The Emerging Role of NANOG as an Early Cancer Risk Biomarker in Patients with Oral Potentially Malignant Disorders
Abstract
1. Introduction
2. Methods and Materials
2.1. Patients and Tissue Specimens
2.2. Tissue Microarray (TMA) Construction
2.3. Immunohistochemistry (IHC)
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
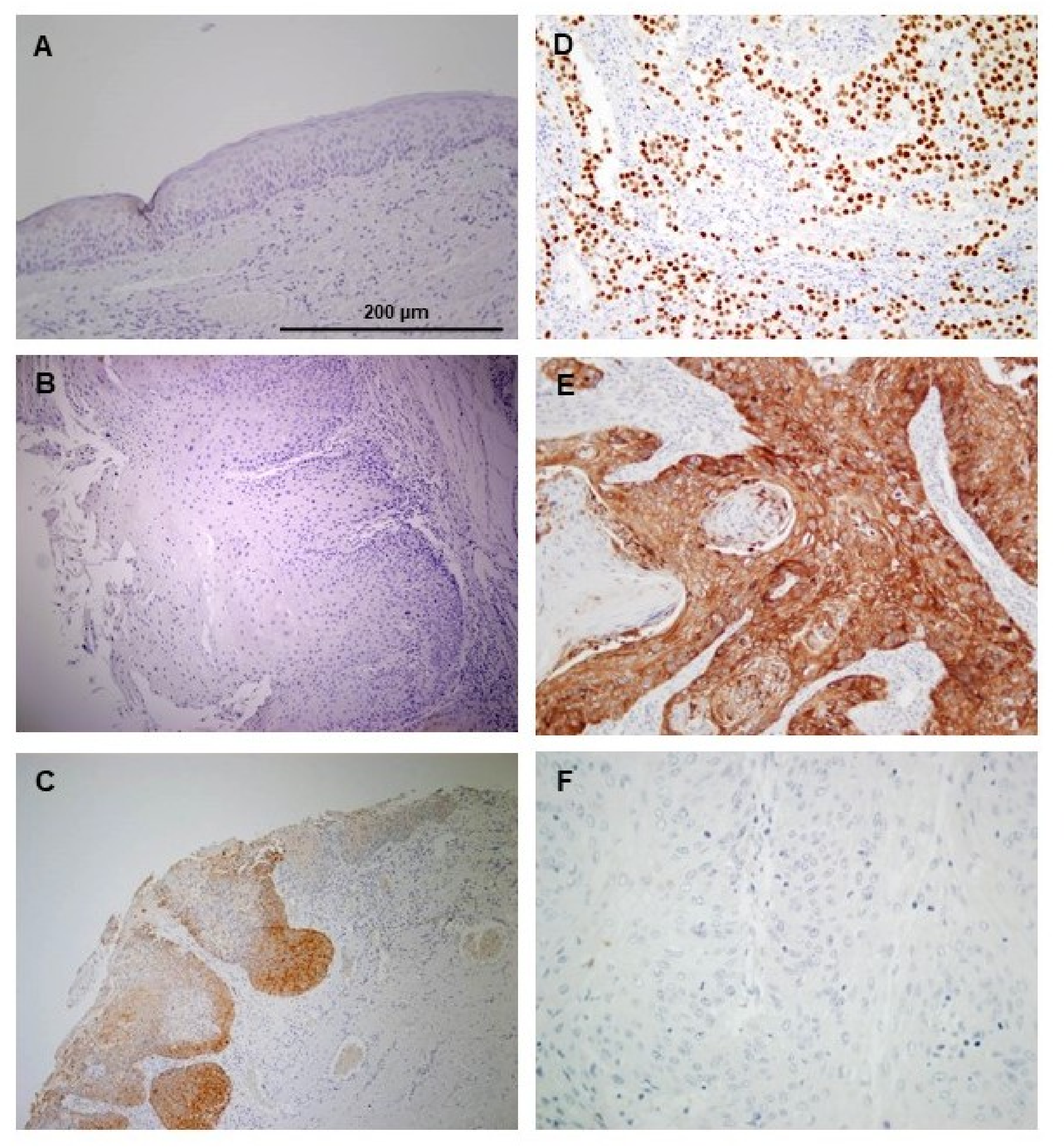
3.2. Immunohistochemical Analysis of NANOG Expression in Oral Epithelial Dysplasias
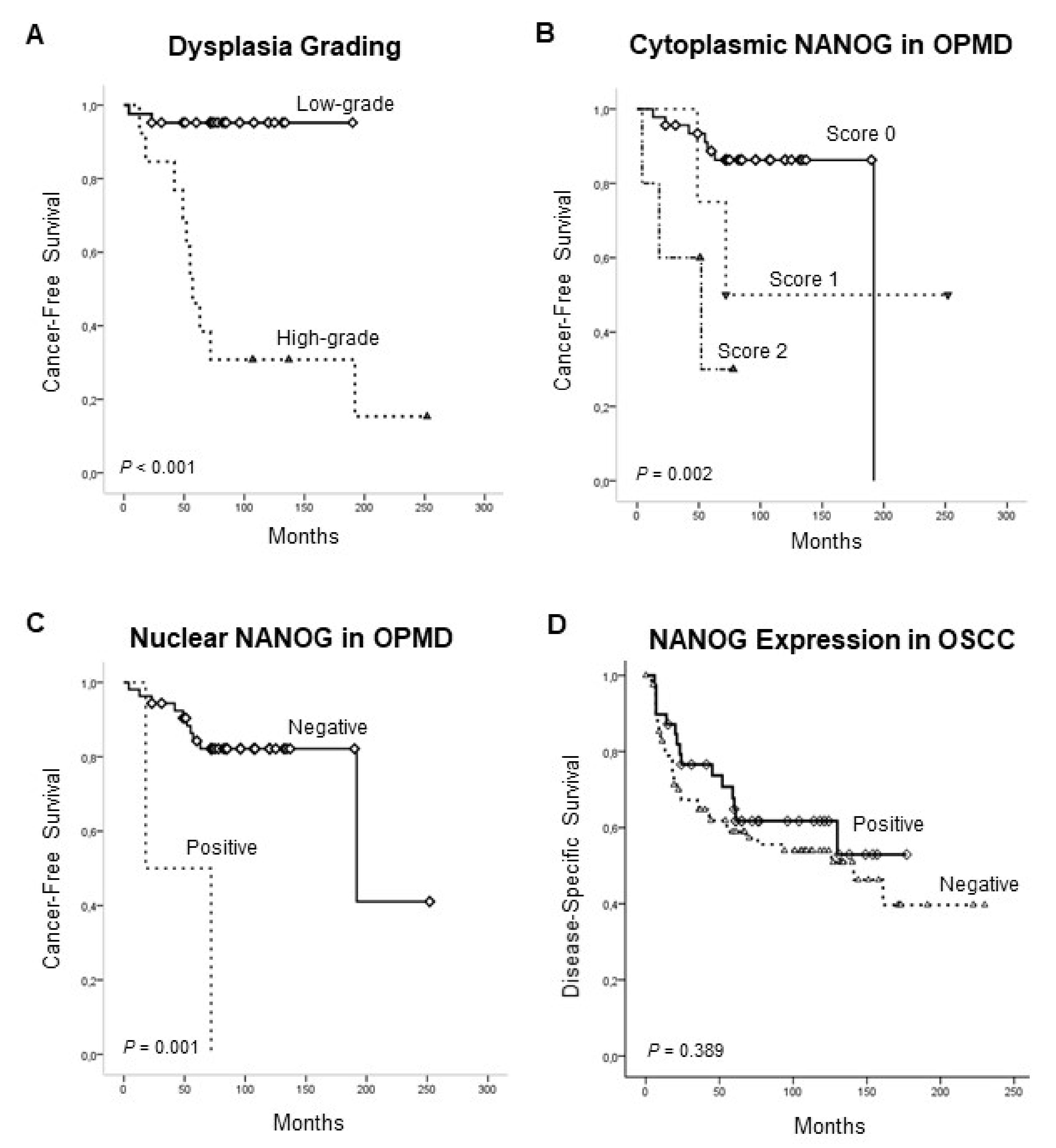
3.3. Association of NANOG Protein Expression with Oral Cancer Risk
3.4. Clinical Significance of NANOG Protein Expression in OSCC Progression and Disease Outcome
3.5. In Silico Analysis of NANOG and OCT4 mRNA Expression Using TCGA Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fu, T.Y.; Hsieh, I.C.; Cheng, J.T.; Tsai, M.H.; Hou, Y.Y.; Lee, J.H.; Liou, H.H.; Huang, S.F.; Chen, H.C.; Yen, L.M.; Tseng, H.H.; Ger, L.P. Association of OCT4, SOX2, and NANOG expression with oral squamous cell carcinoma progression. J. Oral Pathol. Med. 2016, 45, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Sinha, N.; Mukhopadhyay, S.; Das, D.N.; Panda, P.K.; Bhutia, S.K. Relevance of cancer initiating/stem cells in carcinogenesis and therapy resistance in oral cancer. Oral Oncol. 2013, 49, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Califano, J.; Westra, W.H.; Meininger, G.; Corio, R.; Koch, W.M.; Sidransky, D. Genetic progression and clonal relationship of recurrent premalignant head and neck lesions. Clin. Cancer Res. 2000, 6, 347–352. [Google Scholar] [PubMed]
- Mithani, S.K.; Mydlarz, W.K.; Grumbione, F.L.; Smith, I.M.; Califano, J.A. Molecular genetics of premalignant oral lesions. Oral Dis. 2007, 13, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Luiz, S.T.; Modolo, F.; Mozzer, I.; Dos Santos, E.C.; Nagashima, S.; Camargo Martins, A.P.; de Azevedo, M.L.V.; Azevedo Alanis, L.R.; Hardy, A.M.T.G.; de Moraes, R.S.; et al. Immunoexpression of SOX-2 in oral leukoplakia. Oral Dis. 2018, 24, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Teodorczyk, M.; Kleber, S.; Wollny, D.; Sefrin, J.P.; Aykut, B.; Mateos, A.; Herhaus, P.; Sancho-Martinez, I.; Hill, O.; et al. CD95 promotes metastatic spread via Sck in pancreatic ductal adenocarcinoma. Cell Death Differ. 2015, 22, 1192–1202. [Google Scholar] [CrossRef]
