2. Conditional Discrimination Learning Task
2.3. Procedure
3. Results
3.1. Demographic Characteristics
3.2. Data Preparation and Statistical Analysis
3.3. Conditional Discrimination Learning
3.4. Working Memory and Delay Aversion
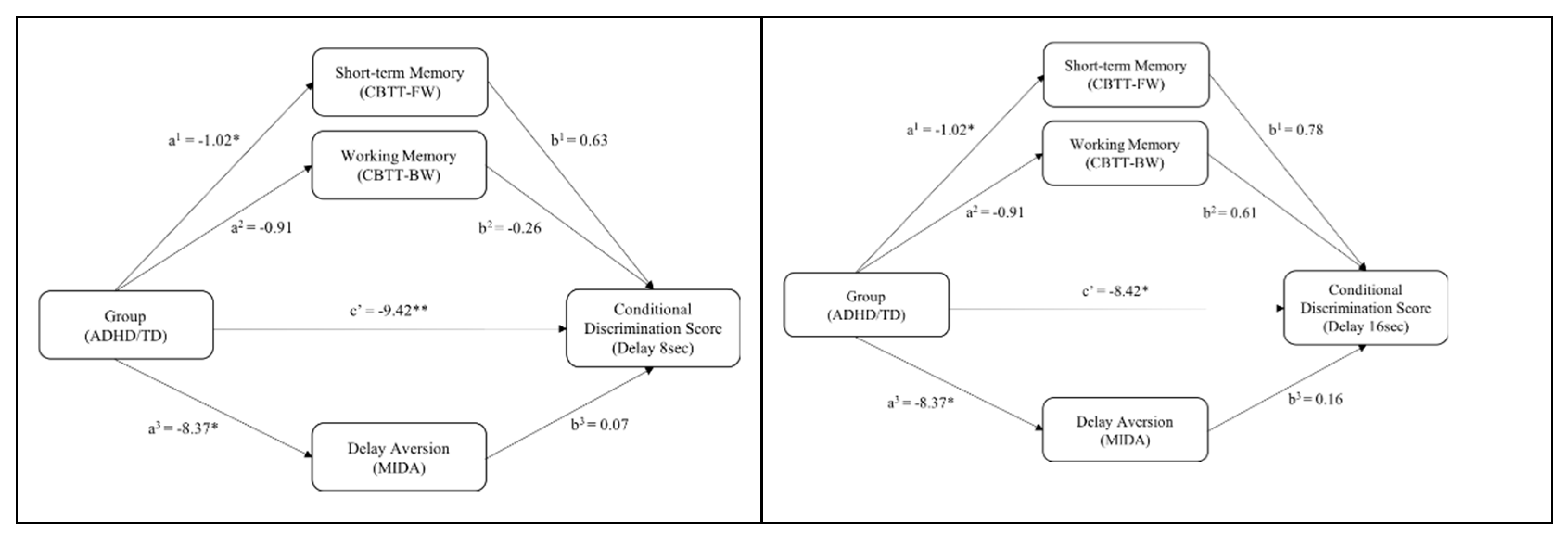
3.5. Mediation Model
4. Discussion
5. Clinical Implications
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mok, L.W.; Estevez, A.F.; Overmier, J.B. Unique Outcome Expectations as a Training and Pedagogical Tool. Psychol. Rec. 2017, 60, 227–247. [Google Scholar] [CrossRef]
- Schultz, W. Multiple reward signals in the brain. Nat. Rev. Neurosci. 2000, 1, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Alsop, B.; Furukawa, E.; Sowerby, P.; Jensen, S.; Moffat, C.; Tripp, G. Behavioral sensitivity to changing reinforcement contingencies in attention-deficit hyperactivity disorder. J. Child Psychol. Psychiatry Allied Discip. 2016, 57, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Nigg, J.T.; Casey, B.J. An integrative theory of attention-deficit/hyperactivity disorder based on the cognitive and affective neurosciences. Dev. Psychopathol. 2005, 17, 785–806. [Google Scholar] [CrossRef]
- De Meyer, H.; Beckers, T.; Tripp, G.; van der Oord, S. Reinforcement Contingency Learning in Children with ADHD: Back to the Basics of Behavior Therapy. J. Abnorm. Child Psychol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.S.; Knights, R.M. The performance of hyperactive and normal boys under differing reward and punishment schedules. J. Pediatr. Psychol. 1978, 3, 195–201. [Google Scholar] [CrossRef]
- Douglas, V.I.; Parry, P.A.; Parrry, P.A. Effects of reward on delayed reaction time task performance of hyperactive children. J. Abnorm. Child Psychol. 1983, 11, 313–326. [Google Scholar] [CrossRef]
- Douglas, V.I.; Parry, P.A. Effects of reward and nonreward on frustration and attention in attention deficit disorder. J. Abnorm. Child Psychol. 1994, 22, 281–302. [Google Scholar] [CrossRef]
- Freibergs, V.; Douglas, V.I. Concept learning in hyperactive and normal children. J. Abnorm. Psychol. 1969, 74, 388–395. [Google Scholar] [CrossRef]
- Segers, E.; Beckers, T.; Geurts, H.; Claes, L.; Danckaerts, M.; van der Oord, S. Working memory and reinforcement schedule jointly determine reinforcement learning in children: Potential implications for behavioral parent training. Front. Psychol. 2018, 9, 394. [Google Scholar] [CrossRef]
- Trapold, M.A. Are expectancies based upon different positive reinforcing events discriminably different? Learn. Motiv. 1970, 1, 129–140. [Google Scholar] [CrossRef]
