Comparison of Radiographic Progression-Free Survival and PSA Response on Sequential Treatment Using Abiraterone and Enzalutamide for Newly Diagnosed Castration-Resistant Prostate Cancer: A Propensity Score Matched Analysis from Multicenter Cohort
Abstract
:1. Introduction
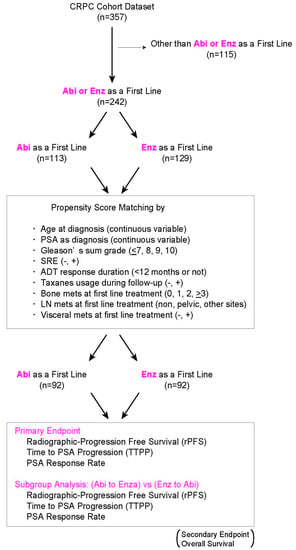
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| rPFS | radiographic progression-free survival |
| OS | overall survival |
| CRPC | castration-resistant prostate cancer |
| ASIs | androgen signaling inhibitors |
| TTPP | time to PSA progression |
| ADT | androgen deprivation therapy |
| RCT | randomized control trial |
| SCC | Spearman’s correlation coefficient |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Mazzu, Y.Z.; Armenia, J.; Chakraborty, G.; Yoshikawa, Y.; Coggins, S.; Nandakumar, S.; Gerke, T.; Pomerantz, M.; Qiu, X.; Zhao, H.; et al. A novel mechanism driving poor-prognosis prostate cancer: Overexpression of the DNA repair gene, ribonucleotide reductase small subunit M2 (RRM2). Clin. Cancer Res. 2019, 25, 4480–4492. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Du, S.Y.; Armenia, J.; Qu, F.; Fan, J.; Wang, X.; Fei, T.; Komura, K.; Liu, S.X.; Lee, G.M.; et al. Expression of lncRNA MIR222HG co-transcribed from the miR-221/222 gene promoter facilitates the development of castration-resistant prostate cancer. Oncogenesis 2018, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Komura, K.; Yoshikawa, Y.; Shimamura, T.; Chakraborty, G.; Gerke, T.A.; Hinohara, K.; Chadalavada, K.; Jeong, S.H.; Armenia, J.; Du, S.Y.; et al. ATR inhibition controls aggressive prostate tumors deficient in Y-linked histone demethylase KDM5D. J. Clin. Invest. 2018, 128, 2979–2995. [Google Scholar] [CrossRef] [PubMed]
- Komura, K.; Jeong, S.H.; Hinohara, K.; Qu, F.; Wang, X.; Hiraki, M.; Azuma, H.; Lee, G.S.; Kantoff, P.W.; Sweeney, C.J. Resistance to docetaxel in prostate cancer is associated with androgen receptor activation and loss of KDM5D expression. Proc. Natl. Acad. Sci. USA 2016, 113, 6259–6264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Bono, J.S.; Logothetis, C.J.; Molina, A.; Fizazi, K.; North, S.; Chu, L.; Chi, K.N.; Jones, R.J.; Goodman, O.B., Jr.; Saad, F.; et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 2011, 364, 1995–2005. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.E.; Sternberg, C.N.; Miller, K.; de Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.E.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Bhattacharya, S.; Carles, J.; Chowdhury, S.; et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 2014, 371, 424–433. [Google Scholar] [CrossRef]
