The Effect of Dietary Supplementation of Crocetin for Myopia Control in Children: A Randomized Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods—Randomized Clinical Trial
2.1. Study Design
2.2. Study Organization
2.3. Participants
2.4. Randomization and Masking
2.5. Intervention
2.6. Procedure for Follow-Up Examinations
2.7. Outcomes
2.8. Choroidal Thickness Measurement
2.9. Statistical Analysis
3. Results—Randomized Clinical Trial
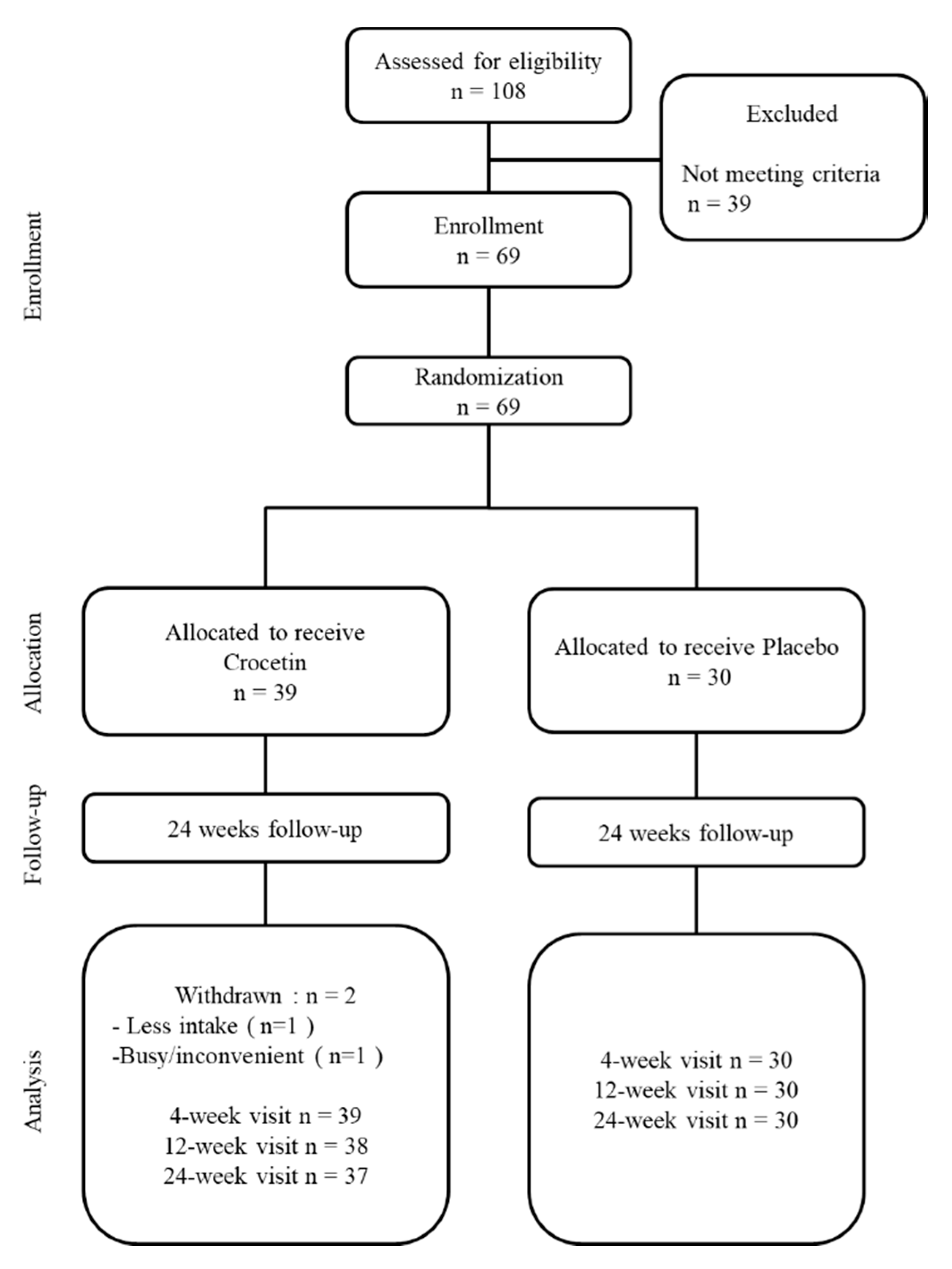
3.1. Flow of Participants
3.2. Participant Profiles
3.3. Adverse Events
3.4. Comparison of Myopia Progression after 24 Weeks
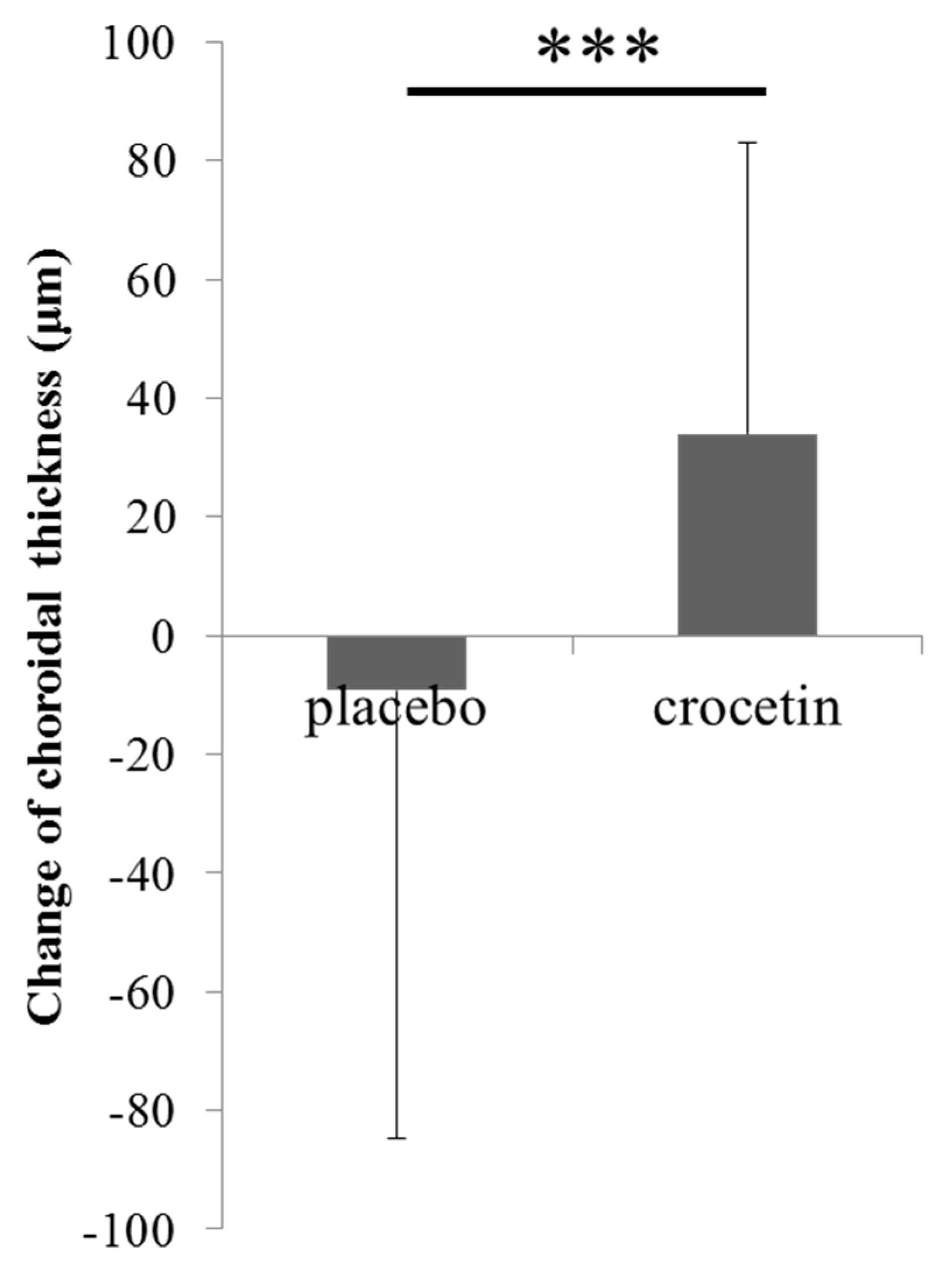
3.5. Change in Choroidal Thickness over 6 Months
4. Discussion
5. Conclusions
6. Patent
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Matsumura, H.; Hirai, H. Prevalence of myopia and refractive changes in students from 3 to 17 years of age. Surv. Ophthalmol. 1999, 44, S109–S115. [Google Scholar] [CrossRef]
- Sawada, A.; Tomidokoro, A.; Araie, M.; Iwase, A.; Yamamoto, T. Refractive errors in an elderly Japanese population: The Tajimi study. Ophthalmology 2008, 115, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.J.; Resnikoff, S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, T.S.; Frick, K.D.; Holden, B.A.; Fricke, T.R.; Naidoo, K.S. Potential lost productivity resulting from the global burden of uncorrected refractive error. Bull. World Health Organ. 2009, 87, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Gwiazda, J.; Hyman, L.; Dong, L.M.; Everett, D.; Norton, T.; Kurtz, D.; Manny, R.; Marsh-Tootle, W.; Scheiman, M. Factors associated with high myopia after 7 years of follow-up in the Correction of Myopia Evaluation Trial (COMET) Cohort. Ophthalmic Epidemiol. 2007, 14, 230–237. [Google Scholar] [CrossRef]
- Wu, P.C.; Chuang, M.N.; Choi, J.; Chen, H.; Wu, G.; Ohno-Matsui, K.; Jonas, J.B.; Cheung, C.M.G. Update in myopia and treatment strategy of atropine use in myopia control. Eye (Lond. Engl.) 2019, 33, 3–13. [Google Scholar] [CrossRef]
- Huang, J.; Wen, D.; Wang, Q.; McAlinden, C.; Flitcroft, I.; Chen, H.; Saw, S.M.; Chen, H.; Bao, F.; Zhao, Y.; et al. Efficacy Comparison of 16 Interventions for Myopia Control in Children: A Network Meta-analysis. Ophthalmology 2016, 123, 697–708. [Google Scholar] [CrossRef]