- Estévez, A.F.; Fuentes, L.J.; Marı́-Beffa, P.; González, C.; Alvarez, D. The Differential Outcome Effect as a Useful Tool to Improve Conditional Discrimination Learning in Children. Learn. Motiv. 2001, 32, 48–64. [Google Scholar] [CrossRef][Green Version]
- Frank, M.J.; Scheres, A.; Sherman, S.J. Understanding decision-making deficits in neurological conditions: Insights from models of natural action selection. Model. Nat. Action Sel. 2011, 330–362. [Google Scholar] [CrossRef]
- Gitten, J.C.; Winer, J.L.; Festa, E.K.; Heindel, W.C. Conditional associative learning of spatial and object information in children with attention deficit/hyperactivity disorder. Child Neuropsychol. 2006, 12, 39–56. [Google Scholar] [CrossRef] [PubMed]
- Sonuga-Barke, E.J.S. The dual pathway model of AD/HD: An elaboration of neuro-developmental characteristics. Neurosci. Biobehav. Rev. 2003, 27, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Wehmeier, P.M.; Schacht, A.; Barkley, R.A. Social and Emotional Impairment in Children and Adolescents with ADHD and the Impact on Quality of Life. J. Adolesc. Health 2010, 46, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Biederman, J.; Monuteaux, M.C.; Doyle, A.E.; Seidman, L.J.; Wilens, T.E.; Ferrero, F.; Morgan, C.L.; Faraone, S.V. Impact of executive function deficits and attention-deficit/hyperactivity disorder (ADHD) on academic outcomes in children. J. Consult. Clin. Psychol. 2004, 72, 757–766. [Google Scholar] [CrossRef]
- Sonuga-Barke, E.J.S.; Sergeant, J.A.; Nigg, J.; Willcutt, E. Executive Dysfunction and Delay Aversion in Attention Deficit Hyperactivity Disorder: Nosologic and Diagnostic Implications. Child Adolesc. Psychiatr. Clin. N. Am. 2008, 17, 367–384. [Google Scholar] [CrossRef]
- Martinussen, R.; Hayden, J.; Hogg-Johnson, S.; Tannock, R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 2005, 44, 377–384. [Google Scholar] [CrossRef]
- Kofler, M.J.; Harmon, S.L.; Aduen, P.A.; Day, T.N.; Austin, K.E.; Spiegel, J.A.; Irwin, L.; Sarver, D.E. Neurocognitive and behavioral predictors of social problems in ADHD: A bayesian framework. Neuropsychology 2018, 32, 344–355. [Google Scholar] [CrossRef]
- Willcutt, E.G.; Doyle, A.E.; Nigg, J.T.; Faraone, S.V.; Pennington, B.F. Validity of the Executive Function Theory of Attention-Deficit/Hyperactivity Disorder: A Meta-Analytic Review. Biol. Psychiatry 2005, 57, 1336–1346. [Google Scholar] [CrossRef]
- Dovis, S.; Van Der Oord, S.; Wiers, R.W.; Prins, P.J.M. What part of working memory is not working in ADHD? short-term memory, the central executive and effects of reinforcement. J. Abnorm. Child Psychol. 2013, 41, 901–917. [Google Scholar] [CrossRef] [PubMed]