- Hussenet, T.; Dali, S.; Exinger, J.; Monga, B.; Jost, B.; Dembelé, D.; Martinet, N.; Thibault, C.; Huelsken, J.; Brambilla, E.; et al. SOX2 is an oncogene activated by recurrent 3q26.3 amplifications in human lung squamous cell carcinomas. PLoS ONE 2010, 5, e8960. [Google Scholar] [CrossRef]
- Habu, N.; Imanishi, Y.; Kameyama, K.; Shimoda, M.; Tokumaru, Y.; Sakamoto, K.; Fujii, R.; Shigetomi, S.; Otsuka, K.; Sato, Y.; et al. Expression of Oct3/4 and Nanog in the head and neck squamous carcinoma cells and its clinical implications for delayed neck metastasis in stage I/II oral tongue squamous cell carcinoma. BMC Cancer 2015, 15, 730. [Google Scholar] [CrossRef]
- Albers, A.E.; Chen, C.; Köberle, B.; Qian, X.; Klussmann, J.P.; Wollenberg, B.; Kaufmann, A.M. Stem cells in squamous head and neck cancer. Crit. Rev. Oncol. Hematol. 2012, 81, 224–240. [Google Scholar] [CrossRef]
- Costea, D.E.; Tsinkalovsky, O.; Vintermyr, O.K.; Johannessen, A.C.; Mackenzie, I.C. Cancer stem cells—New and potentially important targets for the therapy of oral squamous cell carcinoma. Oral Dis. 2006, 12, 443–454. [Google Scholar] [CrossRef]
- Lee, H.J.; Kang, Y.H.; Lee, J.S.; Byun, J.H.; Kim, U.K.; Jang, S.J.; Rho, G.J.; Park, B.W. Positive expression of NANOG, mutant p53, and CD44 is directly associated with clinicopathological features and poor prognosis of oral squamous cell carcinoma. BMC Oral Health 2015, 15, 153. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.L.; Yu, C.C.; Chang, Y.C.; Yu, C.H.; Chou, M.Y. Markedly increased Oct4 and Nanog expression correlates with cisplatin resistance in oral squamous cell carcinoma. J. Oral Pathol. Med. 2011, 40, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Chiou, S.H.; Yu, C.C.; Huang, C.Y.; Lin, S.C.; Liu, C.J.; Tsai, T.H.; Chou, S.H.; Chien, C.S.; Ku, H.H.; Lo, J.F. Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin. Cancer Res. 2008, 14, 4085–4095. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.C.; Nieh, S.; Lai, C.H.; Tsai, C.S.; Chang, L.C.; Hua, C.C.; Chi, W.Y.; Chien, H.P.; Wang, C.W.; Chan, S.C.; et al. A retrospective review of the prognostic value of ALDH-1, Bmi-1 and Nanog stem cell markers in esophageal squamous cell carcinoma. PLoS ONE 2014, 9, e105676. [Google Scholar] [CrossRef] [PubMed]