- Read, S.A.; Collins, M.J.; Vincent, S.J. Light exposure and physical activity in myopic and emmetropic children. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2014, 91, 330–341. [Google Scholar] [CrossRef]
- Rose, K.A.; Morgan, I.G.; Smith, W.; Burlutsky, G.; Mitchell, P.; Saw, S.M. Myopia, lifestyle and schooling in students of Chinese ethnicity in Singapore and Sydney. Arch. Ophthalmol. (Chic. Ill. 1960) 2008, 126, 52–530. [Google Scholar] [CrossRef]
- Wu, P.C.; Chen, C.T.; Lin, K.K.; Sun, C.C.; Kuo, C.N.; Huang, H.M.; Poon, Y.C.; Yang, M.L.; Chen, C.Y.; Huang, J.C.; et al. Myopia Prevention and Outdoor Light Intensity in a School-Based Cluster Randomized Trial. Ophthalmology 2018, 125, 1239–1250. [Google Scholar] [CrossRef] [Green Version]
- Torii, H.; Kurihara, T.; Seko, Y.; Negishi, K.; Ohnuma, K.; Inaba, T.; Kawashima, M.; Jiang, X.; Kondo, S.; Miyauchi, M.; et al. Violet Light Exposure Can Be a Preventive Strategy Against Myopia Progression. EBioMedicine 2017, 15, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Torii, H.; Ohnuma, K.; Kurihara, T.; Tsubota, K.; Negishi, K. Violet Light Transmission is Related to Myopia Progression in Adult High Myopia. Sci. Rep. 2017, 7, 14523. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Schaeffel, F.; Jiang, B.; Feldkaemper, M. Effects of Light of Different Spectral Composition on Refractive Development and Retinal Dopamine in Chicks. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4413–4424. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.J.; McGuire, J.J.; Schaeffel, F.; Stell, W.K. Light and focus-dependent expression of the transcription factor ZENK in the chick retina. Nat. Neurosci. 1999, 2, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.J.; Omar, G.; Walton, N.A.; Verrill, T.A.; Unson, C.G. Glucagon-expressing neurons within the retina regulate the proliferation of neural progenitors in the circumferential marginal zone of the avian eye. J. Neurosci. 2005, 25, 10157–10166. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Kurihara, T.; Miyauchi, M.; Ishida, A.; Jiang, X.; Ikeda, S.I.; Torii, H.; Tsubota, K. Oral crocetin administration suppressed refractive shift and axial elongation in a murine model of lens-induced myopia. Sci. Rep. 2019, 9, 295. [Google Scholar] [CrossRef] [PubMed]