- Conway, A.R.A.; Cowan, N.; Bunting, M.F. The cocktail party phenomenon revisited: The importance of working memory capacity. Psychon. Bull. Rev. 2001, 8, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Luman, M.; Tripp, G.; Scheres, A. Identifying the neurobiology of altered reinforcement sensitivity in ADHD: A review and research agenda. Neurosci. Biobehav. Rev. 2010, 34, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Sagvolden, T.; Johansen, E.B.; Aase, H.; Russell, V.A. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behav. Brain Sci. 2005, 28, 397–419. [Google Scholar] [CrossRef] [PubMed]
- Tripp, G.; Wickens, J.R. Research review: Dopamine transfer deficit: A neurobiological theory of altered reinforcement mechanisms in ADHD. J. Child Psychol. Psychiatry Allied Discip. 2008, 49, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Van Dessel, J.; Sonuga-Barke, E.; Mies, G.; Lemiere, J.; Van der Oord, S.; Morsink, S.; Danckaerts, M. Delay aversion in attention deficit/hyperactivity disorder is mediated by amygdala and prefrontal cortex hyper-activation. J. Child Psychol. Psychiatry Allied Discip. 2018, 59, 888–899. [Google Scholar] [CrossRef]
- Bitsakou, P.; Antrop, I.; Wiersema, J.R.; Sonuga-Barke, E.J.S. Probing the limits of delay intolerance: Preliminary young adult data from the Delay Frustration Task (DeFT). J. Neurosci. Methods 2006, 151, 38–44. [Google Scholar] [CrossRef]
- Coghill, D.; Seth, S.; Matthews, K. A comprehensive assessment of memory, delay aversion, timing, inhibition, decision making and variability in attention deficit hyperactivity disorder: Advancing beyond the three-pathway models. Psychol. Med. 2014, 44, 1989–2001. [Google Scholar] [CrossRef]
- Frank, M.J.; Santamaria, A.; Reilly, R.C.; Willcutt, E.; O’Reilly, R.C.; Willcutt, E. Testing computational models of dopamine and noradrenaline dysfunction in attention deficit/hyperactivity disorder. Neuropsychopharmacology 2007, 32, 1583–1599. [Google Scholar] [CrossRef]
- Adamson, C.; Foster, T.M.; McEwan, J.S.A. Delayed matching to sample: The effects of sample-set size on human performance. Behav. Processes 2000, 49, 149–161. [Google Scholar] [CrossRef]
- Grant, R.A. The relation of perceptual activity to Matching Familiar Figures Test accuracy. Dev. Psychol. 1976, 12, 534–539. [Google Scholar] [CrossRef]
- Etkin, M.; D’Amato, M.R. Delayed matching-to-sample and short-term memory in the capuchin monkey. J. Comp. Physiol. Psychol. 1969, 69, 544–549. [Google Scholar] [CrossRef]
- Chelonis, J.J.; Daniels-Shaw, J.L.; Blake, D.J.; Paule, M.G. Developmental aspects of delayed matching-to-sample task performance in children. Neurotoxicol. Teratol. 2000, 22, 683–694. [Google Scholar] [CrossRef]