- Vaz, M.A.; Martinez, J.C.; Devesa, J.M.; Trill, J.D.; Abraira, V.; Riquelme, A.; Carrato, A. Prognostic value of stem cell quantification in stage II colon cancer. PLoS ONE 2014, 9, e88480. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, J.P.; Villaronga, M.Á.; Menéndez, S.T.; Hermida-Prado, F.; Quer, M.; Vilaseca, I.; Allonca, E.; Pedregal Mallo, D.; Astudillo, A.; García-Pedrero, J.M. A Novel Role For Nanog As An Early Cancer Risk Marker In Patients With Laryngeal Precancerous Lesions. Sci. Rep. 2017, 7, 11110. [Google Scholar] [CrossRef]
- Takata, T.; Slootweg, P.J. Tumors of the Oral Cavity and Mobile Tongue. In WHO Classification of Head and Neck Tumours, 4th ed.; El-Naggar, A.K., Chan, J.K.C., Grandis, J.R., Takata, T., Slootweg, P.J., Eds.; International Agency for Research on Cancer (IARC): Lyon, France, 2017; pp. 105–131. [Google Scholar]
- Piazzolla, D.; Palla, A.R.; Pantoja, C.; Cañamero, M.; de Castro, I.P.; Ortega, S.; Gómez-López, G.; Dominguez, O.; Megías, D.; Roncador, G.; et al. Lineage-restricted function of the pluripotency factor NANOG in stratified epithelia. Nat. Commun. 2014, 5, 4226. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Rodriguez, I.P.; Chakravarthi, B.V.S.K.; Varambally, S. UALCAN: A portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef]
- Abbey, L.M.; Kaugars, G.E.; Gunsolley, J.C.; Burns, J.C.; Page, D.G.; Svirsky, J.A.; Eisenberg, E.; Krutchkoff, D.J.; Cushing, M. Intraexaminer and interexaminer reliability in the diagnosis of oral epithelial dysplasia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1995, 80, 188–191. [Google Scholar] [CrossRef]
- Lauritano, D.; Lucchese, A.; Contaldo, M.; Serpico, R.; Lo Muzio, L.; Biolcati, F.; Carinci, F. Oral squamous cell carcinoma: Diagnostic markers and prognostic indicators. J. Biol. Regul. Homeost. Agents 2016, 30, 169–176. [Google Scholar] [PubMed]
- de Vicente, J.C.; Rodrigo, J.P.; Rodriguez-Santamarta, T.; Lequerica-Fernández, P.; Allonca, E.; García-Pedrero, J.M. Cortactin and focal adhesion kinase as predictors of cancer risk in patients with premalignant oral epithelial lesions. Oral Oncol. 2012, 48, 641–646. [Google Scholar] [CrossRef]
