A Modified Method to Assess Tidal Recruitment by Electrical Impedance Tomography
Abstract
:1. Introduction
2. Animals and Methods
2.1. Animals and Ethics
2.2. Study Protocol
Anesthesia, Ventilatory Settings, and Induction of Lung Injury
2.3. Measurements and Data Analysis
2.3.1. Computed Tomography
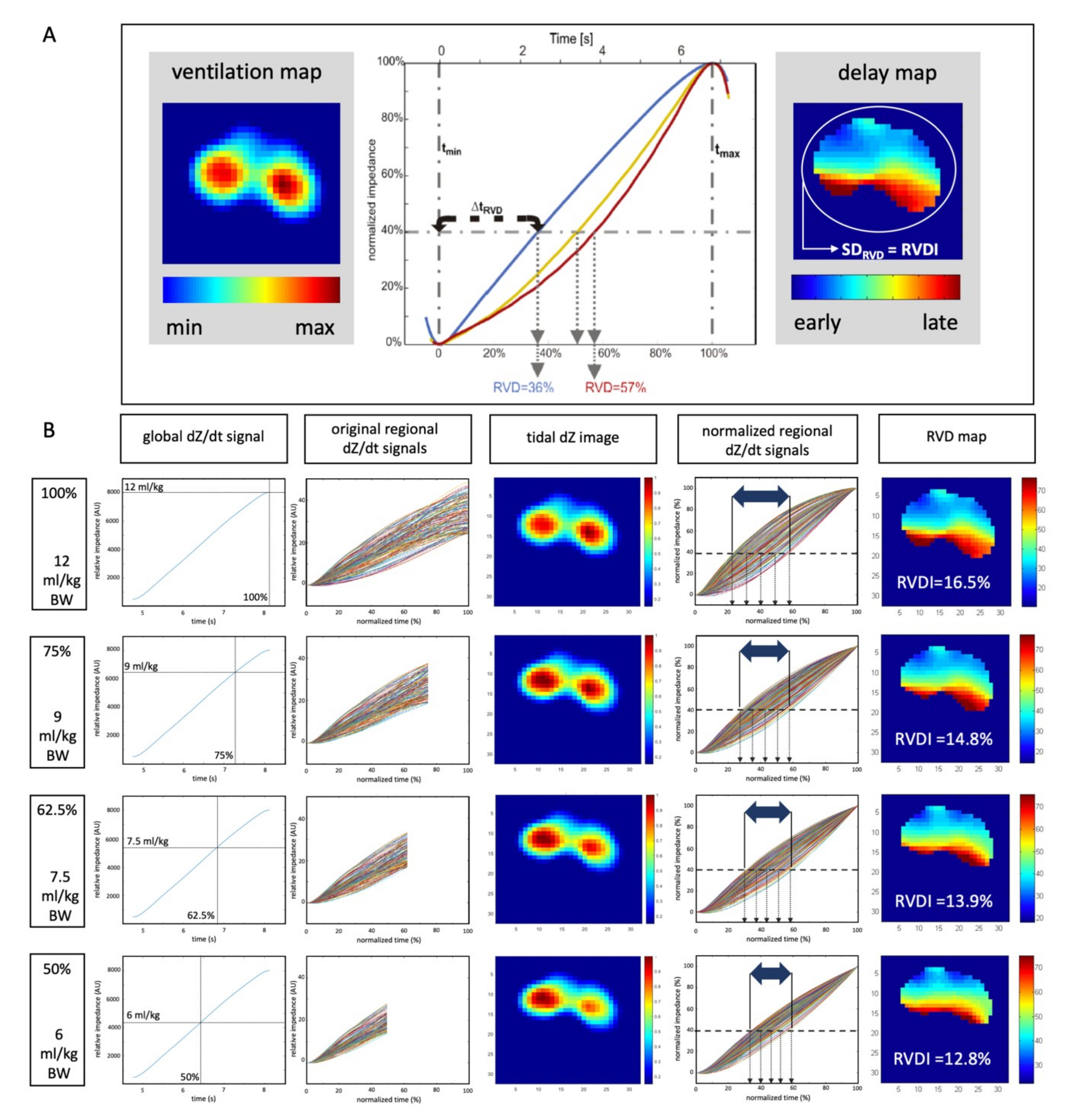
2.3.2. Electrical Impedance Tomography
2.3.3. Quantification of Heterogeneity in Ventilatory Time Courses
2.3.4. Selection of Definite RVDI Thresholds to Predict Tidal Recruitment
2.4. Statistical Analysis
3. Results
3.1. Lung Injury and Cardiopulmonary Effects of Different PEEP Levels
3.2 Temporal Heterogeneity Measured by EIT
3.3. End-Inspiratory Pressures Resulting from Different Slow Inflation VT Maneuvers
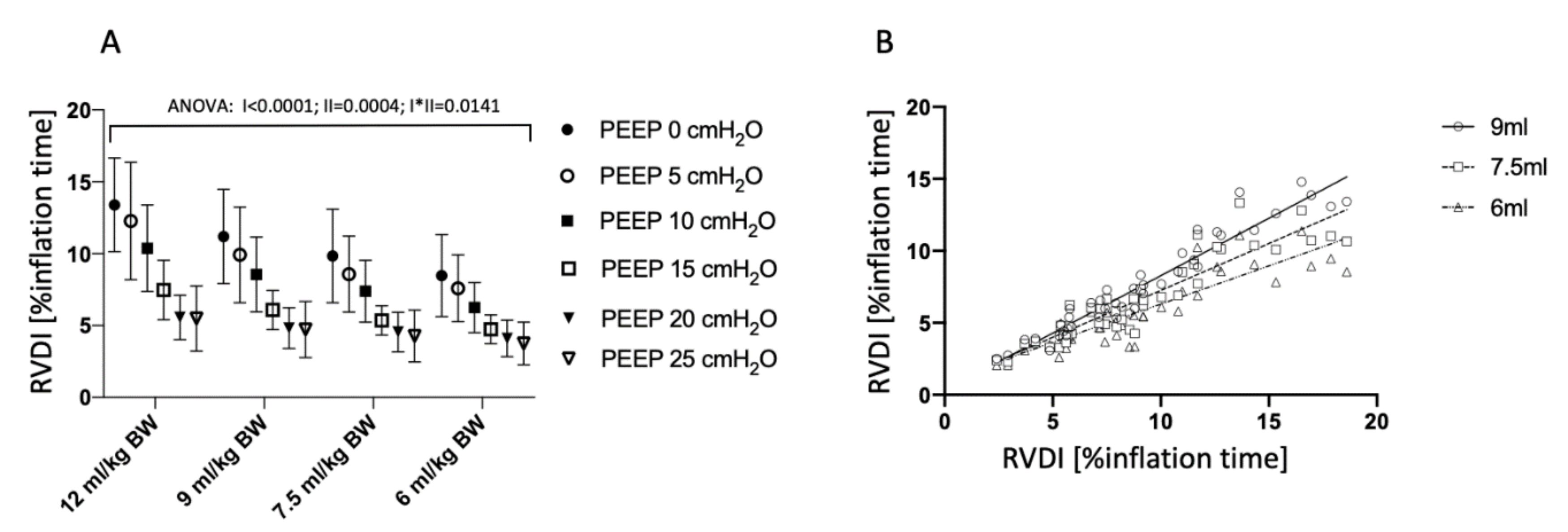
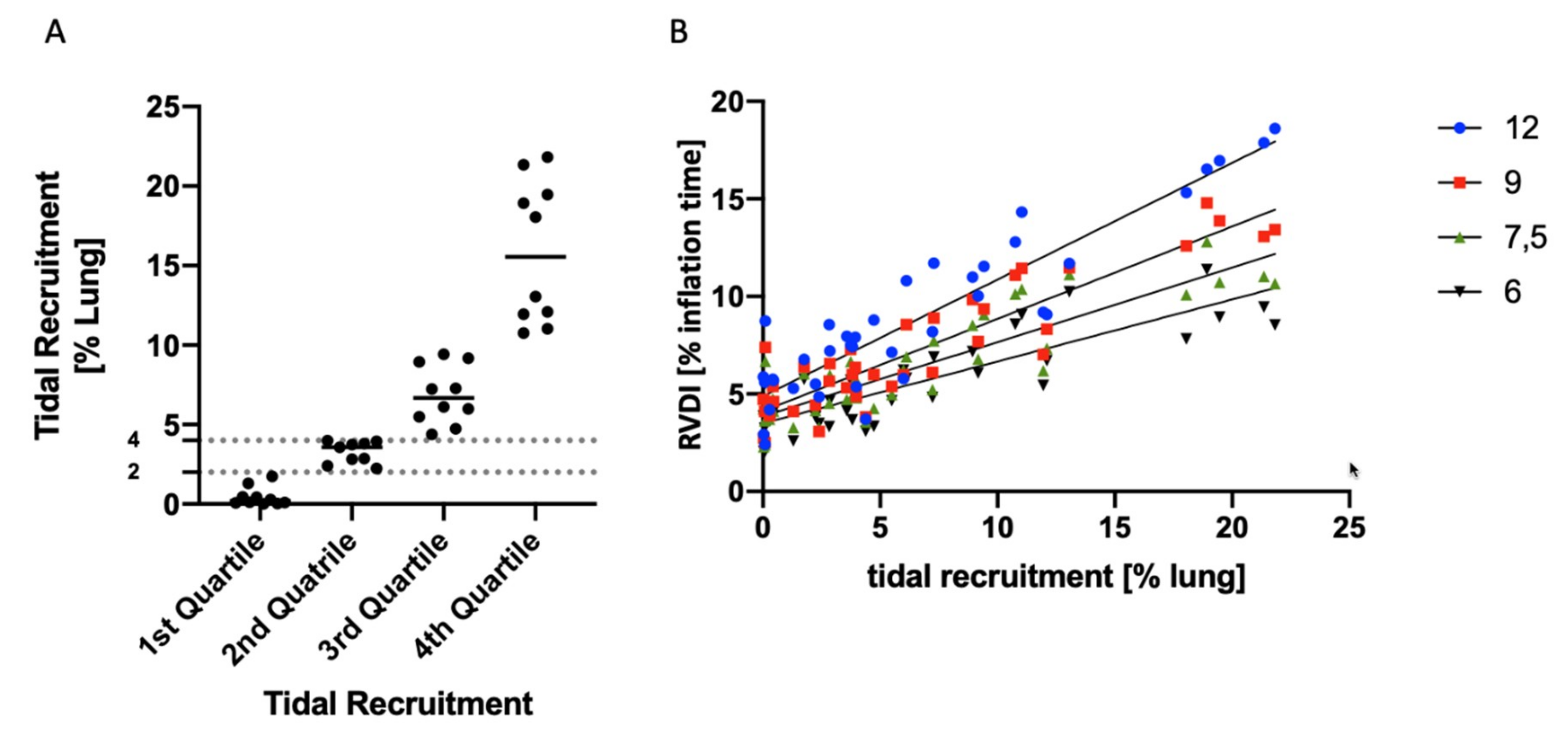
3.4. Tidal Recruitment at Different PEEP Levels
3.5. Correlation of RVDI and Tidal Recruitment Obtained from Different Slow Inflation VT Maneuvers
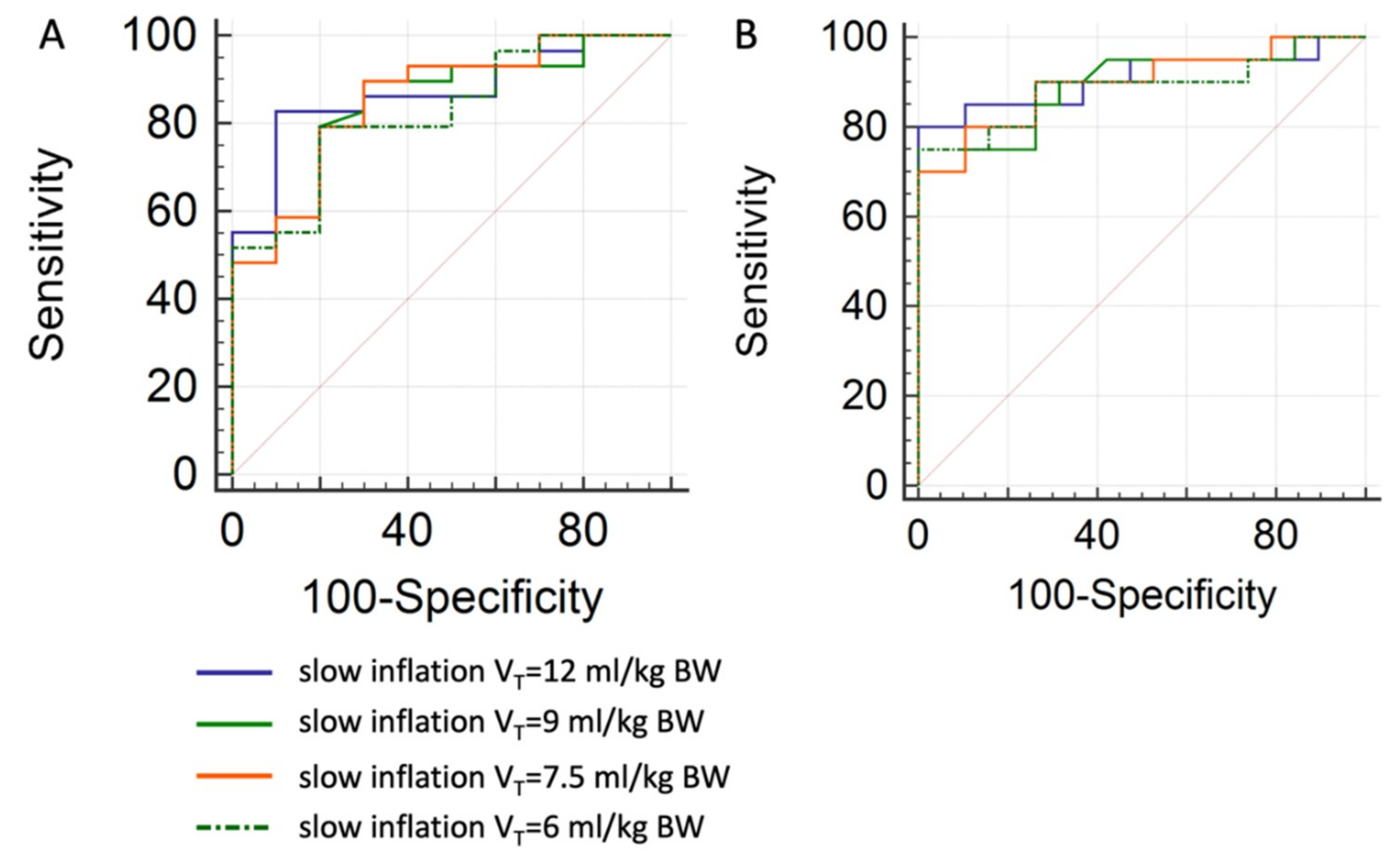
3.6. Prediction of Tidal Recruitment thresholds from RVDI Values
4. Discussion
- RVDI can be derived from slow inflation maneuvers using a low VT of 6 to 9 mL/kg BW, to quantify temporal heterogeneity of regional ventilatory time courses.
- Absolute RVDI values are not comparable when derived from slow inflation maneuvers using different VTs.
- RVDI values from slow inflation maneuvers using low VTs of 6 to 9 mL/kg BW correlate with tidal recruitment.
4.1. Effects of Slow Inflation VT on RVDI-Values
4.2. Assessment of Tidal Recruitment Using RVDI Measurements
4.3. PEEP Titration Using EIT
4.3.1. EIT-Based Approaches to Individually Titrate PEEP
4.3.2. Regional Ventilation Delay Inhomogeneity to Individually Titrate PEEP
4.4. Clinical Applicability of RVDI Measurements
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- This official conference report was cosponsored by the American Thoracic Society the European Society of Intensive Care Medicine and the Societé de Réanimation de Langue Française and approved by the ATS Board of Directors. International Consensus Conferences in Intensive Care Medicine: Ventilator-associated Lung Injury in ARDS. Intensive Care Med. 1999, 25, 1444–1452. [CrossRef]
