Mechanical Ventilation Strategies Targeting Different Magnitudes of Collapse and Tidal Recruitment in Porcine Acid Aspiration-Induced Lung Injury
Abstract
:1. Introduction
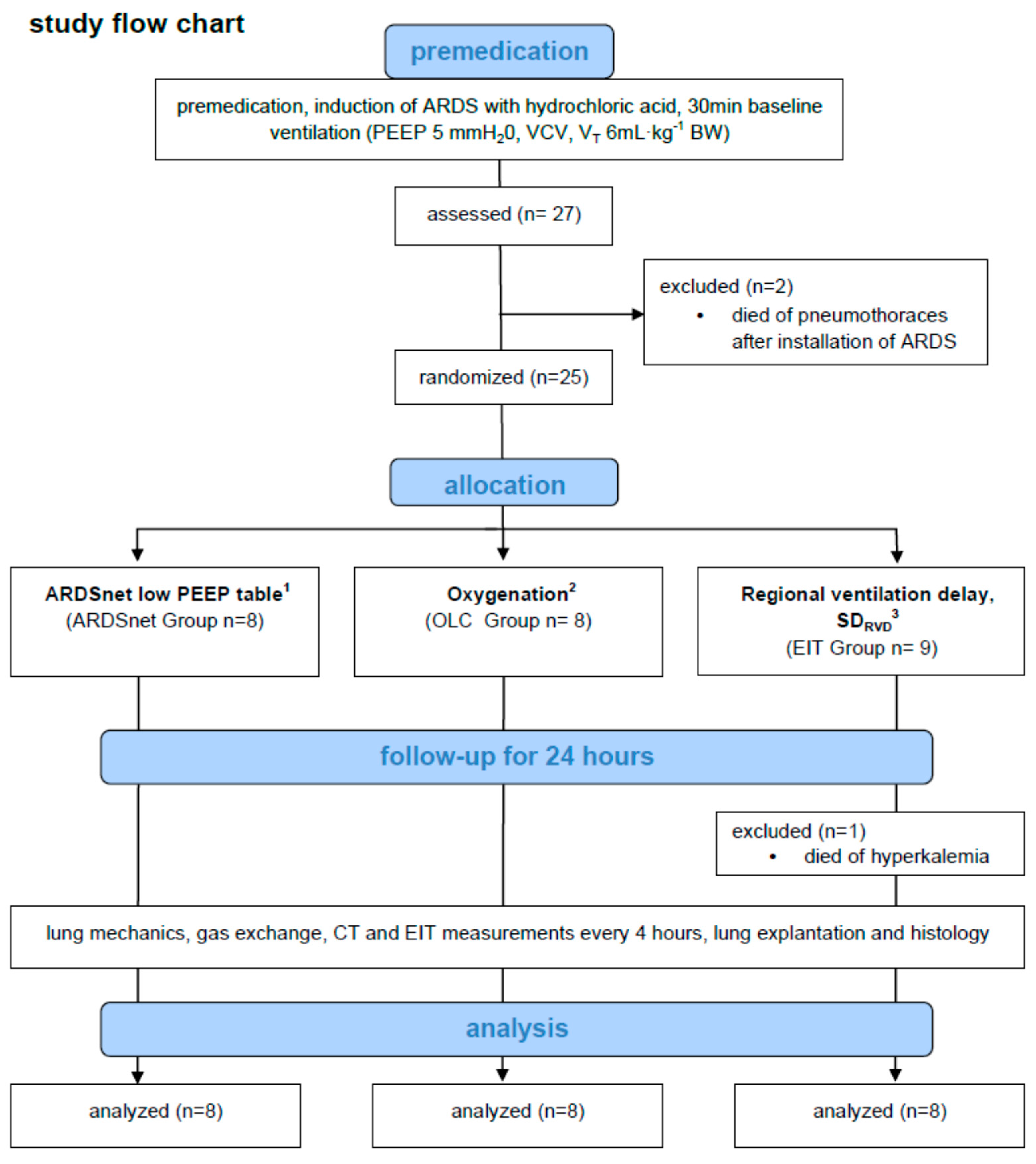
2. Materials and Methods
2.1. Animal Handling and Preparation
2.2. Acid Aspiration-Induced Pulmonary Acute Respiratory Distress Syndrome
2.3. Individualization of PEEP and Mechanical Ventilation
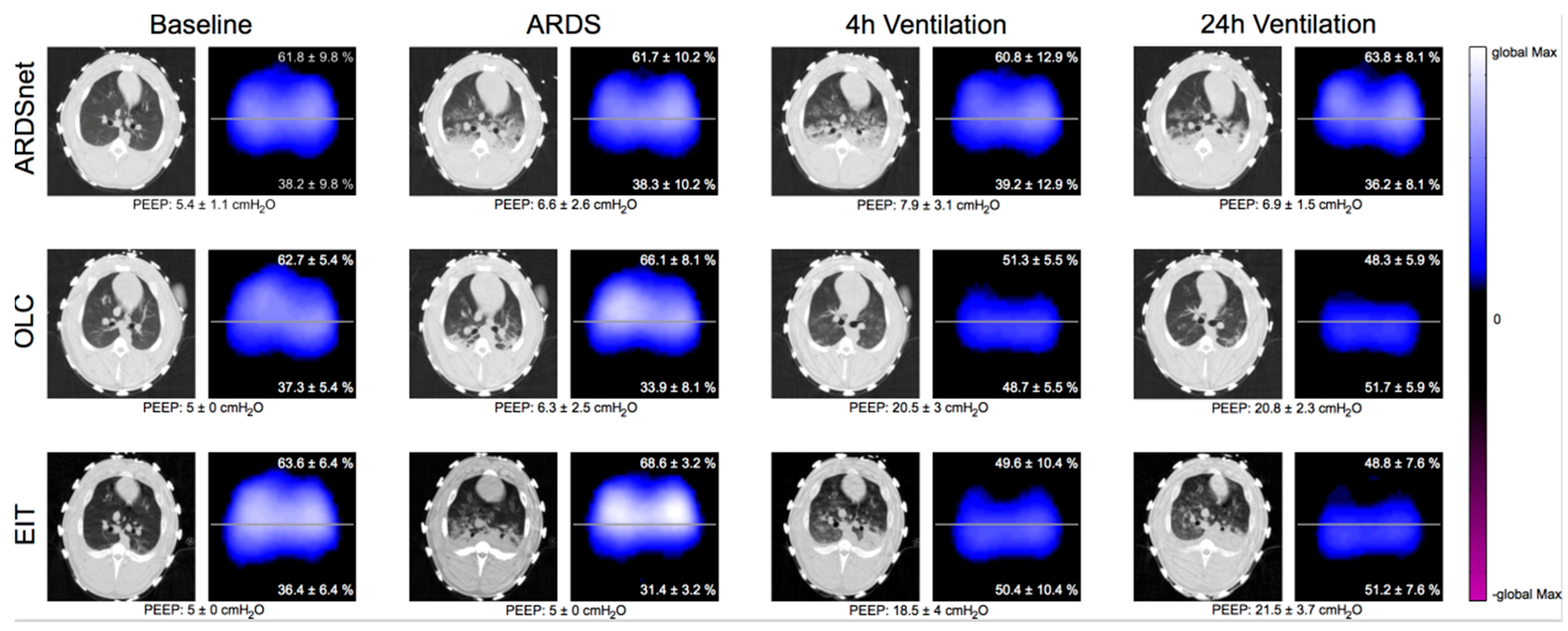
2.4. Quantitative Computed Tomography (qCT)
2.5. Electrical Impedance Tomography
2.6. Tissue Processing and Histological Analysis
2.7. Statistical Analysis
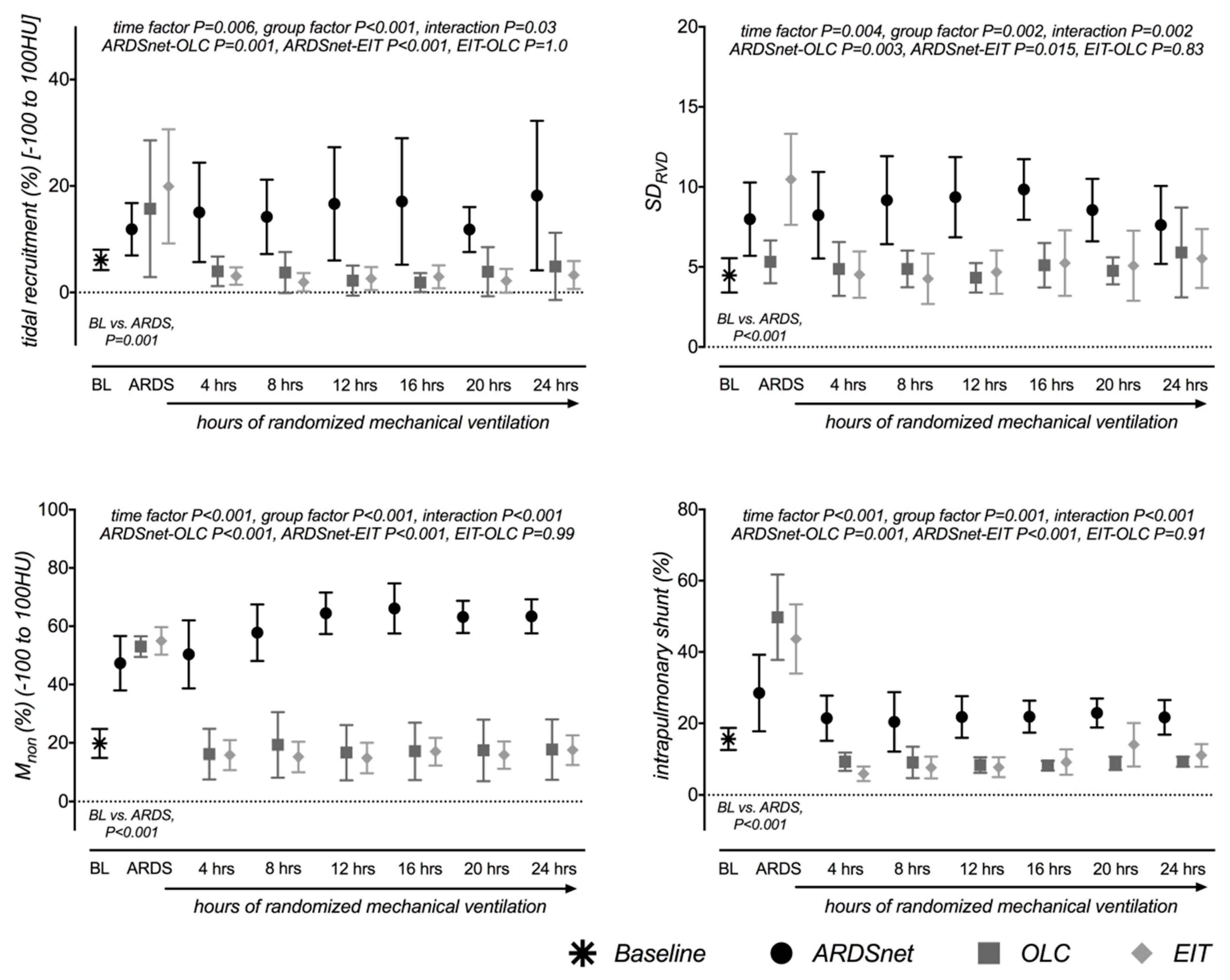
3. Results
3.1. General Aspects and Effects of Induction of ARDS
3.2. Effects on Lung Morphology and Function
3.3. Hemodynamics and Systemic Physiological Parameters
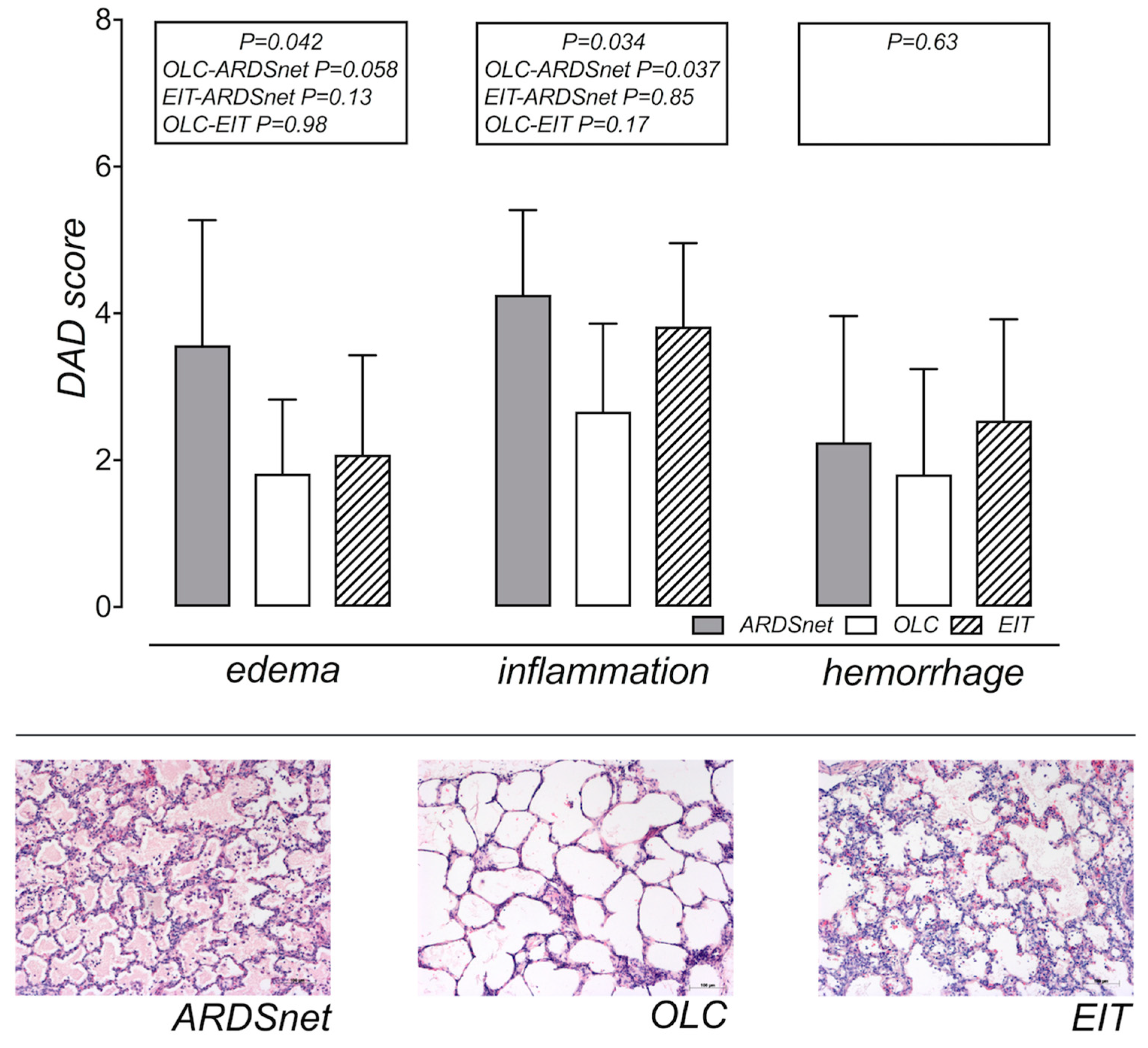
3.4. Assessment of Edema and Histology
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Amato, M.B.; Barbas, C.S.; Medeiros, D.M.; Magaldi, R.B.; Schettino, G.P.; Lorenzi-Filho, G.; Kairalla, R.A.; Deheinzelin, D.; Munoz, C.; Oliveira, R.; et al. Effect of a Protective-Ventilation Strategy on Mortality in the Acute Respiratory Distress Syndrome. N. Engl. J. Med. 1998, 338, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Ware, L.B.; Matthay, M.A. The acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1334–1349. [Google Scholar] [CrossRef] [PubMed]
- Slutsky, A.S.; Ranieri, V.M. Ventilator-induced lung injury. N. Engl. J. Med. 2013, 369, 2126–2136. [Google Scholar] [CrossRef] [PubMed]
- Briel, M.; Meade, M.; Mercat, A.; Brower, R.G.; Talmor, D.; Walter, S.D.; Slutsky, A.S.; Pullenayegum, E.; Zhou, Q.; Cook, D.; et al. Higher vs. lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: Systematic review and meta-analysis. JAMA 2010, 303, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, A.B.; Suzumura, É.A.; Laranjeira, L.N.; Paisani, D.M.; Damiani, L.P.; Guimarães, H.P.; Romano, E.R.; Regenga, M.M.; Taniguchi, L.N.T.; Teixeira, C.; et al. Effect of Lung Recruitment and Titrated Positive End-Expiratory Pressure (PEEP) vs. Low PEEP on Mortality in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2017, 318, 1335–1345. [Google Scholar] [CrossRef] [PubMed]
- Sahetya, S.K.; Brower, R.G. Lung Recruitment and Titrated PEEP in Moderate to Severe ARDS: Is the Door Closing on the Open Lung? JAMA 2017, 318, 1327–1329. [Google Scholar] [CrossRef] [PubMed]