- Elliott, R.; Dolan, R.J. Differential neural responses during performance of matching and nonmatching to sample tasks at two delay intervals. J. Neurosci. 1999, 19, 5066–5073. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Case, J.P.; Laude, J.R.; Zentall, T.R. Delayed matching to sample in pigeons: Effects of delay of reinforcement and illuminated delays. Learn. Motiv. 2015, 49, 51–59. [Google Scholar] [CrossRef]
- Skinner, B.F. Are theories of learning necessary? Psychol. Rev. 1950, 57, 193–216. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, D.; Fisher, P.; Lucas, C.P.; Dulcan, M.K.; Schwab-Stone, M.E. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, Differences From Previous Versions, and Reliability of Some Common Diagnoses. J. Am. Acad. Child Adolesc. Psychiatry 2000, 39, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Kort, W.; Schittekatte, M.; Bosmans, M.; Compaan, E.L.; Dekker, P.H.; Vermeir, G.; Verhaeghe, P. WISC-III: Handleiding En Verantwoording; Pearson: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Sattler, J. Assessment of Children: Cognitive Applications, 4th ed.; Jerome M Sattler Publisher: San Diego, CA, USA, 2001. [Google Scholar]
- Oosterlaan, J.; Baeyens, D.; Scheres, A.; Antrop, I.; Roeyens, H.; Sergeant, J. VvGK6-16: Vragenlijst Voor Gedragsproblemen Bij Kinderen 6 Tot En Met 16 Jaar; Pearson: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Greenhill, L.L. A guide to treatments that work. In A Guide to Treatments That Work; Nathan, P.E., Gorman, J., Eds.; Oxford Universtity Press: New York, NY, USA, 1998; pp. 42–64. [Google Scholar]
- Domjan, M. The Essentials of Conditioning and Learning, 3rd ed.; Cengage Learning: Boston, MA, USA, 2000. [Google Scholar]
- Corsi, P.M. Human memory and the medial temporal region of the brain. Diss. Abstr. Int. 1972, 34, 891. [Google Scholar]
- Kuntsi, J.; Stevenson, J.; Oosterlaan, J.; Sonuga-Barke, E.J.S. Test-retest reliability of a new delay aversion task and executive function measures. Br. J. Dev. Psychol. 2001, 19, 339–348. [Google Scholar] [CrossRef]
- Kuntsi, J.; Oosterlaan, J.; Stevenson, J. Psychological Mechanisms in Hyperactivity: I Response Inhibition Deficit, Working Memory Impairment, Delay Aversion, or Something Else? J. Child Psychol. Psychiatry 2001, 42, S0021963001006709. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR; American Psychiatric Association: Washington, DC, USA, 2000; Available online: https://books.google.be/books/about/Diagnostic_and_Statistical_Manual_of_Men.html?id=oOdGAAAAMAAJ&redir_esc=y (accessed on 31 May 2019).
