Hypoxia Rapidly Induces the Expression of Cardiomyogenic Factors in Human Adipose-Derived Adherent Stromal Cells
Abstract
:1. Introduction
2. Experimental Section
2.1. Adipose Tissue Harvesting, Isolation, and Culture of ADASs and ASCs
2.2. Preparation of Normoxic and Hypoxic Conditioned Cells
2.3. Flow Cytometry
2.4. RNA Isolation, Reverse Transcription (RT)-PCR and Quantitive PCR (qPCR) Analysis
2.5. Immunoblot Analysis
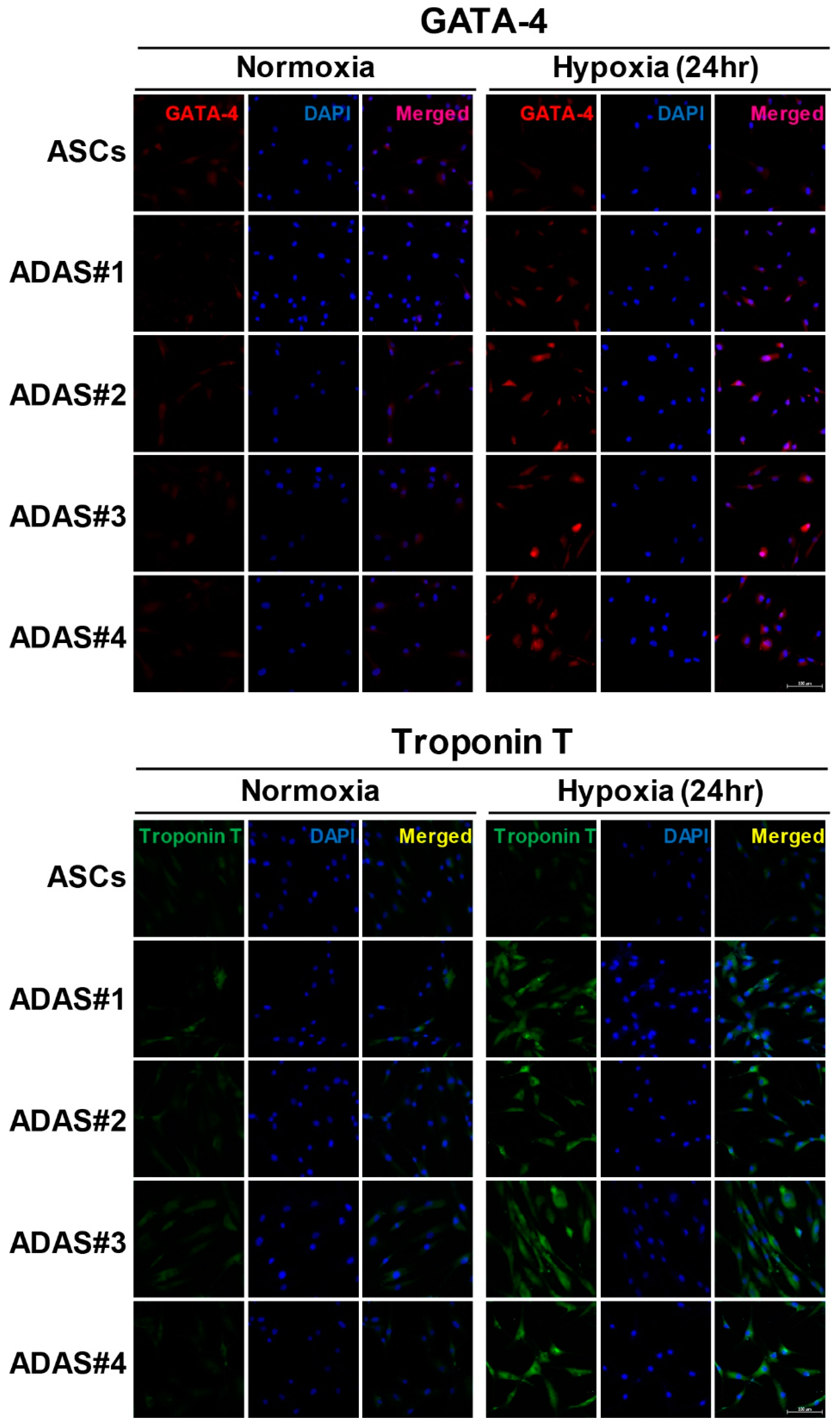
2.6. Immunocytochemistry
2.7. Antibody Array
2.8. Statistical Analysis
3. Results
3.1. Characterization of ADASs
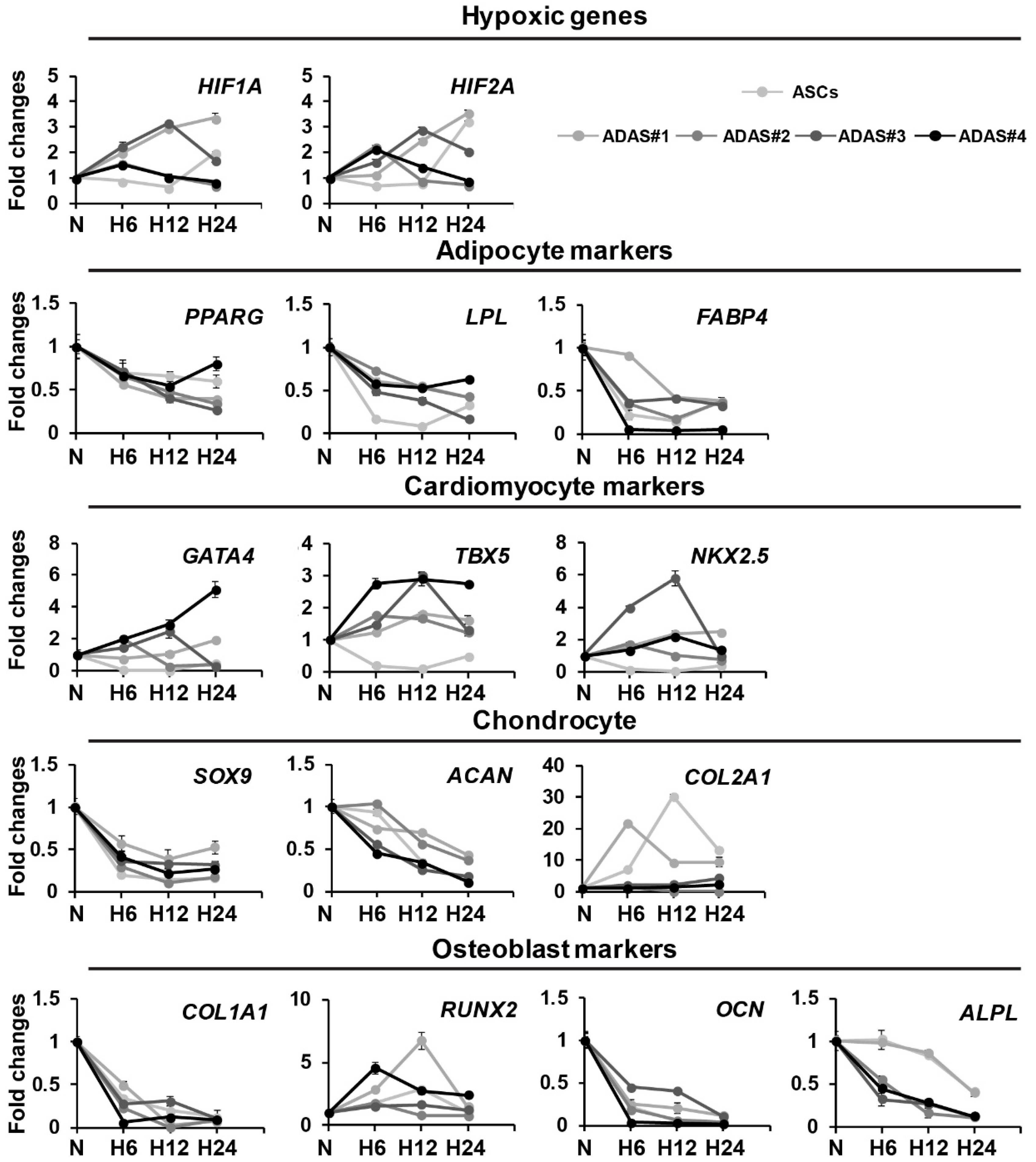
3.2. Time-Dependent Differential Regulation of Differentiation-Related Genes of Cell Lineages in ADASs under Hypoxic Conditions
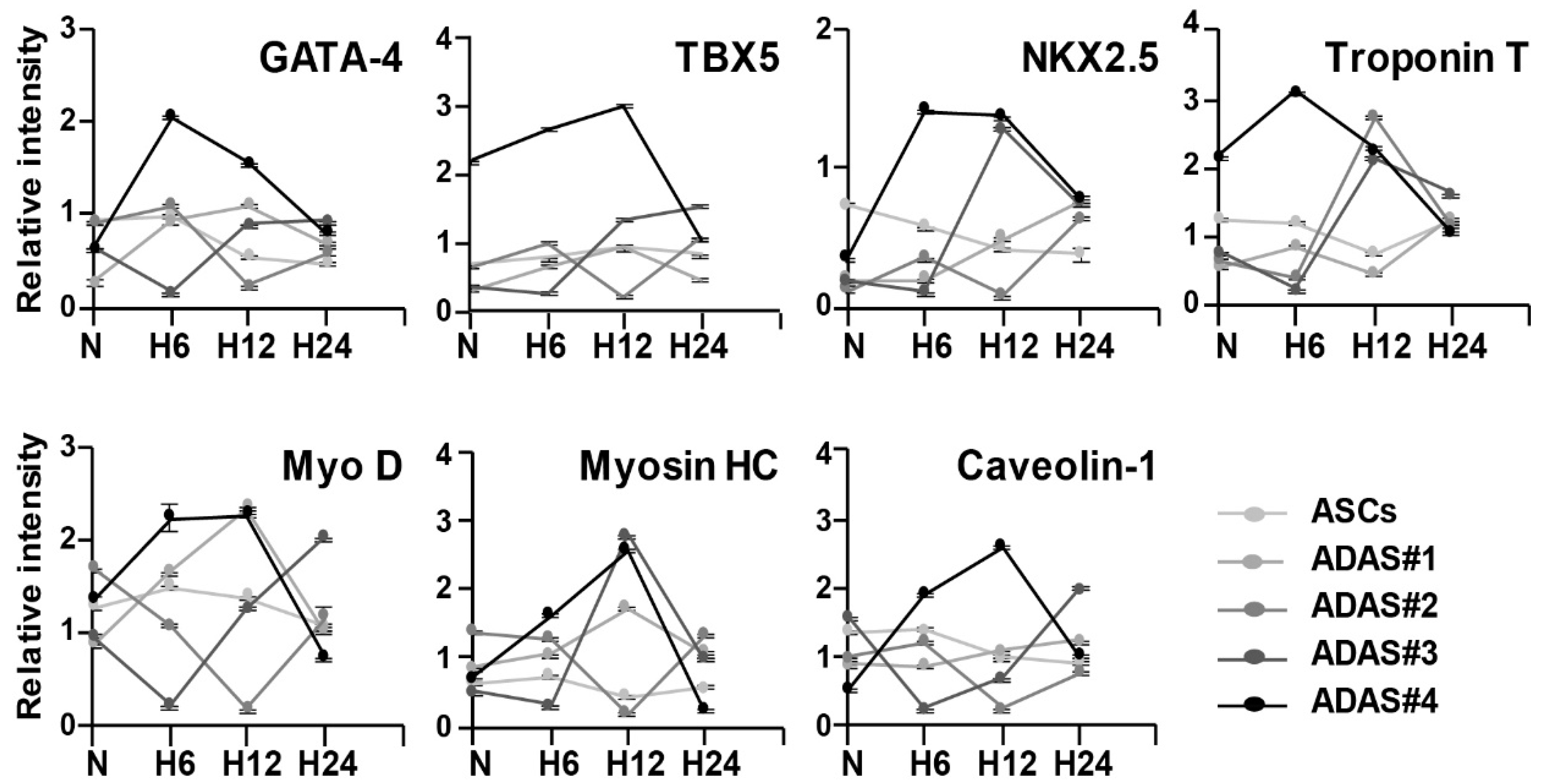
3.3. Time-Dependent Differential Regulation of Cardiomyogenic Proteins in ADASs under Hypoxic Conditions
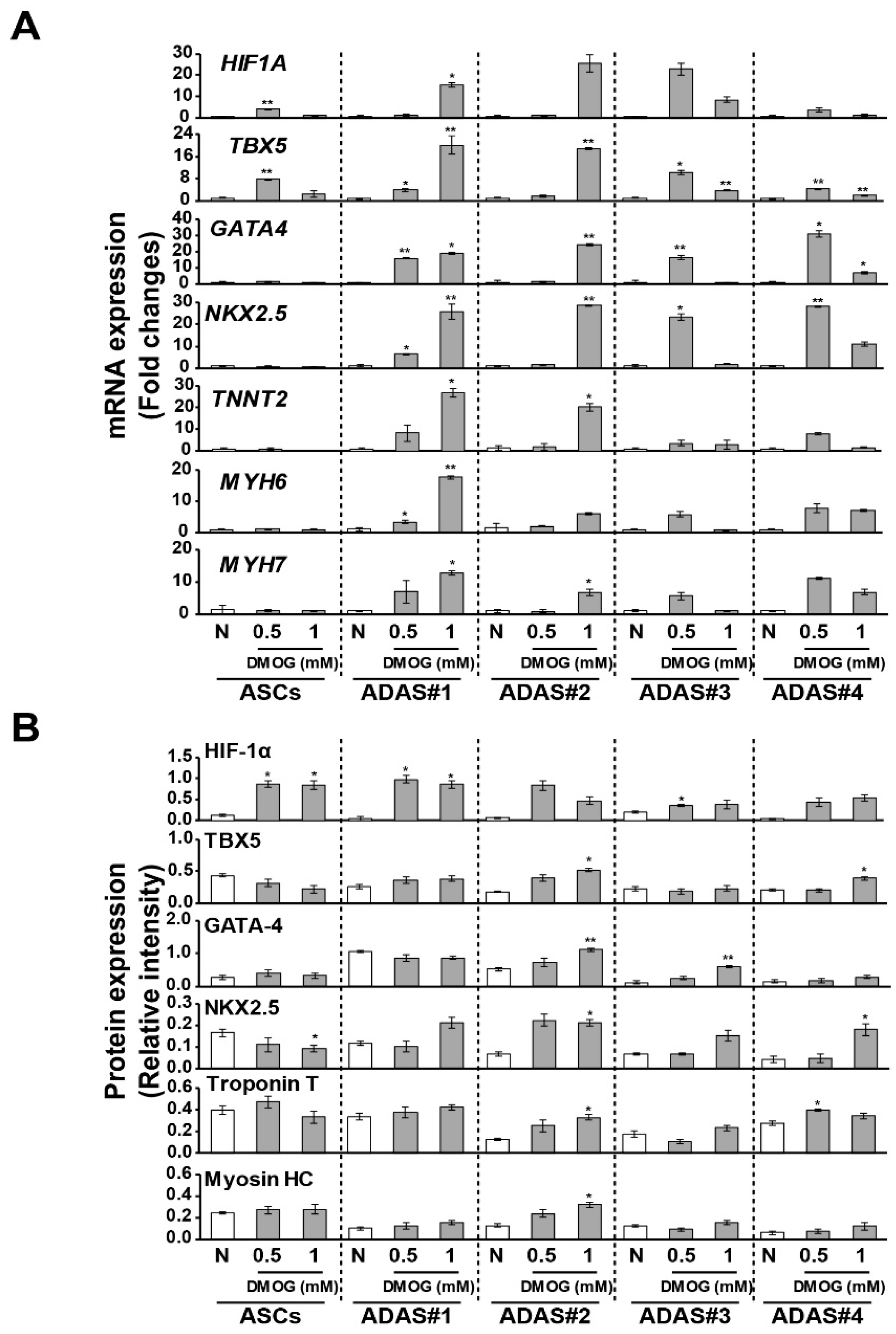
3.4. Changes in Cardiomyogenic Markers in Dimethyloxaloylglycine -Treated ADASs
3.5. Secretome Analysis of ASCs and ADASs under Hypoxic Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Han, S.; Sun, H.M.; Hwang, K.C.; Kim, S.W. Adipose-Derived Stromal Vascular Fraction Cells: Update on Clinical Utility and Efficacy. Crit. Rev. Eukaryot. Gene Expr. 2015, 25, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, E.; Fujita, D.; Takahashi, M.; Oba, S.; Nishimatsu, H. Adipose tissue-derived stem cells as a therapeutic tool for cardiovascular disease. World J. Cardiol. 2015, 7, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Bora, P.; Majumdar, A.S. Adipose tissue-derived stromal vascular fraction in regenerative medicine: A brief review on biology and translation. Stem Cell Res. Ther. 2017, 8, 145. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.; Guo, J.; Banyard, D.A.; Fadavi, D.; Toranto, J.D.; Wirth, G.A.; Paydar, K.Z.; Evans, G.R.; Widgerow, A.D. Stromal vascular fraction: A regenerative reality? Part 1: Current concepts and review of the literature. J. Plast. Reconstr. Aesthet. Surg. 2016, 69, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Nguyen, A.; Banyard, D.A.; Fadavi, D.; Toranto, J.D.; Wirth, G.A.; Paydar, K.Z.; Evans, G.R.; Widgerow, A.D. Stromal vascular fraction: A regenerative reality? Part 2: Mechanisms of regenerative action. J. Plast. Reconstr. Aesthet. Surg. 2016, 69, 180–188. [Google Scholar] [CrossRef]