- de Vicente, J.C.; Rodrigo, J.P.; Rodriguez-Santamarta, T.; Lequerica-Fernández, P.; Allonca, E.; García-Pedrero, J.M. Podoplanin expression in oral leukoplakia: Tumorigenic role. Oral Oncol. 2013, 49, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Stransky, N.; Egloff, A.M.; Tward, A.D.; Kostic, A.D.; Cibulskis, K.; Sivachenko, A.; Kryukov, G.V.; Lawrence, M.S.; Sougnez, C.; McKenna, A.; et al. The mutational landscape of head and neck squamous cell carcinoma. Science 2011, 333, 1157–1160. [Google Scholar] [CrossRef] [PubMed]
- Hedberg, M.L.; Goh, G.; Chiosea, S.I.; Bauman, J.E.; Freilino, M.L.; Zeng, Y.; Wang, L.; Diergaarde, B.B.; Gooding, W.E.; Lui, V.W.; et al. Genetic landscape of metastatic and recurrent head and neck squamous cell carcinoma. J. Clin. Investig. 2016, 126, 1606. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Teijeiro, S.; García-Inclán, C.; Villaronga, M.Á.; Casado, P.; Hermida-Prado, F.; Granda-Díaz, R.; Rodrigo, J.P.; Calvo, F.; Del-Río-Ibisate, N.; Gandarillas, A.; et al. Factors Secreted by Cancer-Associated Fibroblasts that Sustain Cancer Stem Properties in Head and Neck Squamous Carcinoma Cells as Potential Therapeutic Targets. Cancers 2018, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Prasetyanti, P.R.; Medema, J.P. Intra-tumor heterogeneity from a cancer stem cell perspective. Mol. Cancer 2017, 16, 41. [Google Scholar] [CrossRef] [PubMed]
- Biddle, A.; Gammon, L.; Liang, X.; Costea, D.E.; Mackenzie, I.C. Phenotypic Plasticity Determines Cancer Stem Cell Therapeutic Resistance in Oral Squamous Cell Carcinoma. EBioMedicine 2016, 4, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajj, M.; Clarke, M.F. Self-renewal and solid tumor stem cells. Oncogene 2004, 23, 7274–7282. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, N.; Ishii, G.; Kojima, M.; Sanada, M.; Fujii, S.; Ochiai, A. Podoplanin, a novel marker of tumor-initiating cells in human squamous cell carcinoma A431. Biochem. Biophys. Res. Commun. 2008, 373, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, H.; El-Naggar, A.K.; Papadimitrakopoulou, V.; Ren, H.; Fan, Y.H.; Feng, L.; Lee, J.J.; Kim, E.; Hong, W.K.; Lippman, S.M.; et al. Podoplanin: A novel marker for oral cancer risk in patients with oral premalignancy. J. Clin. Oncol. 