- IBM Corp. IBM SPSS Statistics for Macintosh, Version 25.0; IBM Corp: Armonk, NY, USA, 2017. [Google Scholar]
- Zar, J.H. Biostatistical Analysis; Englewood Cliffs, N.J., Ed.; Prentice-Hall: Upper Saddle River, NJ, USA, 1984. [Google Scholar]
- Dennis, M.; Francis, D.J.; Cirino, P.T.; Schachar, R.; Barnes, M.A.; Fletcher, J.M. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. J. Int. Neuropsychol. Soc. 2009, 15, 331. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis, 2nd ed.; The Guilford Press: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 29, 175–191. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- Björne, P.; Balkenius, C. The role of context and inhibition in ADHD. Behav Brain Sci. 2005, 28, 426–427. [Google Scholar] [CrossRef]
- Tripp, G.; Alsop, B. Sensitivity to Reward Frequency in Boys with Attention Deficit Hyperactivity Disorder. J. Clin. Child Adolesc. Psychol. 1999, 28, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Geurts, H.M.; Verté, S.; Oosterlaan, J.; Roeyers, H.; Sergeant, J.A. How specific are executive functioning deficits in attention deficit hyperactivity disorders and autism? J. Child Psychol. Psychiatry Allied Discip. 2004, 45, 836–854. [Google Scholar] [CrossRef] [PubMed]
- Mok, L.W.; Overmier, J.B. The differential outcomes effect in normal human adults using a concurrent-task within-subjects design and sensory outcomes. Psychol. Rec. 2007, 57, 187–200. [Google Scholar] [CrossRef]
- Overmier, J.B.; Linwick, D. Conditional Choice-Unique Outcomes Establish Expectancies That Mediate Choice Behavior. Integr. Physiol. Behav. Sci. 2001, 36, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Holden, J.M.; Overmier, J.B. Performance under differential outcomes: Contributions of Reward-Specific Expectancies. Learn. Motiv. 2014, 45, 1–14. [Google Scholar] [CrossRef]
| ADHD | TD | F/χ2 | p | |
| M (SD) | M (SD) | |||
| Gender N | 3.43 | 0.064 | ||
| Male | 31 | 27 | ||
| Female | 15 | 28 | ||
| Age (years) | 10.28 (0.98) | 10.01 (1.21) | 1.46 | 0.230 |
| FSIQ | 98.04 (11.60) | 104.71 (10.35) | 9.31 | 0.003 ** |