- Riordan, N.H.; Ichim, T.E.; Min, W.P.; Wang, H.; Solano, F.; Lara, F.; Alfaro, M.; Rodriguez, J.P.; Harman, R.J.; Patel, A.N.; et al. Non-expanded adipose stromal vascular fraction cell therapy for multiple sclerosis. J. Transl. Med. 2009, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, C.S.; Farooqi, N.; O’Brien, K.; Gran, B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br. J. Pharmacol. 2011, 164, 1079–1106. [Google Scholar] [CrossRef]
- Rajashekhar, G.; Ramadan, A.; Abburi, C.; Callaghan, B.; Traktuev, D.O.; Evans-Molina, C.; Maturi, R.; Harris, A.; Kern, T.S.; March, K.L. Regenerative therapeutic potential of adipose stromal cells in early stage diabetic retinopathy. PLoS ONE 2014, 9, e84671. [Google Scholar] [CrossRef]
- Atalay, S.; Coruh, A.; Deniz, K. Stromal vascular fraction improves deep partial thickness burn wound healing. Burns 2014, 40, 1375–1383. [Google Scholar] [CrossRef]
- Gimble, J.M.; Bunnell, B.A.; Chiu, E.S.; Guilak, F. Concise review: Adipose-derived stromal vascular fraction cells and stem cells: Let’s not get lost in translation. Stem Cells 2011, 29, 749–754. [Google Scholar] [CrossRef]
- Chung, M.T.; Zimmermann, A.S.; Paik, K.J.; Morrison, S.D.; Hyun, J.S.; Lo, D.D.; McArdle, A.; Montoro, D.T.; Walmsley, G.G.; Senarath-Yapa, K.; et al. Isolation of human adipose-derived stromal cells using laser-assisted liposuction and their therapeutic potential in regenerative medicine. Stem Cells Transl. Med. 2013, 2, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Arana, M.; Gavira, J.J.; Pena, E.; Gonzalez, A.; Abizanda, G.; Cilla, M.; Perez, M.M.; Albiasu, E.; Aguado, N.; Casado, M.; et al. Epicardial delivery of collagen patches with adipose-derived stem cells in rat and minipig models of chronic myocardial infarction. Biomaterials 2014, 35, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Khalpey, Z.; Janardhanan, R.; Konhilas, J.; Hemphill, C. First in man: Adipose-derived stromal vascular fraction cells may promote restorative cardiac function. Am. J. Med. 2014, 127, e11–e12. [Google Scholar] [CrossRef] [PubMed]