- Ranieri, V.M.; Suter, P.M.; Tortorella, C.; De Tullio, R.; Dayer, J.M.; Brienza, A.; Bruno, F.; Slutsky, A.S. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: A randomized controlled trial. JAMA 1999, 282, 54–61. [Google Scholar] [CrossRef]
- Slutsky, A.S. Lung Injury Caused by Mechanical Ventilation. Chest 1999, 116, 9S–15S. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, S. Ventilation-induced lung injury and mechanotransduction: Stretching it too far? Am. J. Physiol. Cell. Mol. Physiol. 2002, 282, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Brower, R.G.; Matthay, M.A.; Morris, A.; Schoenfeld, D.; Thompson, B.T.; Wheeler, A.; Acute Respiratory Distress Syndrome Network. Ventilation with Lower Tidal Volumes as Compared with Traditional Tidal Volumes for Acute Lung Injury and the Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar] [PubMed]
- Gattinoni, L.; Caironi, P.; Cressoni, M.; Chiumello, D.A.; Ranieri, V.M.; Quintel, M.; Russo, S.; Patroniti, N.; Cornejo, R.; Bugedo, G. Lung Recruitment in Patients with the Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2006, 354, 1775–1786. [Google Scholar] [CrossRef] [PubMed]
- Caironi, P.; Cressoni, M.; Chiumello, D.A.; Ranieri, M.; Quintel, M.; Russo, S.G.; Cornejo, R.; Bugedo, G.; Carlesso, E.; Russo, R.; et al. Lung Opening and Closing during Ventilation of Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2010, 181, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Briel, M.; Meade, M.; Mercat, A.; Brower, R.G.; Talmor, D.; Walter, S.D.; Slutsky, A.S.; Pullenayegum, E.; Zhou, Q.; Cook, D.; et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: Systematic review and meta-analysis. JAMA 2010, 303, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Putensen, C.; Theuerkauf, N.; Zinserling, J.; Wrigge, H.; Pelosi, P. Meta-analysis: Ventilation strategies and outcomes of the acute respiratory distress syndrome and acute lung injury. Ann. Intern. Med. 2009, 151, 566–576. [Google Scholar] [CrossRef]