- Ryan, C.J.; Smith, M.R.; de Bono, J.S.; Molina, A.; Logothetis, C.J.; de Souza, P.; Fizazi, K.; Mainwaring, P.; Piulats, J.M.; Ng, S.; et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 2013, 368, 138–148. [Google Scholar] [CrossRef]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Ozguroglu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2017, 377, 352–360. [Google Scholar] [CrossRef]
- James, N.D.; de Bono, J.S.; Spears, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Ritchie, A.W.S.; Amos, C.L.; Gilson, C.; Jones, R.J.; et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N. Engl. J. Med. 2017, 377, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Gillessen, S.; Attard, G.; Beer, T.M.; Beltran, H.; Bossi, A.; Bristow, R.; Carver, B.; Castellano, D.; Chung, B.H.; Clarke, N.; et al. Management of Patients with Advanced Prostate Cancer: The Report of the Advanced Prostate Cancer Consensus Conference APCCC 2017. Eur. Urol. 2017, 73, 178–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiong, E.; Murphy, D.G.; Akaza, H.; Buchan, N.C.; Chung, B.H.; Kanesvaran, R.; Khochikar, M.; Letran, J.; Lojanapiwat, B.; Ng, C.F.; et al. Management of patients with advanced prostate cancer in the Asia Pacific region: ‘real-world’ consideration of results from the Advanced Prostate Cancer Consensus Conference (APCCC) 2017. BJU Int. 2019, 123, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.A.; Eigl, B.J.; Murray, R.N.; Kollmannsberger, C.; Chi, K.N. Efficacy of enzalutamide following abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer patients. Eur. Urol. 2015, 67, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Badrising, S.; van der Noort, V.; van Oort, I.M.; van den Berg, H.P.; Los, M.; Hamberg, P.; Coenen, J.L.; van den Eertwegh, A.J.; de Jong, I.J.; Kerver, E.D.; et al. Clinical activity and tolerability of enzalutamide (MDV3100) in patients with metastatic, castration-resistant prostate cancer who progress after docetaxel and abiraterone treatment. Cancer 2014, 120, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Brasso, K.; Thomsen, F.B.; Schrader, A.J.; Schmid, S.C.; Lorente, D.; Retz, M.; Merseburger, A.S.; von Klot, C.A.; Boegemann, M.; de Bono, J. Enzalutamide Antitumour Activity Against Metastatic Castration-resistant Prostate Cancer Previously Treated with Docetaxel and Abiraterone: A Multicentre Analysis. Eur. Urol. 2015, 68, 317–324. [Google Scholar] [CrossRef] [PubMed]