- Walkey, A.J.; Summer, R.; Ho, V.; Alkana, P. Acute respiratory distress syndrome: Epidemiology and management approaches. Clin. Epidemiol. 2012, 4, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Chiumello, D.A.; Cressoni, M.; Carlesso, E.; Caspani, M.L.; Marino, A.; Gallazzi, E.; Caironi, P.; Lazzerini, M.; Moerer, O.; Quintel, M.; et al. Bedside Selection of Positive End-Expiratory Pressure in Mild, Moderate, and Severe Acute Respiratory Distress Syndrome. Crit. Care Med. 2014, 42, 252–264. [Google Scholar] [CrossRef]
- Tusman, G.; Pesch, T.; Thamm, O.; Reske, A.; Suárez-Sipmann, F.; Böhm, S.H.; Reissmann, H.; Magnusson, A.; Hedenstierna, G. Use of dynamic compliance for open lung positive end-expiratory pressure titration in an experimental study. Crit. Care Med. 2007, 35, 214–221. [Google Scholar] [Green Version]
- Borges, J.B.; Okamoto, V.N.; Matos, G.F.J.; Caramez, M.P.R.; Arantes, P.R.; Barros, F.; Souza, C.E.; Victorino, J.A.; Kacmarek, R.M.; Barbas, C.S.V.; et al. Reversibility of Lung Collapse and Hypoxemia in Early Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2006, 174, 268–278. [Google Scholar] [CrossRef]
- Hickling, K.G. Reinterpreting the pressure-volume curve in patients with acute respiratory distress syndrome. Curr. Opin. Crit. Care 2002, 8, 32–38. [Google Scholar] [CrossRef]
- Lachmann, B. Open up the lung and keep the lung open. Intensiv. Care Med. 1992, 18, 319–321. [Google Scholar] [CrossRef] [Green Version]
- Borges, J.B.; Suarez-Sipmann, F.; Bohm, S.H.; Tusman, G.; Melo, A.; Maripuu, E.; Sandström, M.; Park, M.; Costa, E.L.; Hedenstierna, G.; et al. Regional lung perfusion estimated by electrical impedance tomography in a piglet model of lung collapse. J. Appl. Physiol. (1985) 2012, 112, 225–236. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, A.R.; Spieth, P.M.; Pelosi, P.; Melo, M.F.V.; Koch, T.; Jandre, F.C.; Giannella-Neto, A.; De Abreu, M.G. Ability of dynamic airway pressure curve profile and elastance for positive end-expiratory pressure titration. Intensiv. Care Med. 2008, 34, 2291–2299. [Google Scholar] [CrossRef] [Green Version]
- Suter, P.M.; Fairley, H.B.; Isenberg, M.D. Optimum End-Expiratory Airway Pressure in Patients with Acute Pulmonary Failure. N. Engl. J. Med. 1975, 292, 284–289. [Google Scholar] [CrossRef]
- Lachmann, B.; Jonson, B.; Lindroth, M.; Robertson, B. Modes of artificial ventilation in severe respiratory distress syndrome: Lung function and morphology in rabbits after wash-out of alveolar surfactant. Crit. Care Med. 1982, 10, 721–732. [Google Scholar] [CrossRef]
- Crotti, S.; Mascheroni, D.; Caironi, P.; Pelosi, P.; Ronzoni, G.; Mondino, M.; Marini, J.J.; Gattinoni, L. Recruitment and derecruitment during acute respiratory failure: A clinical study. Am. J. Respir. Crit. Care Med. 2001, 164, 131–140. [Google Scholar] [CrossRef]