- Umigai, N.; Murakami, K.; Ulit, M.V.; Antonio, L.S.; Shirotori, M.; Morikawa, H.; Nakano, T. The pharmacokinetic profile of crocetin in healthy adult human volunteers after a single oral administration. Phytomed. Int. J. Phytother. Phytopharm. 2011, 18, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Gutheil, W.G.; Reed, G.; Ray, A.; Anant, S.; Dhar, A. Crocetin: an agent derived from saffron for prevention and therapy for cancer. Curr. Pharm. Biotechnol. 2012, 13, 173–179. [Google Scholar] [CrossRef]
- Gao, L.; Zhu, B.Y. The Accumulation of Crocin and Geniposide and Transcripts of Phytoene Synthase during Maturation of Gardenia jasminoides Fruit. Evid. Based Complement. Alternat. Med. 2013, 2013, 686351. [Google Scholar] [CrossRef]
- Lee, I.A.; Lee, J.H.; Baek, N.I.; Kim, D.H. Antihyperlipidemic effect of crocin isolated from the fructus of Gardenia jasminoides and its metabolite Crocetin. Biol. Pharm. Bull. 2005, 28, 2106–2110. [Google Scholar] [CrossRef]
- Mizuma, H.; Tanaka, M.; Nozaki, S.; Mizuno, K.; Tahara, T.; Ataka, S.; Sugino, T.; Shirai, T.; Kajimoto, Y.; Kuratsune, H.; et al. Daily oral administration of crocetin attenuates physical fatigue in human subjects. Nutr. Res. (New York) 2009, 29, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Broadhead, G.K.; Chang, A.; Grigg, J.; McCluskey, P. Efficacy and Safety of Saffron Supplementation: Current Clinical Findings. Crit. Rev. Food Sci. Nutr. 2016, 56, 2767–2776. [Google Scholar] [CrossRef] [PubMed]
- Heitmar, R.; Brown, J.; Kyrou, I. Saffron (Crocus sativus L.) in Ocular Diseases: A Narrative Review of the Existing Evidence from Clinical Studies. Nutrients 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Tomioka, K.; Iwamoto, J.; Saeki, K.; Okamoto, N. Reliability and validity of the International Physical Activity Questionnaire (IPAQ) in elderly adults: The Fujiwara-kyo Study. J. Epidemiol. 2011, 21, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.M.; Chang, D.S.; Wu, P.C. The Association between Near Work Activities and Myopia in Children-A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0140419. [Google Scholar] [CrossRef] [PubMed]
- Ku, P.W.; Steptoe, A.; Lai, Y.J.; Hu, H.Y.; Chu, D.; Yen, Y.F.; Liao, Y.; Chen, L.J. The Associations between Near Visual Activity and Incident Myopia in Children: A Nationwide 4-Year Follow-up Study. Ophthalmology 2019, 126, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Sankaridurg, P.; Naduvilath, T.; Zang, J.; Zou, H.; Zhu, J.; Lv, M.; He, X.; Xu, X. Time spent in outdoor activities in relation to myopia prevention and control: A meta-analysis and systematic review. Acta Ophthalmol. 2017, 95, 551–566. [Google Scholar] [CrossRef]