- David, T.; Natalie, C.; Omi, P. Enzalutamide after failure of docetaxel and abiraterone in metastatic castrate resistant prostate cancer (mCRPC): Results from an expanded access program. J. Clin. Oncol. 2014, 32, 188. [Google Scholar]
- Loriot, Y.; Bianchini, D.; Ileana, E.; Sandhu, S.; Patrikidou, A.; Pezaro, C.; Albiges, L.; Attard, G.; Fizazi, K.; De Bono, J.S.; et al. Antitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100). Ann. Oncol. 2013, 24, 1807–1812. [Google Scholar] [CrossRef] [PubMed]
- Maughan, B.L.; Luber, B.; Nadal, R.; Antonarakis, E.S. Comparing Sequencing of Abiraterone and Enzalutamide in Men with Metastatic Castration-Resistant Prostate Cancer: A Retrospective Study. Prostate 2017, 77, 33–40. [Google Scholar] [CrossRef]
- Mori, K.; Kimura, T.; Onuma, H.; Kimura, S.; Yamamoto, T.; Sasaki, H.; Miki, J.; Miki, K.; Egawa, S. Lactate dehydrogenase predicts combined progression-free survival after sequential therapy with abiraterone and enzalutamide for patients with castration-resistant prostate cancer. Prostate 2017, 77, 1144–1150. [Google Scholar] [CrossRef]
- Noonan, K.L.; North, S.; Bitting, R.L.; Armstrong, A.J.; Ellard, S.L.; Chi, K.N. Clinical activity of abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Ann. Oncol. 2013, 24, 1802–1807. [Google Scholar] [CrossRef] [PubMed]
- Schrader, A.J.; Boegemann, M.; Ohlmann, C.H.; Schnoeller, T.J.; Krabbe, L.M.; Hajili, T.; Jentzmik, F.; Stoeckle, M.; Schrader, M.; Herrmann, E.; et al. Enzalutamide in castration-resistant prostate cancer patients progressing after docetaxel and abiraterone. Eur. Urol. 2014, 65, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Terada, N.; Maughan, B.L.; Akamatsu, S.; Kobayashi, T.; Yamasaki, T.; Inoue, T.; Kamba, T.; Ogawa, O.; Antonarakis, E.S. Exploring the optimal sequence of abiraterone and enzalutamide in patients with chemotherapy-naive castration-resistant prostate cancer: The Kyoto-Baltimore collaboration. Int. J. Urol. 2017, 24, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, F.B.; Roder, M.A.; Rathenborg, P.; Brasso, K.; Borre, M.; Iversen, P. Enzalutamide treatment in patients with metastatic castration-resistant prostate cancer progressing after chemotherapy and abiraterone acetate. Scand. J. Urol. 2014, 48, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, N.; Yamada, Y.; Tabata, K.I.; Satoh, T.; Kamiya, N.; Suzuki, H.; Kawahara, T.; Uemura, H.; Yano, A.; Kawakami, S.; et al. Abiraterone Followed by Enzalutamide Versus Enzalutamide Followed by