- Bugedo, G.; Bruhn, A.; Hernández, G.; Rojas, G.; Varela, C.; Tapia, J.C.; Castillo, L. Lung computed tomography during a lung recruitment maneuver in patients with acute lung injury. Intensiv. Care Med. 2003, 29, 218–225. [Google Scholar] [CrossRef]
- De Matos, G.F.; Stanzani, F.; Passos, R.H.; Fontana, M.F.; Albaladejo, R.; Caserta, R.E.; Santos, D.C.; Borges, J.B.; Amato, M.B.; Barbas, C.S. How large is the lung recruitability in early acute respiratory distress syndrome: A prospective case series of patients monitored by computed tomography. Crit. Care 2012, 16, R4. [Google Scholar] [CrossRef]
- Costa, E.L.V.; Borges, J.B.; Melo, A.; Suarez-Sipmann, F.; Toufen, C.; Bohm, S.H.; Amato, M.B.P. Bedside estimation of recruitable alveolar collapse and hyperdistension by electrical impedance tomography. Intensiv. Care Med. 2009, 35, 1132–1137. [Google Scholar] [CrossRef]
- Kobylianskii, J.; Murray, A.; Brace, D.; Goligher, E.; Fan, E. Electrical impedance tomography in adult patients undergoing mechanical ventilation: A systematic review. J. Crit. Care 2016, 35, 33–50. [Google Scholar] [CrossRef]
- Wrigge, H.; Zinserling, J.; Muders, T.; Varelmann, D.; Günther, U.; Von Der Groeben, C.; Magnusson, A.; Hedenstierna, G.; Putensen, C. Electrical impedance tomography compared with thoracic computed tomography during a slow inflation maneuver in experimental models of lung injury. Crit. Care Med. 2008, 36, 903–909. [Google Scholar] [CrossRef]
- Muders, T.; Luepschen, H.; Zinserling, J.; Greschus, S.; Fimmers, R.; Guenther, U.; Buchwald, M.; Grigutsch, D.; Leonhardt, S.; Putensen, C.; et al. Tidal recruitment assessed by electrical impedance tomography and computed tomography in a porcine model of lung injury. Crit. Care Med. 2012, 40, 903–911. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Berggren, S.M. The Oxygen Deficit of Aterial Blood Caused by non- ventilating parts of the lung. Acta Physiol. Scand. 1942, 4, 1–92. [Google Scholar]
- ARDS Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. The Acute Respiratory Distress Syndrome: The Berlin definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Reske, A.W.; Costa, E.L.V.; Reske, A.P.; Rau, A.; Borges, J.B.; Beraldo, M.A.; Gottschaldt, U.; Seiwerts, M.; Schreiter, D.; Petroff, D.; et al. Bedside Estimation of Nonaerated Lung Tissue Using Blood Gas Analysis. Crit. Care Med. 2013, 41, 732–743. [Google Scholar] [CrossRef]
- Reske, A.W.; Reske, A.P.; Heine, T.; Spieth, P.M.; Rau, A.; Seiwerts, M.; Busse, H.; Gottschaldt, U.; Schreiter, D.; Born, S.; et al. Computed tomographic assessment of lung weights in trauma patients with early posttraumatic lung dysfunction. Crit. Care 2011, 15, R71. [Google Scholar] [CrossRef]