- Valina, C.; Pinkernell, K.; Song, Y.H.; Bai, X.; Sadat, S.; Campeau, R.J.; Le Jemtel, T.H.; Alt, E. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur. Heart J. 2007, 28, 2667–2677. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.R.; Trachtenberg, B.; Velazquez, D.L.; McNiece, I.; Altman, P.; Rouy, D.; Mendizabal, A.M.; Pattany, P.M.; Lopera, G.A.; Fishman, J.; et al. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: Functional recovery and reverse remodeling. Circ. Res. 2011, 108, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, H.; Boitard, S.E.; Planat-Benard, V.; Pouly, J.; Neamatalla, H.; Joanne, P.; Perier, M.C.; Bellamy, V.; Casteilla, L.; Li, Z.; et al. Efficacy of epicardially delivered adipose stroma cell sheets in dilated cardiomyopathy. Cardiovasc. Res. 2013, 99, 640–647. [Google Scholar] [CrossRef] [Green Version]
- Moon, M.H.; Kim, S.Y.; Kim, Y.J.; Kim, S.J.; Lee, J.B.; Bae, Y.C.; Sung, S.M.; Jung, J.S. Human adipose tissue-derived mesenchymal stem cells improve postnatal neovascularization in a mouse model of hindlimb ischemia. Cell Physiol. Biochem. 2006, 17, 279–290. [Google Scholar] [CrossRef]
- Gutierrez-Fernandez, M.; Rodriguez-Frutos, B.; Ramos-Cejudo, J.; Teresa Vallejo-Cremades, M.; Fuentes, B.; Cerdan, S.; Diez-Tejedor, E. Effects of intravenous administration of allogenic bone marrow- and adipose tissue-derived mesenchymal stem cells on functional recovery and brain repair markers in experimental ischemic stroke. Stem Cell Res. Ther. 2013, 4, 11. [Google Scholar] [CrossRef]
- Choi, J.W.; Kim, K.E.; Lee, C.Y.; Lee, J.; Seo, H.H.; Lim, K.H.; Choi, E.; Lim, S.; Lee, S.; Kim, S.W.; et al. Alterations in Cardiomyocyte Differentiation-Related Proteins in Rat Mesenchymal Stem Cells Exposed to Hypoxia. Cell Physiol. Biochem. 2016, 39, 1595–1607. [Google Scholar] [CrossRef]
- Choi, J.W.; Shin, S.; Lee, C.Y.; Lee, J.; Seo, H.H.; Lim, S.; Lee, S.; Kim, I.K.; Lee, H.B.; Kim, S.W.; et al. Rapid Induction of Osteogenic Markers in Mesenchymal Stem Cells by Adipose-Derived Stromal Vascular Fraction Cells. Cell Physiol. Biochem. 2017, 44, 53–65. [Google Scholar] [CrossRef]