- Vangeneugden, T.; Laenen, A.; Geys, H.; Renard, D.; Molenberghs, G. Applying linear mixed models to estimate reliability in clinical trial data with repeated measurements. Control. Clin. Trials 2004, 25, 13–30. [Google Scholar] [CrossRef]
- Ayaki, M.; Torii, H.; Tsubota, K.; Negishi, K. Decreased sleep quality in high myopia children. Sci. Rep. 2016, 6, 33902. [Google Scholar] [CrossRef]
- Jee, D.; Morgan, I.G.; Kim, E.C. Inverse relationship between sleep duration and myopia. Acta Ophthalmol. 2016, 94, e204–e210. [Google Scholar] [CrossRef]
- Foster, P.J.; Jiang, Y. Epidemiology of myopia. Eye (Lond. Engl.) 2014, 28, 202–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yam, J.C.; Jiang, Y.; Tang, S.M.; Law, A.K.P.; Chan, J.J.; Wong, E.; Ko, S.T.; Young, A.L.; Tham, C.C.; Chen, L.J.; et al. Low-Concentration Atropine for Myopia Progression (LAMP) Study: A Randomized, Double-Blinded, Placebo-Controlled Trial of 0.05%, 0.025%, and 0.01% Atropine Eye Drops in Myopia Control. Ophthalmology 2019, 126, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.K.; Yeoh, J.; Rahman, W.; Patel, P.J.; Tufail, A.; Da Cruz, L. Topographic variation and interocular symmetry of macular choroidal thickness using enhanced depth imaging optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2012, 53, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, A.; Shiragami, C.; Shirakata, Y.; Manabe, S.; Izumibata, S.; Shiraga, F. Enhanced depth imaging spectral-domain optical coherence tomography of subfoveal choroidal thickness in normal Japanese eyes. Jpn. J. Ophthalmol. 2012, 56, 23–235. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Imamura, Y.; Margolis, R.; Slakter, J.S.; Spaide, R.F. Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am. J. Ophthalmol. 2009, 148, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Ikuno, Y.; Tano, Y. Retinal and choroidal biometry in highly myopic eyes with spectral-domain optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3876–3880. [Google Scholar] [CrossRef] [PubMed]