- Nieman, G.F.; Satalin, J.; Andrews, P.; Aiash, H.; Habashi, N.M.; Gatto, L.A. Personalizing mechanical ventilation according to physiologic parameters to stabilize alveoli and minimize ventilator induced lung injury (VILI). Intensiv. Care Med. Exp. 2017, 5, 1. [Google Scholar] [CrossRef]
- Hickling, K.G. The Pressure–Volume Curve Is Greatly Modified by Recruitment: A Mathematical Model of ARDS Lungs. Am. J. Respir. Crit. Care Med. 1998, 158, 194–202. [Google Scholar] [CrossRef]
- Muders, T.; Luepschen, H.; Putensen, C. Impedance tomography as a new monitoring technique. Curr. Opin. Crit. Care 2010, 16, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Frerichs, I.; Amato, M.B.P.; Van Kaam, A.H.; Tingay, D.G.; Zhao, Z.; Grychtol, B.; Bodenstein, M.; Gagnon, H.; Böhm, S.H.; Teschner, E.; et al. Chest electrical impedance tomography examination, data analysis, terminology, clinical use and recommendations: Consensus statement of the TRanslational EIT developmeNt stuDy group. Thorax 2016, 72, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Wrigge, H.; Zinserling, J.; Muders, T.; Varelmann, D.; Günther, U.; Von Der Groeben, C.; Magnusson, A.; Hedenstierna, G.; Putensen, C. Electrical impedance tomography compared with thoracic computed tomography during a slow inflation maneuver in experimental models of lung injury. Crit. Care Med. 2008, 36, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Muders, T.; Luepschen, H.; Zinserling, J.; Greschus, S.; Fimmers, R.; Guenther, U.; Buchwald, M.; Grigutsch, D.; Leonhardt, S.; Putensen, C.; et al. Tidal recruitment assessed by electrical impedance tomography and computed tomography in a porcine model of lung injury. Crit. Care Med. 2012, 40, 903–911. [Google Scholar] [CrossRef] [PubMed]
- The ARDS Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S.; Ranieri, V.; et al. Acute respiratory distress syndrome: The berlin definition. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Bikker, I.G.; van Bommel, J.; Miranda, D.R.; Bakker, J.; Gommers, D. End-expiratory lung volume during mechanical ventilation: A comparison with reference values and the effect of positive end-expiratory pressure in intensive care unit patients with different lung conditions. Crit. Care 2008, 12, R145. [Google Scholar] [CrossRef]