- Lim, S.; Kim, I.K.; Choi, J.W.; Seo, H.H.; Lim, K.H.; Lee, S.; Lee, H.B.; Kim, S.W.; Hwang, K.C. Gender-dimorphic effects of adipose-derived stromal vascular fractions on HUVECs exposed to oxidative stress. Int. J. Med. Sci. 2017, 14, 911–919. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.; Choi, J.W.; Lim, S.; Lee, S.; Jun, E.Y.; Sun, H.M.; Kim, I.K.; Lee, H.B.; Kim, S.W.; Hwang, K.C. Anti-apoptotic effects of adipose-derived adherent stromal cells in mesenchymal stem cells exposed to oxidative stress. Cell Biochem. Funct. 2018, 36, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.R.; Hare, J.M. Mesenchymal stem cells: Biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ. Res. 2011, 109, 923–940. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Deng, J.; Tian, W.; Xiang, B.; Yang, T.; Li, G.; Wang, J.; Gruwel, M.; Kashour, T.; Rendell, J.; et al. Adipose-derived stem cells are an effective cell candidate for treatment of heart failure: An MR imaging study of rat hearts. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1020–H1031. [Google Scholar] [CrossRef] [PubMed]
- Jurgens, W.J.; Kroeze, R.J.; Zandieh-Doulabi, B.; van Dijk, A.; Renders, G.A.; Smit, T.H.; van Milligen, F.J.; Ritt, M.J.; Helder, M.N. One-step surgical procedure for the treatment of osteochondral defects with adipose-derived stem cells in a caprine knee defect: A pilot study. BioRes. Open Access 2013, 2, 315–325. [Google Scholar] [CrossRef]
- van Dijk, A.; Naaijkens, B.A.; Jurgens, W.J.; Nalliah, K.; Sairras, S.; van der Pijl, R.J.; Vo, K.; Vonk, A.B.; van Rossum, A.C.; Paulus, W.J.; et al. Reduction of infarct size by intravenous injection of uncultured adipose derived stromal cells in a rat model is dependent on the time point of application. Stem Cell Res. 2011, 7, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R.; Pingguan-Murphy, B.; Wan Abas, W.A.; Noor Azmi, M.A.; Omar, S.Z.; Chua, K.H.; Wan Safwani, W.K. Impact of low oxygen tension on stemness, proliferation and differentiation potential of human adipose-derived stem cells. Biochem. Biophys. Res. Commun. 2014, 448, 218–224. [Google Scholar] [CrossRef]
- Baksh, D.; Song, L.; Tuan, R.S. Adult mesenchymal stem cells: Characterization, differentiation, and application in cell and gene therapy. J. Cell. Mol. Med. 2004, 8, 301–316. [Google Scholar] [CrossRef]