2008, 26, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, J.P.; García-Carracedo, D.; González, M.V.; Mancebo, G.; Fresno, M.F.; García-Pedrero, J. Podoplanin expression in the development and progression of laryngeal squamous cell carcinomas. Mol. Cancer 2010, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Chambers, I.; Colby, D.; Robertson, M.; Nichols, J.; Lee, S.; Tweedie, S.; Smith, A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 2003, 113, 643–655. [Google Scholar] [CrossRef]
- Mitsui, K.; Tokuzawa, Y.; Itoh, H.; Segawa, K.; Murakami, M.; Takahashi, K.; Maruyama, M.; Maeda, M.; Yamanaka, S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 2003, 113, 631–642. [Google Scholar] [CrossRef]
- Navarro, P.; Festuccia, N.; Colby, D.; Gagliardi, A.; Mullin, N.P.; Zhang, W.; Karwacki-Neisius, V.; Osorno, R.; Kelly, D.; Robertson, M.; et al. OCT4/SOX2-independent Nanog autorepression modulates heterogeneous Nanog gene expression in mouse ES cells. EMBO J. 2012, 31, 4547–4562. [Google Scholar] [CrossRef]
- Silva, J.; Nichols, J.; Theunissen, T.W.; Guo, G.; van Oosten, A.L.; Barrandon, O.; Wray, J.; Yamanaka, S.; Chambers, I.; Smith, A. Nanog is the gateway to the pluripotent ground state. Cell 2009, 138, 722–737. [Google Scholar] [CrossRef]
- Rasti, A.; Mehrazma, M.; Madjd, Z.; Abolhasani, M.; Saeednejad Zanjani, L.; Asgari, M.L. Co-expression of cancer stem cell markers OCT4 and NANOG predicts poor prognosis in renal cell carcinomas. Sci. Rep. 2018, 8, 11739. [Google Scholar] [CrossRef]
- Wang, M.L.; Chiou, S.H.; Wu, C.W. Targeting cancer stem cells: Emerging role of Nanog transcription factor. Onco Targets Ther. 2013, 6, 1207–1220. [Google Scholar] [CrossRef]
- Okita, K.; Ichisaka, T.; Yamanaka, S. Generation of germline-competent induced pluripotent stem cells. Nature 2007, 448, 313–317. [Google Scholar] [CrossRef]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Abelev, G.I.; Lazarevich, N.L. Control of differentiation in progression of epithelial tumors. Adv. Cancer Res. 2006, 95, 61–113. [Google Scholar] [CrossRef] [PubMed]