- Giaccio, M. Crocetin from saffron: An active component of an ancient spice. Crit. Rev. Food Sci. Nutr. 2004, 44, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Holloway, G.M.; Gainer, J.L. The carotenoid crocetin enhances pulmonary oxygenation. J. Appl. Physiol. (Bethesda Md. 1985) 1988, 65, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Seyde, W.C.; McKernan, D.J.; Laudeman, T.; Gainer, J.L.; Longnecker, D.E. Carotenoid compound crocetin improves cerebral oxygenation in hemorrhaged rats. J. Cereb. Blood Flow Metab. 1986, 6, 703–707. [Google Scholar] [CrossRef]
- Zhou, C.H.; Xiang, M.; He, S.Y.; Qian, Z.Y. Protein kinase C pathway is involved in the inhibition by crocetin of vascular smooth muscle cells proliferation. Phytother. Res. 2010, 24, 1680–1686. [Google Scholar] [CrossRef]
- Cao, W.; Cui, J.; Li, S.; Zhang, D.; Guo, Y.; Li, Q.; Luan, Y.; Liu, X. Crocetin restores diabetic endothelial progenitor cell dysfunction by enhancing NO bioavailability via regulation of PI3K/AKT-eNOS and ROS pathways. Life Sci. 2017, 181, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Fotouhi, A.; Hashemi, H.; Khabazkhoob, M.; Mohammad, K. The prevalence of refractive errors among schoolchildren in Dezful, Iran. Br. J. Ophthalmol. 2007, 91, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Lithander, J. Prevalence of myopia in school children in the Sultanate of Oman: A nation-wide study of 6292 randomly selected children. Acta Ophthalmol. Scand. 1999, 77, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Montes-Mico, R.; Ferrer-Blasco, T. Distribution of refractive errors in Spain. Doc. Ophthalmol. 2000, 101, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Norouzirad, R.; Hashemi, H.; Yekta, A.; Nirouzad, F.; Ostadimoghaddam, H.; Yazdani, N.; Dadbin, N.; Javaherforoushzadeh, A.; Khabazkhoob, M. The prevalence of refractive errors in 6 to 15-year-old schoolchildren in Dezful, Iran. J. Curr. Ophthalmol. 2015, 27, 51–55. [Google Scholar] [CrossRef]
- Kanda, H.; Oshika, T.; Hiraoka, T.; Hasebe, S.; Ohno-Matsui, K.; Ishiko, S.; Hieda, O.; Torii, H.; Varnas, S.R.; Fujikado, T. Effect of spectacle lenses designed to reduce relative peripheral hyperopia on myopia progression in Japanese children: A 2-year multicenter randomized controlled trial. Jpn. J. Ophthalmol. 2018, 62, 537–543. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Category | All | Crocetin | Placebo | p–Value | |
|---|---|---|---|---|---|---|
| Number of cases | 69 | 39 | 30 | |||
| Number of eyes | 138 | 78 | 60 | |||
| Age (years) | 10.2 ± 1.3 | 10.4 ± 1.2 | 10.1 ± 1.3 | 0.352 | † | |
| Sex | boys | 38 (55.1%) | 22 (56.4%) | 16 (53.3%) | 0.812 | †† |