Abiraterone in Chemotherapy-naive Patients with Metastatic Castration-resistant Prostate Cancer. Clin. Genitourin. Cancer 2018, 16, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Miyake, H.; Hara, T.; Tamura, K.; Sugiyama, T.; Furuse, H.; Ozono, S.; Fujisawa, M. Comparative Assessment of Efficacies Between 2 Alternative Therapeutic Sequences with Novel Androgen Receptor-Axis-Targeted Agents in Patients with Chemotherapy-Naive Metastatic Castration-Resistant Prostate Cancer. Clin. Genitourin. Cancer 2017, 15, e591–e597. [Google Scholar] [CrossRef] [PubMed]
- De Bono, J.S.; Chowdhury, S.; Feyerabend, S.; Elliott, T.; Grande, E.; Melhem-Bertrandt, A.; Baron, B.; Hirmand, M.; Werbrouck, P.; Fizazi, K. Antitumour Activity and Safety of Enzalutamide in Patients with Metastatic Castration-resistant Prostate Cancer Previously Treated with Abiraterone Acetate Plus Prednisone for ≥24 weeks in Europe. Eur. Urol. 2018, 74, 37–45. [Google Scholar] [CrossRef]
- Khalaf, D.; Annala, M.; Finch, D.L.; Oja, C.D.; Vergidis, J.; Zulfiqar, M.; Sunderland, K.; Beja, K.; Vandekerkhove, G.R.; Gleave, M.; et al. Phase 2 randomized cross-over trial of abiraterone + prednisone (ABI+P) vs. enzalutamide (ENZ) for patients (pts) with metastatic castration resistant prostate cancer (mCPRC): Results for 2nd-line therapy. J. Clin. Oncol. 2018, 36, 5015. [Google Scholar] [CrossRef]
- Rathkopf, D.E.; Beer, T.M.; Loriot, Y.; Higano, C.S.; Armstrong, A.J.; Sternberg, C.N.; de Bono, J.S.; Tombal, B.; Parli, T.; Bhattacharya, S.; et al. Radiographic Progression-Free Survival as a Clinically Meaningful End Point in Metastatic Castration-Resistant Prostate Cancer: The PREVAIL Randomized Clinical Trial. JAMA Oncol. 2018, 4, 694–701. [Google Scholar] [CrossRef]
- Morris, M.J.; Molina, A.; Small, E.J.; de Bono, J.S.; Logothetis, C.J.; Fizazi, K.; de Souza, P.; Kantoff, P.W.; Higano, C.S.; Li, J.; et al. Radiographic progression-free survival as a response biomarker in metastatic castration-resistant prostate cancer: COU-AA-302 results. J. Clin. Oncol. 2015, 33, 1356–1363. [Google Scholar] [CrossRef]
- Lorente, D.; Castro, E.; Lozano, R.; Puente, J.; Romero-Laorden, N.; Morales-Barrera, R.; Rodriguez Vida, A.; Sáez, M.I.; Mendez-Vidal, M.J.; Fernandez, E.; et al. Correlation between time to PSA progression (TTPP), radiographic progression-free survival (rPFS) and overall survival (OS) in first-line abiraterone/enzalutamide (Abi/Enza) and docetaxel (Doc) treated patients in a prospective cohort study. J. Clin. Oncol. 2019, 37, 267. [Google Scholar] [CrossRef]