- Wan Safwani, W.K.; Wong, C.W.; Yong, K.W.; Choi, J.R.; Mat Adenan, N.A.; Omar, S.Z.; Wan Abas, W.A.; Pingguan-Murphy, B. The effects of hypoxia and serum-free conditions on the stemness properties of human adipose-derived stem cells. Cytotechnology 2016, 68, 1859–1872. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Zachar, V.; Pennisi, C.P.; Fink, T.; Maeda, Y.; Emmersen, J. Hypoxia Enhances Differentiation of Adipose Tissue-Derived Stem Cells toward the Smooth Muscle Phenotype. Int. J. Mol. Sci. 2018, 19, 517. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, S.H.; Song, S.Y.; Kim, W.S.; Song, S.U.; Yi, T.; Jeon, M.S.; Chung, H.M.; Xia, Y.; Sung, J.H. Hypoxia induces adipocyte differentiation of adipose-derived stem cells by triggering reactive oxygen species generation. Cell Biol. Int. 2014, 38, 32–40. [Google Scholar] [CrossRef]
- Shang, T.; Li, S.; Zhang, Y.; Lu, L.; Cui, L.; Guo, F.F. Hypoxia promotes differentiation of adipose-derived stem cells into endothelial cells through demethylation of ephrinB2. Stem Cell Res. Ther. 2019, 10, 133. [Google Scholar] [CrossRef]
- Merceron, C.; Vinatier, C.; Portron, S.; Masson, M.; Amiaud, J.; Guigand, L.; Cherel, Y.; Weiss, P.; Guicheux, J. Differential effects of hypoxia on osteochondrogenic potential of human adipose-derived stem cells. Am. J. Physiol. Cell Physiol. 2010, 298, C355–C364. [Google Scholar] [CrossRef]
- Xie, X.J.; Wang, J.A.; Cao, J.; Zhang, X. Differential effects of hypoxia on osteochondrogenic potential of human adipose-derived stem cells. Acta Pharmacol. Sinica 2006, 27, 1153–1158. [Google Scholar] [CrossRef]
- Wang, J.A.; Chen, T.L.; Jiang, J.; Shi, H.; Gui, C.; Luo, R.H.; Xie, X.J.; Xiang, M.X.; Zhang, X. Hypoxic preconditioning attenuates hypoxia/reoxygenation-induced apoptosis in mesenchymal stem cells. Acta Pharmacol. Sinica 2008, 29, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Chacko, S.M.; Ahmed, S.; Selvendiran, K.; Kuppusamy, M.L.; Khan, M.; Kuppusamy, P. Hypoxic preconditioning induces the expression of prosurvival and proangiogenic markers in mesenchymal stem cells. Am. J. Physiol. Cell Physiol. 2010, 299, C1562–C1570. [Google Scholar] [CrossRef]