- Lundquist, H.; Hedenstierna, G.; Strandberg, Å.; Tokics, L.; Brismar, B. CT-Assessment of Dependent Lung Densities in Man During General Anaesthesia. Acta Radiol. 1995, 36, 626–632. [Google Scholar] [CrossRef]
- Gattinoni, L.; Caironi, P.; Pelosi, P.; Goodman, L.R. What Has Computed Tomography Taught Us about the Acute Respiratory Distress Syndrome? Am. J. Respir. Crit. Care Med. 2001, 164, 1701–1711. [Google Scholar] [CrossRef] [Green Version]
- Frerichs, I.; Dargaville, P.A.; Van Genderingen, H.; Morel, D.R.; Rimensberger, P.C. Lung Volume Recruitment after Surfactant Administration Modifies Spatial Distribution of Ventilation. Am. J. Respir. Crit. Care Med. 2006, 174, 772–779. [Google Scholar] [CrossRef]
- Frerichs, I.; Hahn, G.; Golisch, W.; Kurpitz, M.; Burchardi, H.; Hellige, G. Monitoring perioperative changes in distribution of pulmonary ventilation by functional electrical impedance tomography. Acta Anaesthesiol. Scand. 1998, 42, 721–726. [Google Scholar] [CrossRef]
- Spieth, P.M.; Knels, L.; Kasper, M.; Domingues Quelhas, A.; Wiedemann, B.; Lupp, A.; Hübler, M.; Neto, A.G.; Koch, T.; Gama de Abreu, M. Effects of vaporized perfluorohexane and partial liquid ventilation on regional distribution of alveolar damage in experimental lung injury. Intensive Care Med. 2007, 33, 308–314. [Google Scholar] [CrossRef]
- Quintel, M.; Heine, M.; Hirschl, R.B.; Tillmanns, R.; Wessendorf, V. Effects of partial liquid ventilation on lung injury in a model of acute respiratory failure: A histologic and morphometric analysis. Crit. Care Med. 1998, 26, 833–843. [Google Scholar] [CrossRef]
- Leme, A.C.; Hajjar, L.A.; Volpe, M.S.; Fukushima, J.T.; Santiago, R.R.D.S.; Osawa, E.A.; De Almeida, J.P.; Gerent, A.M.; Franco, R.A.; Feltrim, M.I.Z.; et al. Effect of Intensive vs. Moderate Alveolar Recruitment Strategies Added to Lung-Protective Ventilation on Postoperative Pulmonary Complications. JAMA 2017, 317, 1422–1432. [Google Scholar] [CrossRef]
- Grasso, S.; Mascia, L.; Del Turco, M.; Malacarne, P.; Giunta, F.; Brochard, L.; Slutsky, A.S.; Ranieri, V.M. Effects of Recruiting Maneuvers in Patients with Acute Respiratory Distress Syndrome Ventilated with Protective Ventilatory Strategy. Anesthesiology 2002, 96, 795–802. [Google Scholar] [CrossRef]
- Schreiter, D.; Reske, A.; Stichert, B.; Seiwerts, M.; Bohm, S.H.; Kloeppel, R.; Josten, C. Alveolar recruitment in combination with sufficient positive end-expiratory pressure increases oxygenation and lung aeration in patients with severe chest trauma. Crit. Care Med. 2004, 32, 968–975. [Google Scholar] [CrossRef]