- World Medical, A. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar]
- Scher, H.I.; Halabi, S.; Tannock, I.; Morris, M.; Sternberg, C.N.; Carducci, M.A.; Eisenberger, M.A.; Higano, C.; Bubley, G.J.; Dreicer, R.; et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J. Clin Oncol. 2008, 26, 1148–1159. [Google Scholar] [CrossRef]
- Hussain, M.; Wolf, M.; Marshall, E.; Crawford, E.D.; Eisenberger, M. Effects of continued androgen-deprivation therapy and other prognostic factors on response and survival in phase II chemotherapy trials for hormone-refractory prostate cancer: A Southwest Oncology Group report. J. Clin. Oncol. 1994, 12, 1868–1875. [Google Scholar] [CrossRef]
- Taylor, C.D.; Elson, P.; Trump, D.L. Importance of continued testicular suppression in hormone-refractory prostate cancer. J. Clin. Oncol. 1993, 11, 2167–2172. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Ryan, C.J.; Shah, S.; Efstathiou, E.; Smith, M.R.; Taplin, M.E.; Bubley, G.J.; Logothetis, C.J.; Kheoh, T.; Kilian, C.; Haqq, C.M.; et al. Phase II study of abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer displaying bone flare discordant with serologic response. Clin. Cancer Res. 2011, 17, 4854–4861. [Google Scholar] [CrossRef]
- Komura, K.; Sweeney, C.J.; Inamoto, T.; Ibuki, N.; Azuma, H.; Kantoff, P.W. Current treatment strategies for advanced prostate cancer. Int. J. Urol. 2017, 25, 220–231. [Google Scholar] [CrossRef] [Green Version]
- Chi, K.N.; Annala, M.; Sunderland, K.; Khalaf, D.; Finch, D.; Oja, C.D.; Vergidis, J.; Zulfiqar, M.; Beja, K.; Vandekerkhove, G.; et al. A randomized phase II cross-over study of abiraterone + prednisone (ABI) vs. enzalutamide (ENZ) for patients (pts) with metastatic, castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2017, 35, 5002. [Google Scholar] [CrossRef]
- Qu, F.; Xie, W.; Nakabayashi, M.; Zhang, H.; Jeong, S.H.; Wang, X.; Komura, K.; Sweeney, C.J.; Sartor, O.; Lee, G.M.; et al. Association of AR-V7 and Prostate-Specific Antigen RNA Levels in Blood with Efficacy of Abiraterone Acetate and Enzalutamide Treatment in Men with Prostate Cancer. Clin. Cancer Res. 2017, 23, 726–734. [Google Scholar] [CrossRef]
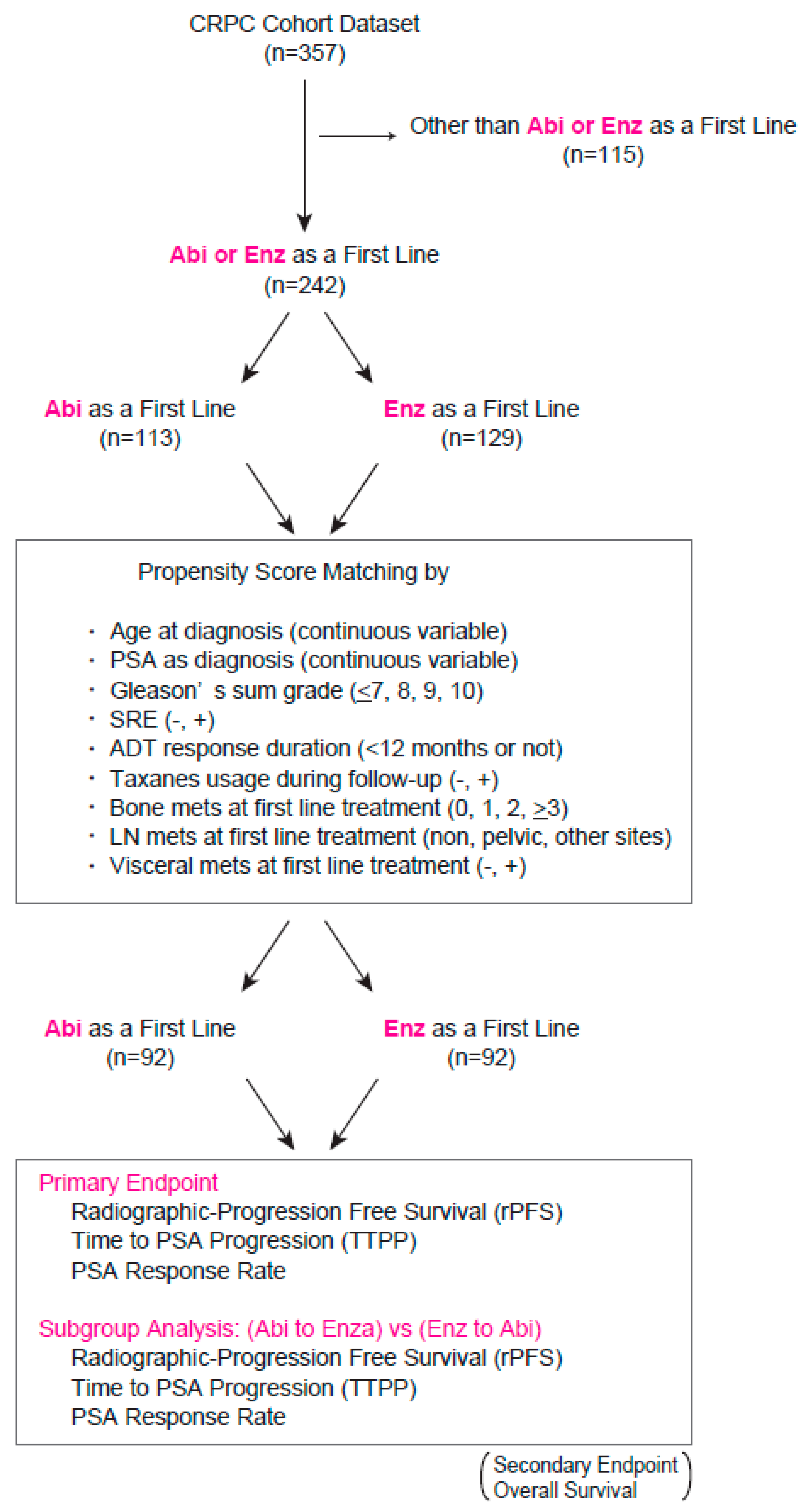



| Variables | Total (n = 184) | Abi (n = 92) | Enz (n = 92) | p Value |
|---|---|---|---|---|
| Age (mean ± SD) | 73.5 + 7.8 | 74.0 + 8.0 | 73.0 + 7.6 | 0.355 |
| SRE during follow-up | ||||
| No (%) | 144 (78.3) | 72 (78.3) | 72 (78.3) | |
| Yes (%) | 40 (21.7) | 20 (21.7) | 20 (21.7) | 1.000 |