- Beegle, J.; Lakatos, K.; Kalomoiris, S.; Stewart, H.; Isseroff, R.R.; Nolta, J.A.; Fierro, F.A. Hypoxic preconditioning of mesenchymal stromal cells induces metabolic changes, enhances survival, and promotes cell retention in vivo. Stem Cells 2015, 33, 1818–1828. [Google Scholar] [CrossRef]
- Grayson, W.L.; Zhao, F.; Bunnell, B.; Ma, T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2007, 358, 948–953. [Google Scholar] [CrossRef]
- Wang, J.; Xiang, B.; Deng, J.X.; Lin, H.Y.; Freed, D.H.; Arora, R.C.; Tian, G.H. Hypoxia enhances the therapeutic potential of superparamagnetic iron oxide-labeled adipose-derived stem cells for myocardial infarction. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2017, 37, 516–522. [Google Scholar] [CrossRef]
- Rasmussen, J.G.; Frobert, O.; Pilgaard, L.; Kastrup, J.; Simonsen, U.; Zachar, V.; Fink, T. Prolonged hypoxic culture and trypsinization increase the pro-angiogenic potential of human adipose tissue-derived stem cells. Cytotherapy 2011, 13, 318–328. [Google Scholar] [CrossRef]
- Bunnell, B.A.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Adipose-derived stem cells: Isolation, expansion and differentiation. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, J.; Ahuja, P.; Bodenmann, S.; Knapik, D.; Perriard, E.; Krek, W.; Perriard, J.C. Essential role of developmentally activated hypoxia-inducible factor 1alpha for cardiac morphogenesis and function. Circ. Res. 2008, 103, 1139–1146. [Google Scholar] [CrossRef]
- Tello-Montoliu, A.; Patel, J.V.; Lip, G.Y. Angiogenin: A review of the pathophysiology and potential clinical applications. J. Thromb. Haemost. 2006, 4, 1864–1874. [Google Scholar] [CrossRef]
- Ascione, R.; Rowlinson, J.; Avolio, E.; Katare, R.; Meloni, M.; Spencer, H.L.; Mangialardi, G.; Norris, C.; Krankel, N.; Spinetti, G.; et al. Migration towards SDF-1 selects angiogenin-expressing bone marrow monocytes endowed with cardiac reparative activity in patients with previous myocardial infarction. Stem Cell Res.Ther. 2015, 6, 53. [Google Scholar] [CrossRef]
- Huang, S.D.; Lu, F.L.; Xu, X.Y.; Liu, X.H.; Zhao, X.X.; Zhao, B.Z.; Wang, L.; Gong, D.J.; Yuan, Y.; Xu, Z.Y. Transplantation of angiogenin-overexpressing mesenchymal stem cells synergistically augments cardiac function in a porcine model of chronic ischemia. J. Thorac. Cardiovasc. Surg. 2006, 132, 1329–1338. [Google Scholar] [CrossRef] [Green Version]