- Cinnella, G.; Grasso, S.; Raimondo, P.; D’antini, D.; Mirabella, L.; Rauseo, M.; Dambrosio, M. Physiological Effects of the Open Lung Approach in Patients with Early, Mild, Diffuse Acute Respiratory Distress Syndrome: An Electrail Impedance Tomography Study. Anesthesiology 2015, 123, 1113–1121. [Google Scholar] [CrossRef]
- Hemmes, S.N.T.; De Abreu, M.G.; Severgnini, P.; Schultz, M.J. High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): A multicentre randomised controlled trial. Lancet 2014, 384, 495–503. [Google Scholar]
- Gattinoni, L.; Caironi, P.; Cressoni, M.; Chiumello, D.A.; Ranieri, V.M.; Quintel, M.; Russo, S.; Patroniti, N.; Cornejo, R.; Bugedo, G. Lung Recruitment in Patients with the Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2006, 354, 1775–1786. [Google Scholar] [CrossRef]
- Maiolo, G.; Collino, F.; Vasques, F.; Rapetti, F.; Tonetti, T.; Romitti, F.; Cressoni, M.; Chiumello, D.; Mörer, O.; Herrmann, P.; et al. Reclassifying Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2018, 197, 1586–1595. [Google Scholar] [CrossRef]
- Sinha, P.; Network, F.T.N.A.; Delucchi, K.L.; Thompson, B.T.; McAuley, D.F.; Matthay, M.A.; Calfee, C.S. Latent class analysis of ARDS subphenotypes: A secondary analysis of the statins for acutely injured lungs from sepsis (SAILS) study. Intensiv. Care Med. 2018, 44, 1859–1869. [Google Scholar] [CrossRef]
- Muscedere, J.G.; Mullen, J.B.; Gan, K.; Slutsky, A.S. Tidal ventilation at low airway pressures can augment lung injury. Am. J. Respir. Crit. Care Med. 1994, 149, 1327–1334. [Google Scholar] [CrossRef]
- Pelosi, P.; Rocco, P.R.M.; De Abreu, M.G. Close down the lungs and keep them resting to minimize ventilator-induced lung injury. Crit. Care 2018, 22, 72. [Google Scholar] [CrossRef]
- Goligher, E.C.; Hodgson, C.L.; Adhikari Neill, K.J.; Meade, M.O.; Wunsch, H.; Uleryk, E.; Gajic, O.; Amato, M.P.B.; Ferguson, N.D.; Rubenfeld, G.D.; et al. Lung Recruitment Maneuvers for Adult Patients with Acute Respiratory Distress Syndrome. A Systematic Review and Meta-Analysis. Ann. Am. Thorac. Soc. 2017, 14 (Suppl. 4), S304–S311. [Google Scholar] [CrossRef]
- Silva, P.L.; Cruz, F.F.; Fujisaki, L.C.; Oliveira, G.P.; Samary, C.S.; Ornellas, D.S.; Maron-Gutierrez, T.; Rocha, N.N.; Goldenberg, R.; Garcia, C.S.; et al. Hypervolemia induces and potentiates lung damage after recruitment maneuver in a model of sepsis-induced acute lung injury. Crit. Care 2010, 14, R114. [Google Scholar] [CrossRef]
- Katira, B.H.; Engelberts, D.; Otulakowski, G.; Giesinger, R.E.; Yoshida, T.; Post, M.; Kuebler, W.M.; Connelly, K.A.; Kavanagh, B.P. Abrupt Deflation after Sustained Inflation Causes Lung Injury. Am. J. Respir. Crit. Care Med. 2018, 198, 1165–1176. [Google Scholar] [CrossRef]