| Taxanes during follow-up | ||||
| No (%) | 159 (86.4) | 80 (87.0) | 79 (85.9) | |
| Yes (%) | 25 (13.6) | 12 (13.0) | 13 (14.1) | 0.809 |
| ADT response duration | ||||
| ≥12months (%) | 136 (73.9) | 65 (70.6) | 71 (77.2) | |
| <12 months (%) | 48 (26.1) | 27 (29.4) | 21 (22.8) | 0.809 |
| Median PSA level at diagnosis (ng/mL) (quartile) | 124.0 (29.3, 395.6) | 124.3 (41.9, 327.1) | 93.8 (25.7, 574.8) | 0.685 |
| Median PSA level at first line treatment (ng/mL) (quartile) | 6.8 (2.3, 30.1) | 6.8 (2.0, 30.4) | 6.8 (2.5, 30.1) | 0.918 |
| Gleason sum score (%) | ||||
| ≤7 | 20 (10.9) | 9 (9.8) | 11 (12.0) | |
| 8 | 41 (22.3) | 20 (21.7) | 21 (22.8) | |
| 9 | 113 (61.4) | 56 (60.9) | 57 (62.0) | |
| 10 | 10 (5.4) | 7 (7.6) | 3 (3.3) | 0.598 |
| Local treatment prior to ADT (%) | ||||
| Non | 156 (84.8) | 77 (83.7) | 79 (85.9) | |
| Prostatectomy | 16 (8.7) | 9 (9.8) | 7 (7.6) | |
| Radiation | 8 (4.3) | 5 (5.4) | 3 (3.3) | |
| Others | 4 (2.2) | 1 (1.1) | 3 (3.3) | 0.311 |
| Initial ADT (%) | ||||
| LHRH analog + NAs | 162 (88.0) | 78 (84.8) | 84 (91.3) | |
| LHRH analog | 11 (6.0) | 7 (7.6) | 4 (4.3) | |
| NAs | 7 (3.8) | 5 (5.4) | 2 (2.2) | |
| Others | 4 (2.2) | 2 (2.2) | 2 (2.2) | 0.113 |
| Mets at first line treatment (%) | ||||
| M0 | 57 (31.0) | 27 (29.4) | 30 (32.6) | |
| M1 | 127 (69.0) | 65 (70.7) | 62 (67.4) | 0.345 |
| Visceral mets at first line treatment (%) | ||||
| No | 165 (89.7) | 82 (89.1) | 83 (90.2) | |
| Yes | 19 (10.3) | 10 (10.9) | 9 (9.8) | 0.809 |
| LN mets at first line treatment (%) | ||||
| Non | 117 (63.6) | 55 (59.8) | 62 (67.4) | |
| Regional | 45 (24.5) | 23 (25.0) | 22 (23.9) | |
| Non-regional | 22 (12.0) | 14 (15.2) | 8 (8.7) | 0.350 |
| No. of bone mets at first line treatment (%) | ||||
| 0 | 83 (45.1) | 40 (43.5) | 43 (46.7) | |
| 1 | 23 (12.5) | 11 (12.0) | 12 (13.0) | |
| 2 | 15 (8.2) | 8 (8.7) | 7 (7.6) | |
| >3 | 63 (34.2) | 33 (35.9) | 30 (32.6) | 0.948 |
| ECOG-PS (%) | ||||
| 0 | 104 (56.5) | 51 (55.4) | 53 (57.6) | |
| 1 | 66 (35.9) | 35 (38.0) | 31 (33.7) | |
| ≥2 | 14 (7.6) | 6 (6.6) | 8 (8.7) | 0.684 |
| Neutrophil-lymphocyte ratio at first line treatment (mean ± SD) | 2.99 ± 2.49 | 3.08 ± 2.93 | 2.84 ± 1.59 | 0.648 |
| Hb at first line treatment (g/dL) (mean ± SD) | 12.2 ± 1.8 | 12.1 ± 1.8 | 12.3 ± 1.8 | 0.627 |
| Platelet count at first line treatment (103/uL) (mean ± SD) | 213 ± 75 | 215 ± 80 | 210 ± 71 | 0.640 |
| ALP at first line treatment (U/L) (quartile) | 248 (202, 350) | 252 (199, 370) | 246 (213, 344) | 0.884 |