- Meier, T.; Luepschen, H.; Karsten, J.; Leibecke, T.; Großherr, M.; Gehring, H.; Leonhardt, S.; Grossherr, M. Assessment of regional lung recruitment and derecruitment during a PEEP trial based on electrical impedance tomography. Intensiv. Care Med. 2007, 34, 543–550. [Google Scholar] [CrossRef]
- Costa, E.L.V.; Borges, J.B.; Melo, A.; Suarez-Sipmann, F.; Toufen, C.; Bohm, S.H.; Amato, M.B.P. Bedside estimation of recruitable alveolar collapse and hyperdistension by electrical impedance tomography. Intensiv. Care Med. 2009, 35, 1132–1137. [Google Scholar] [CrossRef]
- Dargaville, P.A.; Rimensberger, P.C.; Frerichs, I. Regional tidal ventilation and compliance during a stepwise vital capacity manoeuvre. Intensiv. Care Med. 2010, 36, 1953–1961. [Google Scholar] [CrossRef]
- Lowhagen, K.; Lundin, S.; Stenqvist, O. Regional intratidal gas distribution in acute lung injury and acute respiratory distress syndrome—Assessed by electric impedance tomography. Minerva. Anestesiol. 2010, 76, 1024–1035. [Google Scholar] [PubMed]
- Zhao, Z.; Pulletz, S.; Frerichs, I.; Müller-Lisse, U.; Möller, K. The EIT-based global inhomogeneity index is highly correlated with regional lung opening in patients with acute respiratory distress syndrome. BMC Res. Notes 2014, 7, 82. [Google Scholar] [CrossRef] [PubMed]
- Scaramuzzo, G.; Spadaro, S.; Waldmann, A.D.; Böhm, S.H.; Ragazzi, R.; Marangoni, E.; Alvisi, V.; Spinelli, E.; Mauri, T.; Volta, C.A. Heterogeneity of regional inflection points from pressure-volume curves assessed by electrical impedance tomography. Crit. Care 2019, 23, 119. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, S.; Mauri, T.; Böhm, S.H.; Scaramuzzo, G.; Turrini, C.; Waldmann, A.D.; Ragazzi, R.; Pesenti, A.; Volta, C.A. Variation of poorly ventilated lung units (silent spaces) measured by electrical impedance tomography to dynamically assess recruitment. Crit. Care 2018, 22, 26. [Google Scholar] [CrossRef] [PubMed]