- Marini, J.J.; Gattinoni, L. Protecting the Ventilated Lung: Vascular Surge and Deflation Energetics. Am. J. Respir. Crit. Care Med. 2018, 198, 1112–1114. [Google Scholar] [CrossRef]
- Zigelman, C.; Briskin, A.; Hersch, M. Extremely High-Pressure Lung Recruitment Maneuver May Be Life Saving in the Most Severe Cases of Acute Lung Injury/Acute Respiratory Distress Syndrome Letters to the Editor. Crit. Care Med. 2004, 32, 1441–1442. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Burch, R.L.; Hume, C.W. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959; p. 64. [Google Scholar]
- Li, R.; Lewis, J.H.; Cerviño, L.I.; Jiang, S.B. 4D CT sorting based on patient internal anatomy. Phys. Med. Biol. 2009, 54, 4821–4833. [Google Scholar] [CrossRef]
- Li, G.; Xie, H.; Ning, H.; Lu, W.; Low, D.; Citrin, D.; Kaushal, A.; Zach, L.; Camphausen, K.; Miller, R.W. A novel analytical approach to the prediction of respiratory diaphragm motion based on external torso volume change. Phys. Med. Biol. 2009, 54, 4113–4130. [Google Scholar] [CrossRef]
- Chiumello, D.; Marino, A.; Brioni, M.; Menga, F.; Cigada, I.; Lazzerini, M.; Andrisani, M.C.; Biondetti, P.; Cesana, B.; Gattinoni, L. Visual anatomical lung CT scan assessment of lung recruitability. Intensive Care Med. 2013, 39, 66–73. [Google Scholar] [CrossRef]
- Fehrenbach, A.; Fehrenbach, H.; Wittwer, T.; Ochs, M.; Wahlers, T.; Richter, J. Evaluation of Pulmonary Edema: Stereological versus Gravimetrical Analysis. Eur. Surg. Res. 2001, 33, 270–278. [Google Scholar] [CrossRef]
- Spieth, P.M.; Güldner, A.; Carvalho, A.R.; Kasper, M.; Pelosi, P.; Uhlig, S.; Koch, T.; De Abreu, M.G. Open lung approach vs. acute respiratory distress syndrome network ventilation in experimental acute lung injury. Br. J. Anaesth. 2011, 107, 388–397. [Google Scholar] [CrossRef]
- Norman, G. Likert scales, levels of measurement and the “laws” of statistics. Adv. Health Sci. Educ. 2010, 15, 625–632. [Google Scholar] [CrossRef]
- Boone, H.N.; Boone, D.A. Analyzing Likert Data. J. Ext. 2012, 50, 1–5. [Google Scholar]
- Evans, B.A.; Feng, Z.; Peterson, A.V. A comparison of generalized linear mixed model procedures with estimating equations for variance and covariance parameter estimation in longitudinal studies and group randomized trials. Stat. Med. 2001, 20, 3353–3373. [Google Scholar] [CrossRef]
| Group | Baseline | ARDS | 4 h | 8 h | 12 h | 16 h | 20 h | 24 h | |
|---|---|---|---|---|---|---|---|---|---|
| HR aa, bbb (min−1) | ARDSnet OLC EIT | 103 ± 23 | 80 ± 12 | 105 ± 15 | 104 ± 13 | 106 ± 9 | 108 ± 16 | 105 ± 20 | 114 ± 12 |
| 93 ± 13 | 113 ± 16 | 115 ± 13 | 109 ± 11 | 110 ± 17 | 117 ± 10 | 116 ± 18 | |||