| girls | 31 (44.9%) | 17 (43.6%) | 14 (46.7%) | |||
| Parental myopia | both parents | 46 (66.7%) | 28 (71.8%) | 18 (60.0%) | ||
| only father | 11 (15.9%) | 5 (12.8%) | 6 (20.0%) | 0.588 | †† | |
| only mother | 12 (17.4%) | 6 (15.4%) | 6 (20.0%) | |||
| one parent | 23 (33.3%) | 11 (28.2%) | 12 (40.0%) | 0.318 | †† | |
| Height (cm) | 140.8 ± 9.3 | 141.9 ± 9.6 | 139.3 ± 9.0 | 0.250 | † | |
| Weight (kg) | 34.6 ± 7.7 | 34.9 ± 7.8 | 34.2 ± 7.5 | 0.703 | † | |
| Best corrected visual acuity (log MAR) | −0.12 ± 0.06 | −0.12 ± 0.07 | −0.12 ± 0.06 | 0.718 | ||
| SER (D) | −3.45 ± 0.99 | −3.45 ± 0.98 | −3.45 ± 1.00 | 0.756 | ||
| Corneal curvature radius (mm) | 7.83 ± 0.24 | 7.84 ± 0.25 | 7.83 ± 0.23 | 0.577 | ||
| Axial length (mm) | 24.95 ± 0.83 | 24.91 ± 0.89 | 24.99 ± 0.77 | 0.837 | ||
| IOP (mmHg) | 17.2 ± 3.8 | 17.8 ± 3.8 | 16.4 ± 3.6 | 0.050 | ||
| BUT (sec) | 7.9 ± 2.1 | 7.8 ± 2.2 | 8.0 ± 2.0 | 0.829 | ||
| Environmental factors | ||||||
| Time of near-work (min/day) | 205.5 ± 88.2 | 190.3 ± 70.9 | 225.2 ± 104.6 | 0.258 | ||
| Time of sunlight exposure (min/day) | 53.6 ± 42.3 | 63.6 ± 43.2 | 40.5 ± 38.0 | 0.017 | ||
| Time of sleeping (hours/day) | 8.8 ± 0.7 | 8.8 ± 0.7 | 8.7 ± 0.7 | 0.305 | ||
| IPAQ (METS *min/day) | 209.1 ± 186.5 | 239.5 ± 203.4 | 169.6 ± 156.5 | 0.133 | ||
| Means | Estimate Value, D | Standard Error, D | 95% CI | p-Value | |
|---|---|---|---|---|---|
| Treatments | Crocetin | 0.08 | 0.04 | 0.000~0.155 | 0.049 |
| Placebo | reference | - | - | ||
| Age (years) | 6 | −0.12 | 0.16 | −0.442~0.196 | 0.446 |
| 7 | −0.23 | 0.13 | −0.497~0.031 | 0.083 | |
| 8 | −0.21 | 0.08 | −0.366~-0.056 | 0.008 | |
| 9 | 0.03 | 0.06 | −0.082~0.151 | 0.561 | |
| 10 | −0.01 | 0.06 | −0.120~0.097 | 0.838 | |
| 11 | 0.10 | 0.05 | −0.003~0.202 | 0.056 | |
| 12 | reference | - | - | ||
| Sex | Boys | 0.02 | 0.03 | −0.047~0.089 | 0.537 |
| Girls | reference | - | - | ||
| Time of near-work (min/day) | 30−<90 | −0.10 | 0.12 | −0.330~0.141 | 0.428 |
| 90−<150 | −0.05 | 0.08 | −0.206~0.116 | 0.581 | |
| 150−<210 | −0.11 | 0.08 | −0.270~0.051 | 0.178 | |
| 210−<270 | −0.05 | 0.08 | −0.211~0.115 | 0.563 | |
| 270−<330 | 0.01 | 0.10 | −0.180~0.196 | 0.934 | |
| 330−<390 | −0.08 | 0.12 | −0.311~0.149 | 0.489 | |
| 390− | reference | - | - | ||
| Time of sunlight exposure (min/day) | 0−<30 | −0.18 | 0.09 | −0.364~0.001 | 0.052 |
| 30−<90 | −0.22 | 0.09 | −0.400~−0.034 | 0.021 | |