- Hinz, J.; Gehoff, A.; Moerer, O.; Frerichs, I.; Hahn, G.; Hellige, G.; Quintel, M. Regional filling characteristics of the lungs in mechanically ventilated patients with acute lung injury. Eur. J. Anaesthesiol. 2007, 24, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Pulletz, S.; Adler, A.; Kott, M.; Elke, G.; Gawelczyk, B.; Schädler, D.; Zick, G.; Weiler, N.; Frerichs, I. Regional lung opening and closing pressures in patients with acute lung injury. J. Crit. Care 2012, 27, e11–e18. [Google Scholar] [CrossRef]
- Becher, T.H.; Bui, S.; Zick, G.; Bläser, D.; Schädler, D.; Weiler, N.; Frerichs, I. Assessment of respiratory system compliance with electrical impedance tomography using a positive end-expiratory pressure wave maneuver during pressure support ventilation: A pilot clinical study. Crit. Care 2014, 18, 679. [Google Scholar] [CrossRef]
- Victorino, J.A.; Borges, J.B.; Okamoto, V.N.; Matos, G.F.J.; Tucci, M.R.; Caramez, M.P.R.; Tanaka, H.; Sipmann, F.S.; Santos, D.C.B.; Barbas, C.S.V.; et al. Imbalances in regional lung ventilation: A validation study on electrical impedance tomography. Am. J. Respir. Crit. Care Med. 2004, 169, 791–800. [Google Scholar] [CrossRef]
- Blankman, P.; Hasan, D.; Erik, G.J.; Gommers, D. Detection of ‘best’ positive end-expiratory pressure derived from electrical impedance tomography parameters during a decremental positive end-expiratory pressure trial. Crit. Care 2014, 18, R95. [Google Scholar] [CrossRef]
- Bickenbach, J.; Czaplik, M.; Polier, M.; Marx, G.; Marx, N.; Dreher, M. Electrical impedance tomography for predicting failure of spontaneous breathing trials in patients with prolonged weaning. Crit. Care 2017, 21, 177. [Google Scholar] [CrossRef]