| 84 ± 15 | 103 ± 23 | 110 ± 16 | 114 ± 15 | 116 ± 25 | 115 ± 15 | 114 ± 23 | |||
| MAP bbb (mmHg) | ARDSnet OLC EIT | 87 ± 20 | 82 ± 12 | 89 ± 13 | 83 ± 11 | 81 ± 13 | 79 ± 13 | 75 ± 9 | 70 ± 8 |
| 94 ± 10 | 76 ± 14 | 75 ± 8 | 78 ± 7 | 73 ± 11 | 76 ± 12 | 71 ± 10 | |||
| 91 ± 17 | 80 ± 15 | 75 ± 12 | 71 ± 5 | 72 ± 11 | 70 ± 5 | 63 ± 11 | |||
| CO (L·min−1) | ARDSnet OLC EIT | 5.6 ± 1.6 | 5.0 ± 1.6 | 5.8 ± 2.6 | 5.2 ± 1.7 | 5.3 ± 0.9 | 5.4 ± 0.9 | 5.4 ± 1.1 | 6.2 ± 0.8 |
| 5.6 ± 1.3 | 4.3 ± 1.0 | 4.2 ± 1.1 | 4.3 ± 0.8 | 4.6 ± 0.8 | 4.8 ± 0.6 | 4.7 ± 0.9 | |||
| 4.8 ± 1.3 | 4.0 ± 1.2 | 4.8 ± 1.1 | 4.6 ± 1.5 | 4.6 ± 1.4 | 4.7 ± 1.2 | 5.0 ± 1.2 | |||
| SVR a, bbb (dyn·s·cm−5) | ARDSnet OLC EIT | 1186 ± 430 | 1660 ± 954 | 1334 ± 524 | 1370 ± 539 | 1151 ± 314 | 1078 ± 162 | 1041 ± 201 | 888 ± 212 |
| 1331 ± 338 | 1510 ± 478 | 1353 ± 499 | 1246 ± 289 | 1185 ± 331 | 1145 ± 222 | 1047 ± 154 | |||
| 1409 ± 227 | 1604 ± 1046 | 1206 ± 214 | 1201 ± 475 | 1156 ± 491 | 1123 ± 496 | 1069 ± 450 | |||
| PVR aaa, bb (dyn·s·cm−5) | ARDSnet OLC EIT | 167 ± 73 | 391 ± 173 | 394 ± 157 | 412 ± 115 | 350 ± 112 | 324 ± 120 | 249 ± 90 | 268 ± 55 |
| 234 ± 69 | 323 ± 156 | 260 ± 140 | 274 ± 133 | 270 ± 90 | 250 ± 92 | 245 ± 76 | |||
| 333 ± 128 | 431 ± 252 | 352 ± 129 | 291 ± 131 | 219 ± 77 | 240 ± 81 | 235 ± 112 | |||
| PEEP bbb, ccc, ddd (cmH20) | ARDSnet eee, fff OLC EIT | 5.0 ± 0 | 5.0 ± 0 | 7.9 ± 3.1 | 7.1 ± 1.9 | 6.1 ± 1.6 | 6.4 ± 2 | 6.9 ± 1.6 | 6.5 ± 1.6 |
| 5.0 ± 0 | 18 ± 3.5 | 18.0 ± 3.5 | 19.0 ± 3.2 | 19.0 ± 3.2 | 19.0 ± 3.2 | 19.0 ± 3.2 | |||
| 5.0 ± 0 | 19.5 ± 2.1 | 20.0 ± 3.2 | 21.8 ± 3.3 | 21.5 ± 2.6 | 22.0 ± 3.0 | 21.8 ± 3.5 | |||
| Driving aaa, bbb, pressure ccc, ddd (cmH20) | ARDSnet eee, fff OLC EIT | 10.7 ± 2.1 | 21.2 ± 2.8 | 19.1 ± 3.9 | 19.1 ± 2.6 | 18.1 ± 3.0 | 18.9 ± 4.8 | 18.1 ± 4.3 | 18.4 ± 3.4 |
| 20.3 ± 2.2 | 12.6 ± 2.3 | 12.7 ± 2.4 | 11.6 ± 2.2 | 11.2 ± 1.8 | 11.5 ± 1.6 | 11.5 ± 1.6 | |||
| 23.3 ± 1.6 | 12.6 ± 3.5 | 11.5 ± 3.0 | 12.6 ± 3.3 | 12.3 ± 2.1 | 12.0 ± 2.2 | 12.3 ± 3.0 | |||
| Paw-plat aaa, bb, ccc, dd (cmH20) | ARDSnet fff OLC EIT | 16.5 ± 2.1 | 28.1 ± 3.6 | 28.1 ± 4.0 | 27.0 ± 3.4 | 26.3 ± 3.5 | 26.1 ± 3.2 | 27.8 ± 3.8 | 28.0 ± 3.2 |
| 27.4 ± 3.3 | 32.4 ± 4.6 | 32.7 ± 3.8 | 32.0 ± 3.9 | 31.6 ± 4.0 | 31.1 ± 3.6 | 31.9 ± 3.7 | |||
| 30.8 ± 3.3 | 33.6 ± 3.5 | 34.8 ± 4.3 | 36.7 ± 3.7 | 36.0 ± 4.0 | 36.1 ± 4.2 | 36.4 ± 5.1 | |||
| Paw-peak aaa, ccc (cmH20) | ARDSnet fff, g OLC EIT | 21.1 ± 3.5 | 33.4 ± 3.9 | 37.5 ± 4.3 | 37.4 ± 3.5 | 36.6 ± 2.9 | 36.1 ± 2.9 | 40.5 ± 14.2 | 38.4 ± 5.9 |
| 33.4 ± 3.9 | 37.5 ± 4.3 | 37.4 ± 3.5 | 36.6 ± 3.0 | 36.1 ± 2.9 | 41.5 ± 14.2 | 38.4 ± 5.9 | |||
| 37.8 ± 8.3 | 40.3 ± 4.7 | 41.8 ± 4.8 | 43.0 ± 4.1 | 42.5 ± 4.3 | 42.8 ± 4.7 | 44.1 ± 6.9 | |||