- Kurosu, H.; Kuro, O.M. Endocrine fibroblast growth factors as regulators of metabolic homeostasis. Biofactors 2009, 35, 52–60. [Google Scholar] [CrossRef]
- Beenken, A.; Mohammadi, M. The FGF family: Biology, pathophysiology and therapy. Nat. Rev. Drug Discov. 2009, 8, 235–253. [Google Scholar] [CrossRef]
- Itoh, N.; Ohta, H.; Nakayama, Y.; Konishi, M. Roles of FGF Signals in Heart Development, Health, and Disease. Front. Cell Dev. Biol. 2016, 4, 110. [Google Scholar] [CrossRef] [Green Version]
- Hao, Y.; Zhou, J.; Zhou, M.; Ma, X.; Lu, Z.; Gao, M.; Pan, X.; Tang, J.; Bao, Y.; Jia, W. Serum levels of fibroblast growth factor 19 are inversely associated with coronary artery disease in chinese individuals. PLoS ONE 2013, 8, e72345. [Google Scholar] [CrossRef]
- Burger-Kentischer, A.; Goebel, H.; Seiler, R.; Fraedrich, G.; Schaefer, H.E.; Dimmeler, S.; Kleemann, R.; Bernhagen, J.; Ihling, C. Expression of macrophage migration inhibitory factor in different stages of human atherosclerosis. Circulation 2002, 105, 1561–1566. [Google Scholar] [CrossRef]
- Calandra, T.; Echtenacher, B.; Roy, D.L.; Pugin, J.; Metz, C.N.; Hultner, L.; Heumann, D.; Mannel, D.; Bucala, R.; Glauser, M.P. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat. Med. 2000, 6, 164–170. [Google Scholar] [CrossRef]
- Miller, E.J.; Li, J.; Leng, L.; McDonald, C.; Atsumi, T.; Bucala, R.; Young, L.H. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature 2008, 451, 578–582. [Google Scholar] [CrossRef]
- Qi, D.; Hu, X.; Wu, X.; Merk, M.; Leng, L.; Bucala, R.; Young, L.H. Cardiac macrophage migration inhibitory factor inhibits JNK pathway activation and injury during ischemia/reperfusion. J. Clin. Investig. 2009, 119, 3807–3816. [Google Scholar] [CrossRef] [Green Version]


| Genes | Primer Sequence (5′–3′) | |
|---|---|---|
| HIF1A | F 1 | ACTTGGCAACCTTGGATTGG |
| R 2 | GTGCTGAGTAACCACCACTTAT | |
| HIF2A | F | CCTGAGACTGTATGGTCAGCTC |
| R | AGGACGGAGAGAAGGGAACC | |
| PPARG | F | GCAAACCCCTATTCCATGCTG |
| R | ACGGAGCTGATCCCAAAGTT | |
| LPL | F | CGAGCGCTCCATTCATCTCT |
| R | CCAGATTGTTGCAGCGGTTC | |
| FABP4 | F | CCTTAGATGGGGGTGTCCTG |
| R | AACGTCCCTTGGCTTATGCT | |
| GATA4 | F | GCCTCTCGGTGTGACGAGT |
| R | TGGTTCCGGAAGCTGATGTAG | |