- Nestler, C.; Simon, P.; Petroff, D.; Hammermüller, S.; Kamrath, D.; Wolf, S.; Dietrich, A.; Camilo, L.M.; Beda, A.; Carvalho, A.R.; et al. Individualized positive end-expiratory pressure in obese patients during general anaesthesia: A randomized controlled clinical trial using electrical impedance tomography. Br. J. Anaesth. 2017, 119, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Muders, T.; Luepschen, H.; Meier, T.; Leonhardt, S.; Hedenstierna, G.; Putensen, C.; Wrigge, H. Influence of different PEEP setting strategies on regional distribution of pulmonary ventilation, blood flow and strain in porcine lung injury. Int. Care Med. 2010, 36, S206. [Google Scholar]
- Gattinoni, L.; Eleonora, C.; Caironi, P. Monitoring of pulmonary mechanics in acute respiratory distress syndrome to titrate therapy. Curr. Opin. Crit. Care 2005, 11, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Hata, J.S.; Togashi, K.; Kumar, A.B.; Hodges, L.D.; Kaiser, E.F.; Tessmann, P.B.; Faust, C.A.; Sessler, D.I. The Effect of the Pressure–Volume Curve for Positive End-Expiratory Pressure Titration on Clinical Outcomes in Acute Respiratory Distress Syndrome: A Systematic Review. J. Intensive Care Med. 2014, 29, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Matute-Bello, G.; Frevert, C.W.; Martin, T.R. Animal models of acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 295, L379–L399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves-De-Albuquerque, C.F.; Silva, A.R.; Burth, P.; Castro-Faria, M.V.; Castro-Faria-Neto, H.C. Acute Respiratory Distress Syndrome: Role of Oleic Acid-Triggered Lung Injury and Inflammation. Mediat. Inflamm. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ballard-Croft, C.; Wang, D.; Sumpter, L.R.; Zhou, X.; Zwischenberger, J.B. Large-Animal Models of Acute Respiratory Distress Syndrome. Ann. Thorac. Surg. 2012, 93, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bodenstein, M.; Markstaller, K. Overview of the Pathology of Three Widely Used Animal Models of Acute Lung Injury. Eur. Surg. Res. 2008, 40, 305–316. [Google Scholar] [CrossRef]
- Regli, A.; Pelosi, P.; Malbrain, M.L.N.G. Ventilation in patients with intra-abdominal hypertension: What every critical care physician needs to know. Ann. Intensiv. Care 2019, 9, 52. [Google Scholar] [CrossRef]