| PaO2) aaa, bbb, ccc, ddd (mmHg, 100% 02) | ARDSnet eee, fff OLC EIT | 475 ± 70 | 129 ± 64 | 226 ± 100 | 236 ± 112 | 192 ± 98 | 155 ± 64 | 134 ± 58 | 184 ± 95 |
| 66 ± 18 | 442 ± 64 | 454 ± 79 | 477 ± 40 | 471 ± 38 | 444 ± 42 | 446 ± 31 | |||
| 77 ± 32 | 507 ± 56 | 502 ± 51 | 500 ± 40 | 466 ± 49 | 430 ± 36 | 422 ± 74 | |||
| PaCO2 aaa (mmHg) | ARDSnet OLC EIT | 51.4 ± 9.7 | 58.3 ± 15.0 | 67.3 ± 21.4 | 62.4 ± 10.0 | 58.5 ± 8.5 | 59.9 ± 9.5 | 59.0 ± 8.6 | 62.1 ± 12.1 |
| 60.2 ± 11.9 | 59.1 ± 9.6 | 54.7 ± 6.3 | 57.1 ± 7.2 | 53.7 ± 6.3 | 55.9 ± 8.7 | 56.4 ± 7.7 | |||
| 58.5 ± 7.9 | 69.7 ± 10.4 | 67.8 ± 10.2 | 68.4 ± 12.4 | 68.2 ± 16.5 | 65.1 ± 10.4 | 67.3 ± 11 | |||
| Compliance aaa, bbb, (mL·cmH2O−1) ccc, dd | ARDSnet eee, ff OLC EIT | 26.5 ± 4.7 | 12.4 ± 2.1 | 11.6 ± 2.1 | 11.4 ± 2.4 | 12.2 ± 2.9 | 12.0 ± 3.6 | 11.93.2 | 11.4 ± 2.3 |
| 13.6 ± 2. | 19.8 ± 4.1 | 19.7 ± 4.5 | 21.5 ± 7.1 | 21.9 ± 6.7 | 21.9 ± 6.1 | 21.9 ± 5.4 | |||
| 14.5 ± 4. | 18.7 ± 3.8 | 19.9 ± 4.2 | 18.9 ± 4.0 | 18.8 ± 2.7 | 19.8 ± 3.9 | 18.9 ± 4.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haase, J.; Buchloh, D.C.; Hammermüller, S.; Salz, P.; Mrongowius, J.; Carvalho, N.C.; Beda, A.; Rau, A.; Starke, H.; Spieth, P.M.; et al. Mechanical Ventilation Strategies Targeting Different Magnitudes of Collapse and Tidal Recruitment in Porcine Acid Aspiration-Induced Lung Injury. J. Clin. Med. 2019, 8, 1250. https://doi.org/10.3390/jcm8081250
Haase J, Buchloh DC, Hammermüller S, Salz P, Mrongowius J, Carvalho NC, Beda A, Rau A, Starke H, Spieth PM, et al. Mechanical Ventilation Strategies Targeting Different Magnitudes of Collapse and Tidal Recruitment in Porcine Acid Aspiration-Induced Lung Injury. Journal of Clinical Medicine. 2019; 8(8):1250. https://doi.org/10.3390/jcm8081250
Chicago/Turabian StyleHaase, Juliane, Dorina C. Buchloh, Sören Hammermüller, Peter Salz, Julia Mrongowius, Nadja C. Carvalho, Alessandro Beda, Anna Rau, Henning Starke, Peter M. Spieth, and et al. 2019. "Mechanical Ventilation Strategies Targeting Different Magnitudes of Collapse and Tidal Recruitment in Porcine Acid Aspiration-Induced Lung Injury" Journal of Clinical Medicine 8, no. 8: 1250. https://doi.org/10.3390/jcm8081250
APA StyleHaase, J., Buchloh, D. C., Hammermüller, S., Salz, P., Mrongowius, J., Carvalho, N. C., Beda, A., Rau, A., Starke, H., Spieth, P. M., Gittel, C., Muders, T., Wrigge, H., & Reske, A. W. (2019). Mechanical Ventilation Strategies Targeting Different Magnitudes of Collapse and Tidal Recruitment in Porcine Acid Aspiration-Induced Lung Injury. Journal of Clinical Medicine, 8(8), 1250. https://doi.org/10.3390/jcm8081250