| 90−<150 | −0.24 | 0.10 | −0.436~−0.040 | 0.019 | |
| 150− | reference | - | - | ||
| Time of sleeping (hours/day) | 7 | −0.09 | 0.11 | −0.300~0.118 | 0.391 |
| 8 | −0.01 | 0.06 | −0.125~0.104 | 0.859 | |
| 8.5 | 0.04 | 0.16 | −0.277~0.353 | 0.812 | |
| 9 | −0.03 | 0.05 | −0.130~0.079 | 0.627 | |
| 9.5 | 0.13 | 0.09 | −0.055~0.317 | 0.167 | |
| 10 | reference | - | - | ||
| Eye | Left | 0.04 | 0.03 | −0.021~0.090 | 0.221 |
| Right | reference | - | - | ||
| Visit weeks | 4 | 0.32 | 0.03 | 0.256~0.381 | <0.001 |
| 24 | reference | - | - | ||
| SER at baseline | −0.01 | 0.02 | −0.043~0.020 | 0.468 | |
| Interaction effects | |||||
| Visit weeks by group (= crocetin) | 4 | 0.01 | 0.04 | −0.075~0.092 | 0.842 |
| 24 | reference | - | - | ||
| Visit weeks by group (= placebo) | 4 | reference | - | - | |
| 24 | reference | - | - | ||
| a | The Adjusted Mean of Cycloplegic SER Change in both Eyes at Each Visit | ||
| Placebo | Crocetin | ||
| Visit, weeks | Progression, D | Progression, D | p-value |
| 4 | −0.09 ± 0.05 | −0.01 ± 0.05 | 0.028 |
| 24 | −0.41 ± 0.05 | −0.33 ± 0.05 | 0.049 |
| b | The Adjusted Mean of AL Change in both Eyes at Each Visit | ||
| Placebo | Crocetin | ||
| Visit, weeks | Elongation, mm | Elongation, mm | p-value |
| 4 | 0.08 ± 0.02 | 0.06 ± 0.02 | 0.280 |
| 12 | 0.16 ± 0.02 | 0.12 ± 0.02 | 0.020 |
| 24 | 0.21 ± 0.02 | 0.18 ± 0.02 | 0.046 |
| Means | Estimate Value, mm | Standard Error, mm | 95% CI | p-Value | |
|---|---|---|---|---|---|
| Treatments | Crocetin | −0.03 | 0.01 | −0.053~−0.001 | 0.046 |
| Placebo | reference | - | - | ||
| Age (years) | 6 | 0.20 | 0.06 | 0.081~0.318 | 0.002 |
| 7 | 0.10 | 0.05 | 0.003~0.200 | 0.043 | |
| 8 | 0.12 | 0.03 | 0.065~0.181 | <0.001 | |
| 9 | 0.02 | 0.02 | −0.028~0.060 | 0.469 | |
| 10 | 0.02 | 0.02 | −0.020~0.059 | 0.331 | |
| 11 | 0.00 | 0.02 | −0.036~0.039 | 0.931 | |
| 12 | reference | - | - | ||
| Sex | Boys | −0.03 | 0.01 | −0.060~−0.003 | 0.029 |
| Girls | reference | - | - | ||
| Time of near-work (min/day) | 30–<90 | 0.02 | 0.04 | −0.072~0.103 | 0.718 |
| 90–<150 | 0.01 | 0.03 | −0.048~0.072 | 0.685 | |
| 150–<210 | 0.04 | 0.03 | −0.021~0.098 | 0.201 | |
| 210–<270 | 0.03 | 0.03 | −0.031~0.090 | 0.332 | |
| 270–<330 | 0.02 | 0.04 | −0.046~0.094 | 0.497 | |
| 330–<390 | 0.04 | 0.04 | −0.045~0.126 | 0.349 | |
| 390− | reference | - | - | ||
| Time of sunlight exposure (min/day) | 0–<30 | 0.00 | 0.03 | −0.067~0.069 | 0.977 |