- Dowell, K.G.; Simons, A.K.; Bai, H.; Kell, B.; Wang, Z.Z.; Yun, K.; Hibbs, M.A. Novel insights into embryonic stem cell self-renewal revealed through comparative human and mouse systems biology networks. Stem Cells 2014, 32, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, G.; Sawant, S.S.; Hague, A.; Kingsley, K.; Devaraj, H. Association of differential β-catenin expression with Oct-4 and Nanog in oral squamous cell carcinoma and their correlation with clinicopathological factors and prognosis. Head Neck 2015, 37, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Ohnishi, Y.; Inoue, H.; Wato, M.; Tanaka, A.; Kakudo, K.; Nozaki, M. NANOG expression correlates with differentiation, metastasis and resistance to preoperative adjuvant therapy in oral squamous cell carcinoma. Oncol. Lett. 2014, 7, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Fan, H.; Xu, J.; Zhao, E. Prognostic implication of NOTCH1 in early stage oral squamous cell cancer with occult metastases. Clin. Oral Investig. 2018, 22, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.A.; Kiang, A.; Wang-Rodriguez, J.; Rahimy, E.; Haas, M.; Yu, V.; Ellies, L.G.; Chen, J.; Fan, J.B.; Brumund, K.T.; et al. Nicotine promotes acquisition of stem cell and epithelial-to-mesenchymal properties in head and neck squamous cell carcinoma. PLoS ONE 2012, 7, e51967. [Google Scholar] [CrossRef]
- Gu, T.T.; Liu, S.Y.; Zheng, P.S. Cytoplasmic NANOG-positive stromal cells promote human cervical cancer progression. Am. J. Pathol. 2012, 181, 652–661. [Google Scholar] [CrossRef]
- Luo, W.; Li, S.; Peng, B.; Ye, Y.; Deng, X.; Yao, K. Embryonic stem cells markers SOX2, OCT4 and Nanog expression and their correlations with epithelial-mesenchymal transition in nasopharyngeal carcinoma. PLoS ONE 2013, 8, e56324. [Google Scholar] [CrossRef]

| Characteristic | Cytoplasmic NANOG Protein Staining Scores | p * | Nuclear NANOG Expression Negative Positive | p† | |||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | |||||
| Age (years), Mean (SD) | 63 (12) | 57 (18) | 65 (14) | 0.69 | 62 (13) | 69 (13) | 0.46 |
| Gender, number (%) | |||||||
| Female | 26 (90) | 1 (3) | 2 (7) | 0.42 | 28 (97) | 1 (3) | |
| Male | 20 (77) | 3 (11) | 3 (11) | 25 (96) | 1 (4) | ||
| Smoking, number (%) | |||||||
| Yes | 7 (70) | 1 (10) | 2 (20) | 1.00 | 10 (100) | 0 (0) | 1.00 |
| No | 16 (76) | 2 (10) | 3 (14) | 19 (90) | 2 (10) | ||