- Gattinoni, L.; Pelosi, P.; Suter, P.M.; Pedoto, A.; Vercesi, P.; Lissoni, A. Acute Respiratory Distress Syndrome Caused by Pulm and extrapulmonary disease. Different syndromes? Am. J. Respir. Crit. Care Med. 1998, 158, 3–11. [Google Scholar] [CrossRef]
- Reske, A.W.; Busse, H.; Amato, M.B.P.; Jaekel, M.; Kahn, T.; Schwarzkopf, P.; Schreiter, D.; Gottschaldt, U.; Seiwerts, M. Image reconstruction affects computer tomographic assessment of lung hyperinflation. Intensiv. Care Med. 2008, 34, 2044–2053. [Google Scholar] [CrossRef] [PubMed]


| Comparison of RVDI Values | Slow Inflation Tidal Volume of 12 mL/kg BW vs. | ||
|---|---|---|---|
| 9 mL/kg BW | 7.5 mL/kg BW | 6 mL/kg BW | |
| inter-individual comparison, all animals (n = 7) | |||
| linear correlation, R2, p < 0.001 | 0.93 | 0.84 | 0.76 |
| Bland Altman analysis | |||
| Bias ± SD | −1.5 ± 1.2 | −2.4 ± 1.9 | −3.3 ± 2.3 |
| 95% limits of agreement | −4.0 to 1.0 | −6.1 to 1.2 | −7.8 to 1.2 |
| intra-individual comparison | |||
| linear correlation, R2, p < 0.001, respectively | |||
| pig 1 | 0.91 | 0.79 | 0.69 |
| pig 2 | 0.9 | 0.8 | 0.73 |
| pig 3 | 0.97 | 0.94 | 0.86 |
| pig 4 | 0.99 | 0.99 | 0.96 |
| pig 5 | 0.99 | 0.99 | 0.97 |
| pig 6 | 0.85 | 0.62 | 0.37 |
| pig 7 | 0.98 | 0.94 | 0.86 |
| Comparison of Tidal Recruitment Versus | RVDI Values Calculated from a Slow Inflation Tidal Volume of | |||
|---|---|---|---|---|
| 12 mL/kg BW | 9 mL/kg BW | 7.5 mL/kg BW | 6 mL/kg BW | |
| inter-individual comparison, all animals (n = 7) | ||||
| linear correlation, R2, p < 0.001 | 0.83 | 0.83 | 0.76 | 0.71 |
| intra-individual comparison | ||||
| linear correlation, R2, p < 0.001, respectively | ||||
| pig 1 | 0.97 | 0.91 | 0.81 | 0.72 |
| pig 2 | 0.91 | 0.95 | 0.95 | 0.94 |
| pig 3 | 0.96 | 0.94 | 0.94 | 0.86 |
| pig 4 | 0.95 | 0.95 | 0.91 | 0.82 |
| pig 5 | 0.83 | 0.77 | 0.75 | 0.74 |
| pig 6 | 0.87 | 0.62 | 0.33 | 0.12 |
| pig 7 | 0.9 | 0.93 | 0.98 | 0.78 |
| ROC Curve Analysis | RVDI Calculated from a Slow Inflation Tidal Volume of | |||
|---|---|---|---|---|
| 12 mL/kg BW | 9 mL/kg BW | 7.5 mL/kg BW | 6 mL/kg BW | |
| tidal recruitment above 2% of lung volume | ||||