| TBX5 | F | AAGAGTTCCCTCCTCTCCCC |
| R | GTCTTGGCCCCGGGAATAAA | |
| NKX2.5 | F | CAACATGACCCTGAGTCCCC |
| R | TAATCGCCGCCACAAACTCT | |
| SOX9 | F | AACAACCCGTCTACACACAGCTCA |
| R | TGGGTAATGCGCTTGGATAGGTCA | |
| ACAN | F | GTGGTGATGATCTGGCACGAG |
| R | CGTTTGTAGGTGGTGGCTGTG | |
| COL2A1 | F | TGGTCTTGGTGGAAACTTTGCTGC |
| R | AGGTTCACCAGGTTCACCAGGATT | |
| COL1A1 | F | CCGGAAACAGACAAGCAACCCAAA |
| R | AAAGGAGCAGAAAGGGCAGCATTG | |
| RUNX2 | F | AAGGGTCCACTCTGGCTTTG |
| R | CTAGGCGCATTTCAGGTGCT | |
| OCN | F | TCACACTCCTCGCCCTATT |
| R | TGAAAGCCGATGTGGTCAG | |
| ALPL | F | GACCCTTGACCCCCACAAT |
| R | CGCCTCGTACTGCATGTCCCCT | |
| TNNT2 | F | GACAGAGCGGAAAAGTGGGA |
| R | CACAGCTCCTTGGCCTTCTC | |
| MYH6 | F | ACCAACCTGTCCAAGTTCCG |
| MYH7 | F | GACACACTTGAGTAGCCCAGG |
| R | CTTCTAGCCGCTCCTTCTCTG | |
| Internal control | ||
| GAPDH | F | CATGGGTGTGAACCATGAGA |
| R | GGTCATGAGTCCTTCCACGA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.-W.; Moon, H.; Jung, S.E.; Lim, S.; Lee, S.; Kim, I.-K.; Lee, H.-B.; Lee, J.; Song, B.-W.; Kim, S.W.; et al. Hypoxia Rapidly Induces the Expression of Cardiomyogenic Factors in Human Adipose-Derived Adherent Stromal Cells. J. Clin. Med. 2019, 8, 1231. https://doi.org/10.3390/jcm8081231
Choi J-W, Moon H, Jung SE, Lim S, Lee S, Kim I-K, Lee H-B, Lee J, Song B-W, Kim SW, et al. Hypoxia Rapidly Induces the Expression of Cardiomyogenic Factors in Human Adipose-Derived Adherent Stromal Cells. Journal of Clinical Medicine. 2019; 8(8):1231. https://doi.org/10.3390/jcm8081231
Chicago/Turabian StyleChoi, Jung-Won, Hanbyeol Moon, Seung Eun Jung, Soyeon Lim, Seahyoung Lee, Il-Kwon Kim, Hoon-Bum Lee, Jiyun Lee, Byeong-Wook Song, Sang Woo Kim, and et al. 2019. "Hypoxia Rapidly Induces the Expression of Cardiomyogenic Factors in Human Adipose-Derived Adherent Stromal Cells" Journal of Clinical Medicine 8, no. 8: 1231. https://doi.org/10.3390/jcm8081231
APA StyleChoi, J.-W., Moon, H., Jung, S. E., Lim, S., Lee, S., Kim, I.-K., Lee, H.-B., Lee, J., Song, B.-W., Kim, S. W., & Hwang, K.-C. (2019). Hypoxia Rapidly Induces the Expression of Cardiomyogenic Factors in Human Adipose-Derived Adherent Stromal Cells. Journal of Clinical Medicine, 8(8), 1231. https://doi.org/10.3390/jcm8081231