| Ethanol intake, number (%) | |||||||
| Yes | 1 (25) | 2 (50) | 1 (25) | 0.02 | 4 (100) | 0 (0) | 1.00 |
| No | 22 (81) | 1 (4) | 4 (15) | 25 (93) | 2 (7) | ||
| Dysplasia grading | |||||||
| Low-grade | 38 (90) | 1 (3) | 3 (7) | 0.02 | 42 (100) | 0 (0) | 0.05 |
| High-grade | 8 (62) | 3 (23) | 2 (15) | 11 (85) | 2 (15) | ||
| Characteristic | Number of Cases (%) | Progression to Carcinoma (%) | p |
|---|---|---|---|
| Dysplasia Grade | <0.001 † | ||
| Low-grade | 42 (76) | 2 (5) | |
| High-grade | 13 (24) | 10 (77) | |
| Cytoplasmic NANOG | 0.02 * | ||
| Score 0 | 46 (84) | 7 (15) | |
| Score 1 | 4 (7) | 2 (50) | |
| Score 2 | 5 (9) | 3 (60) | |
| Nuclear NANOG expression | 0.04 † | ||
| Negative | 53 (96) | 10 (19) | |
| Positive | 2 (4) | 2 (100) | |
| Characteristic | No Cases | Censored Patients (%) | Mean Cancer-Free Survival Time (95% CI) | p | Hazard Ratio | 95% Confidence Interval |
|---|---|---|---|---|---|---|
| Dysplasia Grade | ||||||
| Low-grade | 42 | 40 (95) | 181.59 (170.21–192.98) | <0.001 | Reference | |
| High-grade | 13 | 3 (23) | 100.69 (54.14–147.24) | 19.08 | 4.09–89.01 | |
| Cytoplasmic NANOG | ||||||
| Score 0 | 46 | 39 (85) | 171.57 (155.07–188.07) | 0.002 | Reference | |
| Score 1 | 4 | 2 (50) | 156.25 (62.07–250.43) | 2.30 | 0.41–12.86 | |
| Score 2 | 5 | 2 (40) | 43.40 (17.52–69.27) | 8.13 | 2.02–32.64 | |
| Nuclear NANOG | ||||||
| Negative | 53 | 43 (81) | 189.58 (150.36–228.81) | 0.001 | Reference | |
| Positive | 2 | 0 (0) | 45.00 (0.00–97.92) | 8.13 | 1.78–38.79 |
| Variable | p | Hazard Ratio | 95% Confidence Interval |
|---|---|---|---|
| Histology (High-grade vs. low-grade dysplasia) | <0.001 | 17.88 | 3.59–89.04 |
| Cytoplasmic NANOG | 0.082 | ||
| Score 0 | Reference | Reference | |
| Score 1 | 0.54 | 0.55 | 0.08–3.63 |
| Score 2 | 0.07 | 4.45 | 0.88–22.42 |
| Nuclear NANOG (positive vs. negative) | 0.48 | 2.014 | 0.28–14.25 |
| Variable | No Cases | Positive NANOG Expression (%) | p |
|---|---|---|---|
| Gender | |||
| Men | 79 | 31 (39) | 0.02 |
| Women | 43 | 8 (18) | |
| Tobacco use | |||
| Smoker | 82 | 33 (40) | 0.005 |
| Non-smoker | 40 | 6 (15) | |
| Alcohol use | |||
| Drinker | 67 | 29 (43) | 0.003 |
| Non-drinker | 55 | 10 (18) | |
| pT | |||
| pT1 + 2 | 79 | 26 (33) | 0.76 |