| LDH at first line treatment (U/L) (quartile) | 200 (182, 238) | 201 (177, 231) | 200 (185, 240) | 0.675 |
| Albumin (g/dL) (quartile) | 4.1 (3.8, 4.4) | 4.1 (3.6, 4.3) | 4.2 (3.9, 4.4) | 0.126 |
| CRP (mg/dL) (quartile) | 0.1 (0.05, 0.32) | 0.1 (0.05, 0.23) | 0.1 (0.05, 0.48) | 0.670 |
| Variables | Total (n = 84) | Abi to Enz (n = 46) | Enz to Abi (n = 38) | p Value |
|---|---|---|---|---|
| Age (mean ± SD) | 73.0 + 7.9 | 71.8 + 7.3 | 74.4 + 8.4 | ns |
| SRE during follow-up | ||||
| No (%) | 63 (75.0) | 32 (69.6) | 31 (81.6) | |
| Yes (%) | 21 (25.0) | 14 (30.4) | 7 (18.4) | ns |
| Median PSA level at 2nd line treatment (ng/mL) (quartile) | 21.3 (3.8, 93.2) | 21.5 (8.1, 61.3) | 21.0 (3.4, 94.3) | ns |
| Gleason sum score (%) | ||||
| ≤7 | 11 (13.1) | 8 (17.4) | 3 (7.9) | |
| 8 | 16 (19.0) | 6 (13.0) | 10 (26.3) | |
| 9 | 53 (63.1) | 30 (65.2) | 23 (60.5) | |
| 10 | 4 (4.8) | 2 (4.4) | 2 (5.3) | ns |
| Mets at 2nd line treatment (%) | ||||
| M0 | 16 (19.0) | 9 (19.6) | 7 (18.4) | |
| M1 | 68 (81.0) | 37 (80.4) | 31 (81.6) | ns |
| Visceral mets at 2nd line treatment (%) | ||||
| No | 75 (88.2) | 39 (84.8) | 36 (94.7) | |
| Yes | 9 (11.8) | 7 (15.2) | 2 (5.3) | ns |
| LN mets at 2nd line treatment (%) | ||||
| No | 59 (70.2) | 31 (67.4) | 28 (73.7) | |
| Yes | 25 (29.8) | 15 (32.6) | 10 (26.3) | ns |
| Bone mets at 2nd line treatment (%) | ||||
| No | 25 (29.8) | 14 (30.4) | 11 (29.0) | |
| Yes | 59 (70.2) | 32 (69.6) | 27 (71.1) | ns |
| Taxanes during follow-up | ||||
| No | 72 (85.7) | 41 (89.1) | 30 (79.0) | |
| Yes | 12 (14.3) | 5 (10.9) | 8 (21.0) | ns |
| ECOG-PS (%) | ||||
| 0 | 34 (40.5) | 17 (37.0) | 17 (44.7) | |
| 1 | 45 (53.6) | 27 (58.7) | 18 (47.4) | |
| ≥2 | 5 (5.9) | 2 (4.4) | 3 (7.9) | ns |
| PSA decline ≥50% at first line treatment | ||||
| No (%) | 43 (51.2) | 28 (60.9) | 15 (39.5) | |
| Yes (%) | 41 (48.8) | 18 (39.1) | 23 (60.5) | 0.037 |
| TTPP at 2nd Line | Radiographic PFS | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | HR | 95%CI | p Value | HR | 95%CI | p Value | ||
| Treatment sequence | ||||||||
| Abi to Enz | Ref | Ref | ||||||
| Enz to Abi | 1.791 | 1.091 | 3.163 | 0.043 | 1.538 | 0.855 | 3.019 | 0.219 |
| Visceral mets at 2nd line treatment | ||||||||
| No | ||||||||
| Yes | 3.647 | 1.003 | 23.634 | 0.049 | 3.647 | 1.182 | 19.278 | 0.032 |
| LN mets at 2nd line treatment | ||||||||
| No | Ref | Ref | ||||||
| Yes | 1.663 | 0.847 | 3.406 | 0.141 | 1.233 | 0.784 | 2.392 | 0.221 |
| Bone mets at 2nd line treatment | ||||||||
| No | Ref | Ref | ||||||
| Yes | 1.946 | 0.972 | 4.071 | 0.06 | 1.392 | 0.872 | 4.281 | 0.099 |