| 30–<90 | −0.01 | 0.03 | −0.076~0.060 | 0.818 | |
| 90–<150 | −0.02 | 0.04 | −0.091~0.056 | 0.633 | |
| 150- | reference | - | - | ||
| Time of sleeping (hours/day) | 7 | 0.01 | 0.04 | −0.064~0.091 | 0.726 |
| 8 | −0.03 | 0.02 | −0.071~0.013 | 0.165 | |
| 8.5 | −0.05 | 0.06 | −0.165~0.070 | 0.423 | |
| 9 | −0.01 | 0.02 | −0.052~0.026 | 0.491 | |
| 9.5 | −0.04 | 0.03 | −0.104~0.034 | 0.310 | |
| 10 | reference | - | - | ||
| Eye | Left | 0.00 | 0.01 | −0.011~0.010 | 0.973 |
| Right | reference | - | - | ||
| Visit weeks | 4 | −0.13 | 0.01 | −0.144~−0.115 | <0.001 |
| 12 | −0.05 | 0.01 | −0.065~−0.039 | <0.001 | |
| 24 | reference | - | - | ||
| AL at baseline | 0.02 | 0.01 | 0.005~0.037 | 0.011 | |
| Interaction effects | |||||
| Visit weeks by group (= crocetin) | 4 | 0.01 | 0.01 | −0.007~0.032 | 0.205 |
| 12 | −0.01 | 0.01 | −0.022~0.013 | 0.609 | |
| 24 | reference | - | - | ||
| Visit weeks by group (= placebo) | 4 | reference | - | - | |
| 12 | reference | - | - | ||
| 24 | reference | - | - | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mori, K.; Torii, H.; Fujimoto, S.; Jiang, X.; Ikeda, S.-i.; Yotsukura, E.; Koh, S.; Kurihara, T.; Nishida, K.; Tsubota, K. The Effect of Dietary Supplementation of Crocetin for Myopia Control in Children: A Randomized Clinical Trial. J. Clin. Med. 2019, 8, 1179. https://doi.org/10.3390/jcm8081179
Mori K, Torii H, Fujimoto S, Jiang X, Ikeda S-i, Yotsukura E, Koh S, Kurihara T, Nishida K, Tsubota K. The Effect of Dietary Supplementation of Crocetin for Myopia Control in Children: A Randomized Clinical Trial. Journal of Clinical Medicine. 2019; 8(8):1179. https://doi.org/10.3390/jcm8081179
Chicago/Turabian StyleMori, Kiwako, Hidemasa Torii, Satoko Fujimoto, Xiaoyan Jiang, Shin-ichi Ikeda, Erisa Yotsukura, Shizuka Koh, Toshihide Kurihara, Kohji Nishida, and Kazuo Tsubota. 2019. "The Effect of Dietary Supplementation of Crocetin for Myopia Control in Children: A Randomized Clinical Trial" Journal of Clinical Medicine 8, no. 8: 1179. https://doi.org/10.3390/jcm8081179
APA StyleMori, K., Torii, H., Fujimoto, S., Jiang, X., Ikeda, S.-i., Yotsukura, E., Koh, S., Kurihara, T., Nishida, K., & Tsubota, K. (2019). The Effect of Dietary Supplementation of Crocetin for Myopia Control in Children: A Randomized Clinical Trial. Journal of Clinical Medicine, 8(8), 1179. https://doi.org/10.3390/jcm8081179