| pT3 + 4 | 43 | 13 (30) | |
| pN | |||
| pN0 | 75 | 26 (35) | 0.41 |
| pN+ | 47 | 13 (28) | |
| Clinical stage | |||
| I + II | 51 | 20 (39) | 0.14 |
| III + IV | 71 | 19 (27) | |
| G status | |||
| G1 | 77 | 21 (27) | 0.14 |
| G2 + G3 | 45 | 18 (40) | |
| Tumor location | |||
| Tongue | 50 | 14 (28) | 0.43 |
| Other sites | 72 | 25 (35) | |
| Tumor location | |||
| Floor of the mouth | 36 | 13 (36) | 0.52 |
| Other sites | 86 | 26 (30) | |
| Tumor recurrence | |||
| No | 68 | 26 (38) | 0.09 |
| Yes | 54 | 13 (24) | |
| Second primary carcinoma | |||
| No | 104 | 32 (31) | 0.49 |
| Yes | 18 | 7 (39) | |
| Clinical status at the end of the follow-up | |||
| Alive without recurrence | 50 | 19 (38) | 0.48 * |
| Dead of index cancer | 53 | 15 (28) | |
| Censored | 19 | 5 (26) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Vicente, J.C.; Rodríguez-Santamarta, T.; Rodrigo, J.P.; Allonca, E.; Vallina, A.; Singhania, A.; Donate-Pérez del Molino, P.; García-Pedrero, J.M. The Emerging Role of NANOG as an Early Cancer Risk Biomarker in Patients with Oral Potentially Malignant Disorders. J. Clin. Med. 2019, 8, 1376. https://doi.org/10.3390/jcm8091376
de Vicente JC, Rodríguez-Santamarta T, Rodrigo JP, Allonca E, Vallina A, Singhania A, Donate-Pérez del Molino P, García-Pedrero JM. The Emerging Role of NANOG as an Early Cancer Risk Biomarker in Patients with Oral Potentially Malignant Disorders. Journal of Clinical Medicine. 2019; 8(9):1376. https://doi.org/10.3390/jcm8091376
Chicago/Turabian Stylede Vicente, Juan C., Tania Rodríguez-Santamarta, Juan P. Rodrigo, Eva Allonca, Aitana Vallina, Anusha Singhania, Paula Donate-Pérez del Molino, and Juana M. García-Pedrero. 2019. "The Emerging Role of NANOG as an Early Cancer Risk Biomarker in Patients with Oral Potentially Malignant Disorders" Journal of Clinical Medicine 8, no. 9: 1376. https://doi.org/10.3390/jcm8091376
APA Stylede Vicente, J. C., Rodríguez-Santamarta, T., Rodrigo, J. P., Allonca, E., Vallina, A., Singhania, A., Donate-Pérez del Molino, P., & García-Pedrero, J. M. (2019). The Emerging Role of NANOG as an Early Cancer Risk Biomarker in Patients with Oral Potentially Malignant Disorders. Journal of Clinical Medicine, 8(9), 1376. https://doi.org/10.3390/jcm8091376