| PSA decline ≥50% at first line | ||||||||
| No | Ref | Ref | ||||||
| Yes | 0.641 | 0.401 | 0.933 | 0.038 | 0.865 | 0.431 | 1.283 | 0.492 |
| ECOG-PS | ||||||||
| 0 | Ref | Ref | ||||||
| >1 | 2.154 | 1.163 | 4.154 | 0.014 | 1.538 | 0.699 | 2.193 | 0.293 |
| Variables | Spearman’s Correlation Coefficient (SCC) (95%CI) | p Value |
|---|---|---|
| rPFS | 0.601 (0.411–0.722) | <0.001 |
| TTPP | 0.468 (0.275–0.625) | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komura, K.; Fujiwara, Y.; Uchimoto, T.; Saito, K.; Tanda, N.; Matsunaga, T.; Ichihashi, A.; Tsutsumi, T.; Tsujino, T.; Yoshikawa, Y.; et al. Comparison of Radiographic Progression-Free Survival and PSA Response on Sequential Treatment Using Abiraterone and Enzalutamide for Newly Diagnosed Castration-Resistant Prostate Cancer: A Propensity Score Matched Analysis from Multicenter Cohort. J. Clin. Med. 2019, 8, 1251. https://doi.org/10.3390/jcm8081251
Komura K, Fujiwara Y, Uchimoto T, Saito K, Tanda N, Matsunaga T, Ichihashi A, Tsutsumi T, Tsujino T, Yoshikawa Y, et al. Comparison of Radiographic Progression-Free Survival and PSA Response on Sequential Treatment Using Abiraterone and Enzalutamide for Newly Diagnosed Castration-Resistant Prostate Cancer: A Propensity Score Matched Analysis from Multicenter Cohort. Journal of Clinical Medicine. 2019; 8(8):1251. https://doi.org/10.3390/jcm8081251
Chicago/Turabian StyleKomura, Kazumasa, Yuya Fujiwara, Taizo Uchimoto, Kenkichi Saito, Naoki Tanda, Tomohisa Matsunaga, Atsushi Ichihashi, Takeshi Tsutsumi, Takuya Tsujino, Yuki Yoshikawa, and et al. 2019. "Comparison of Radiographic Progression-Free Survival and PSA Response on Sequential Treatment Using Abiraterone and Enzalutamide for Newly Diagnosed Castration-Resistant Prostate Cancer: A Propensity Score Matched Analysis from Multicenter Cohort" Journal of Clinical Medicine 8, no. 8: 1251. https://doi.org/10.3390/jcm8081251
APA StyleKomura, K., Fujiwara, Y., Uchimoto, T., Saito, K., Tanda, N., Matsunaga, T., Ichihashi, A., Tsutsumi, T., Tsujino, T., Yoshikawa, Y., Nishimoto, Y., Takai, T., Minami, K., Taniguchi, K., Tanaka, T., Uehara, H., Hirano, H., Nomi, H., Ibuki, N., ... Azuma, H. (2019). Comparison of Radiographic Progression-Free Survival and PSA Response on Sequential Treatment Using Abiraterone and Enzalutamide for Newly Diagnosed Castration-Resistant Prostate Cancer: A Propensity Score Matched Analysis from Multicenter Cohort. Journal of Clinical Medicine, 8(8), 1251. https://doi.org/10.3390/jcm8081251