| Dyscalculia (%) | 2.17 | 0 | 1.21 | 0.272 |
| Dyslexia (%) | 8.70 | 0 | 4.98 | 0.026 * |
| DBDRS | ||||
| Attention | 14.95 (1.79) | 10.52 (0.99) | 241.84 | <0.001 *** |
| Hyperactive/impulsive | 14.36 (2.17) | 10.54 (0.97) | 135.46 | <0.001 *** |
| ODD | 12.55 (2.13) | 10.72 (1.24) | 28.06 | <0.001 *** |
| CD | 11.45 (1.53) | 10.93 (1.28) | 3.86 | 0.052 |
| ADHD | TD | ||||
|---|---|---|---|---|---|
| M (SD) | M (SD) | F1 | p1 | η2p | |
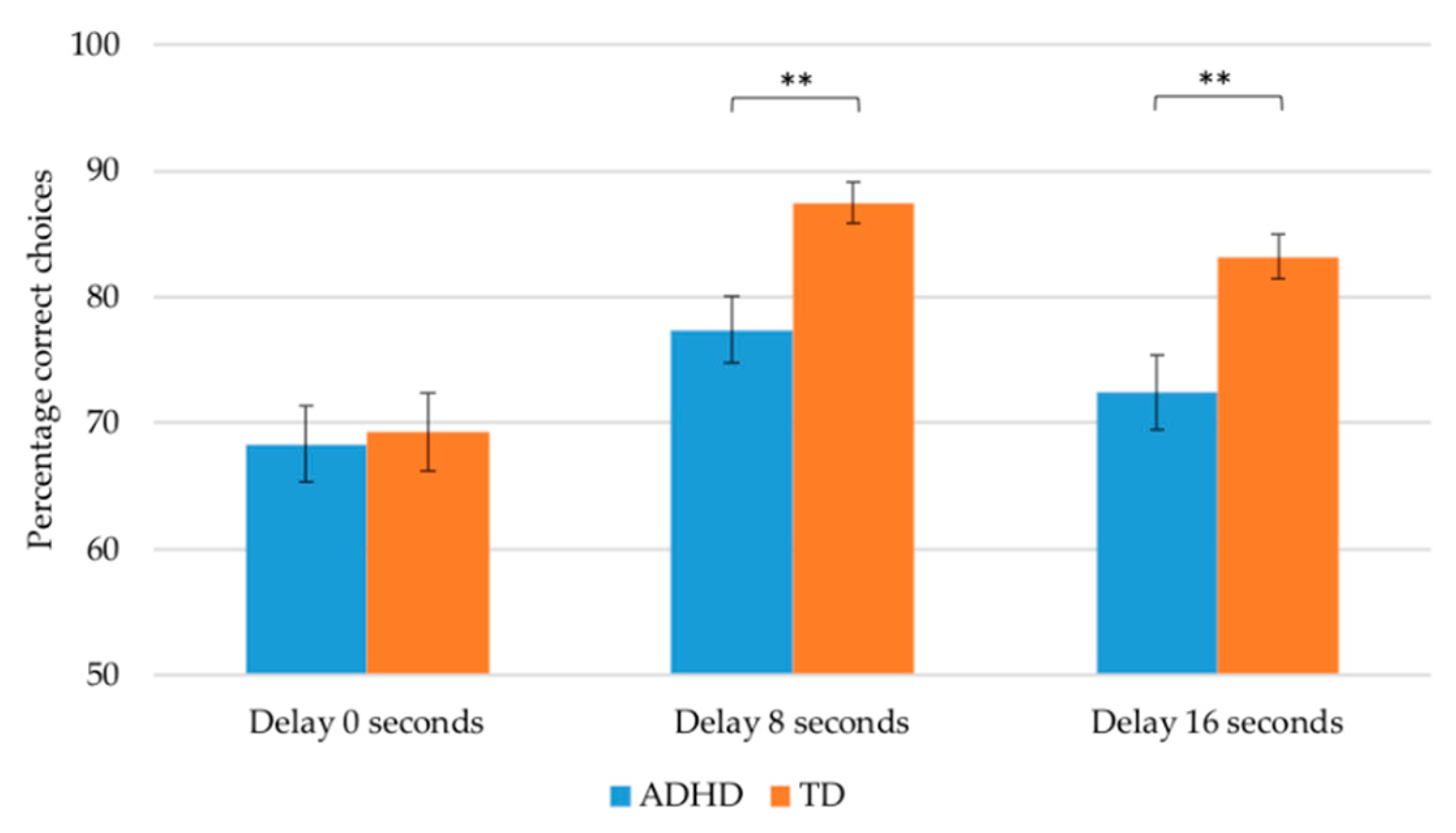
| Delay 0 | 68.34 (20.68) | 69.26 (23.10) | 0.14 | 0.711 | 0.001 |
| Delay 8 | 77.39 (17.76) | 87.45 (12.13) | 12.94 | 0.001 ** | 0.116 |
| Delay 16 | 72.39 (20.10) | 83.18 (13.07) | 7.60 | 0.007 ** | 0.071 |
| Short-term Memory 2 | 42.47 (13.62) | 48.11 (11.24) | 4.62 | 0.034 * | 0.050 |
| Working Memory 2 | 43.58 (13.81) | 48.61 (15.63) | 2.50 | 0.121 | 0.027 |
| Delay Aversion 2 | 81.39 (19.75) | 89.76 (14.30) | 4.04 | 0.048 * | 0.044 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Meyer, H.; Beckers, T.; Tripp, G.; van der Oord, S. Deficits in Conditional Discrimination Learning in Children with ADHD Are Independent of Delay Aversion and Working Memory. J. Clin. Med. 2019, 8, 1381. https://doi.org/10.3390/jcm8091381
De Meyer H, Beckers T, Tripp G, van der Oord S. Deficits in Conditional Discrimination Learning in Children with ADHD Are Independent of Delay Aversion and Working Memory. Journal of Clinical Medicine. 2019; 8(9):1381. https://doi.org/10.3390/jcm8091381
Chicago/Turabian StyleDe Meyer, Hasse, Tom Beckers, Gail Tripp, and Saskia van der Oord. 2019. "Deficits in Conditional Discrimination Learning in Children with ADHD Are Independent of Delay Aversion and Working Memory" Journal of Clinical Medicine 8, no. 9: 1381. https://doi.org/10.3390/jcm8091381
APA StyleDe Meyer, H., Beckers, T., Tripp, G., & van der Oord, S. (2019). Deficits in Conditional Discrimination Learning in Children with ADHD Are Independent of Delay Aversion and Working Memory. Journal of Clinical Medicine, 8(9), 1381. https://doi.org/10.3390/jcm8091381