| AUC | 0.869 | 0.847 | 0.855 | 0.828 |
| SE | 0.0597 | 0.0688 | 0.0680 | 0.0708 |
| 95% CI | 0.722 to 0.955 | 0.695 to 0.942 | 0.706 to 0.947 | 0.673 to 0.929 |
| tidal recruitment above 4% of lung volume | ||||
| AUC | 0.908 | 0.891 | 0.897 | 0.887 |
| SE | 0.0533 | 0.0537 | 0.0521 | 0.0583 |
| 95% CI | 0.771 to 0.977 | 0.749 to 0.968 | 0.758 to 0.971 | 0.744 to 0.966 |
| RVDI-threshold-based prediction of an increase in tidal recruitment above 2 (% lung) during decremental PEEP titration | ||||
| RVDI criterion (% inflation time) | >5.36 | >4.39 | >4.15 | >3.34 |
| Sensitivity (%) | 90 | 90 | 90 | 90 |
| Specificity (%) | 40 | 50 | 60 | 40 |
| RVDI-threshold-based prediction of an increase in tidal recruitment above 4 (% lung) during decremental PEEP titration | ||||
| RVDI criterion (% inflation time) | >6.77 | >5.66 | >4.88 | >4.66 |
| Sensitivity (%) | 90 | 90 | 90 | 90 |
| Specificity (%) | 63 | 68 | 74 | 74 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muders, T.; Hentze, B.; Simon, P.; Girrbach, F.; Doebler, M.R.G.; Leonhardt, S.; Wrigge, H.; Putensen, C. A Modified Method to Assess Tidal Recruitment by Electrical Impedance Tomography. J. Clin. Med. 2019, 8, 1161. https://doi.org/10.3390/jcm8081161
Muders T, Hentze B, Simon P, Girrbach F, Doebler MRG, Leonhardt S, Wrigge H, Putensen C. A Modified Method to Assess Tidal Recruitment by Electrical Impedance Tomography. Journal of Clinical Medicine. 2019; 8(8):1161. https://doi.org/10.3390/jcm8081161
Chicago/Turabian StyleMuders, Thomas, Benjamin Hentze, Philipp Simon, Felix Girrbach, Michael R.G. Doebler, Steffen Leonhardt, Hermann Wrigge, and Christian Putensen. 2019. "A Modified Method to Assess Tidal Recruitment by Electrical Impedance Tomography" Journal of Clinical Medicine 8, no. 8: 1161. https://doi.org/10.3390/jcm8081161
APA StyleMuders, T., Hentze, B., Simon, P., Girrbach, F., Doebler, M. R. G., Leonhardt, S., Wrigge, H., & Putensen, C. (2019). A Modified Method to Assess Tidal Recruitment by Electrical Impedance Tomography. Journal of Clinical Medicine, 8(8), 1161. https://doi.org/10.3390/jcm8081161








