Biomarkers of Acute Lung Injury The Individualized Approach: for Phenotyping, Risk Stratification and Treatment Surveillance
Abstract
1. Introduction; Acute Lung Injury and Biomarkers to Characterize This Condition, and to Assist in Treatment Strategies
2. Biomarkers of Alveolar and Bronchiolar Injury—Surfactant Protein D, Club Cell Secretory Protein and Others
2.1. Biomarkers of Alveolar Injury
2.2. Biomarkers of Bronchiolar Injury
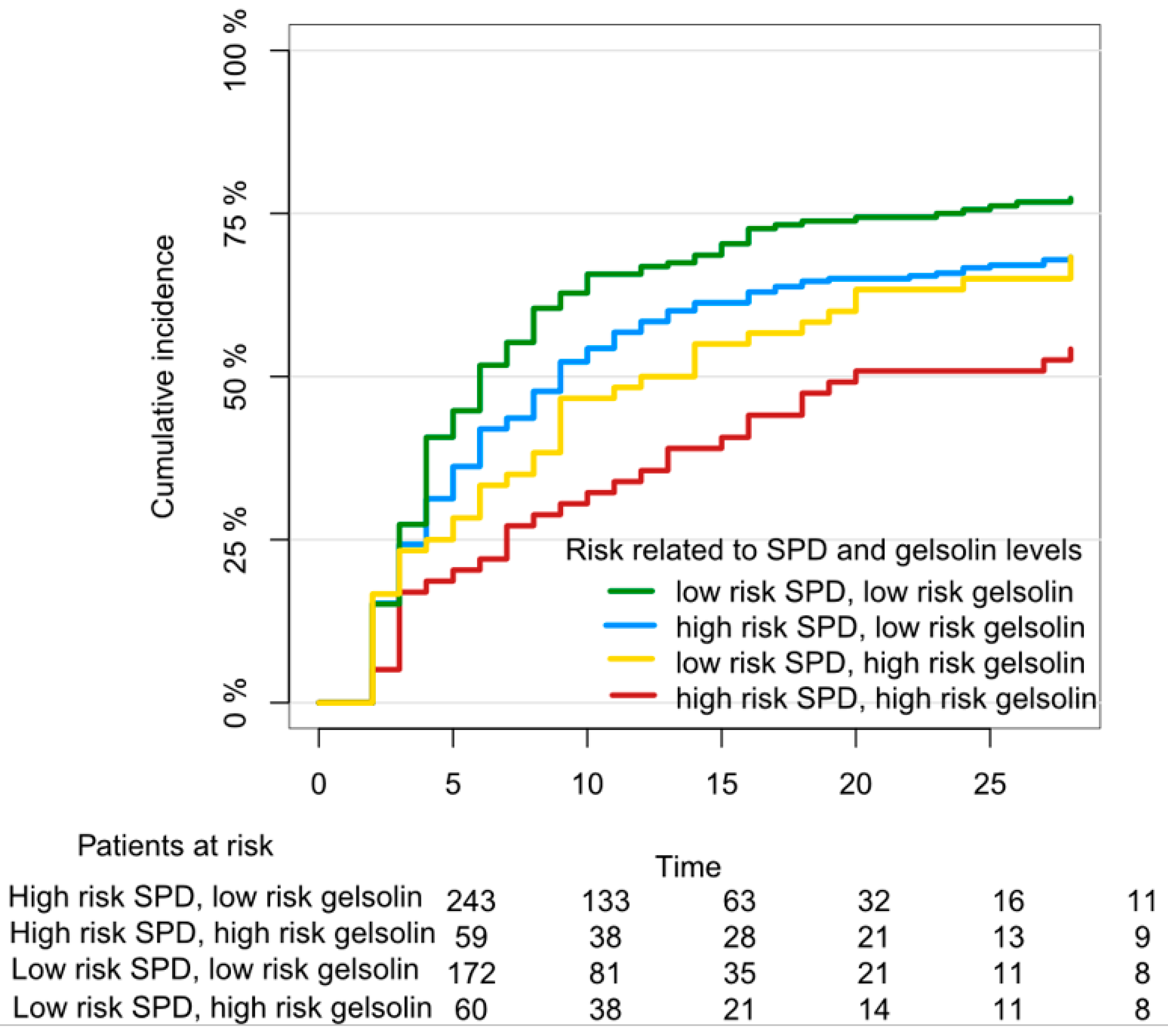
2.3. Biomarkers of Endothelial Injury
3. Biomarkers of Lung Infection: Procalcitonin
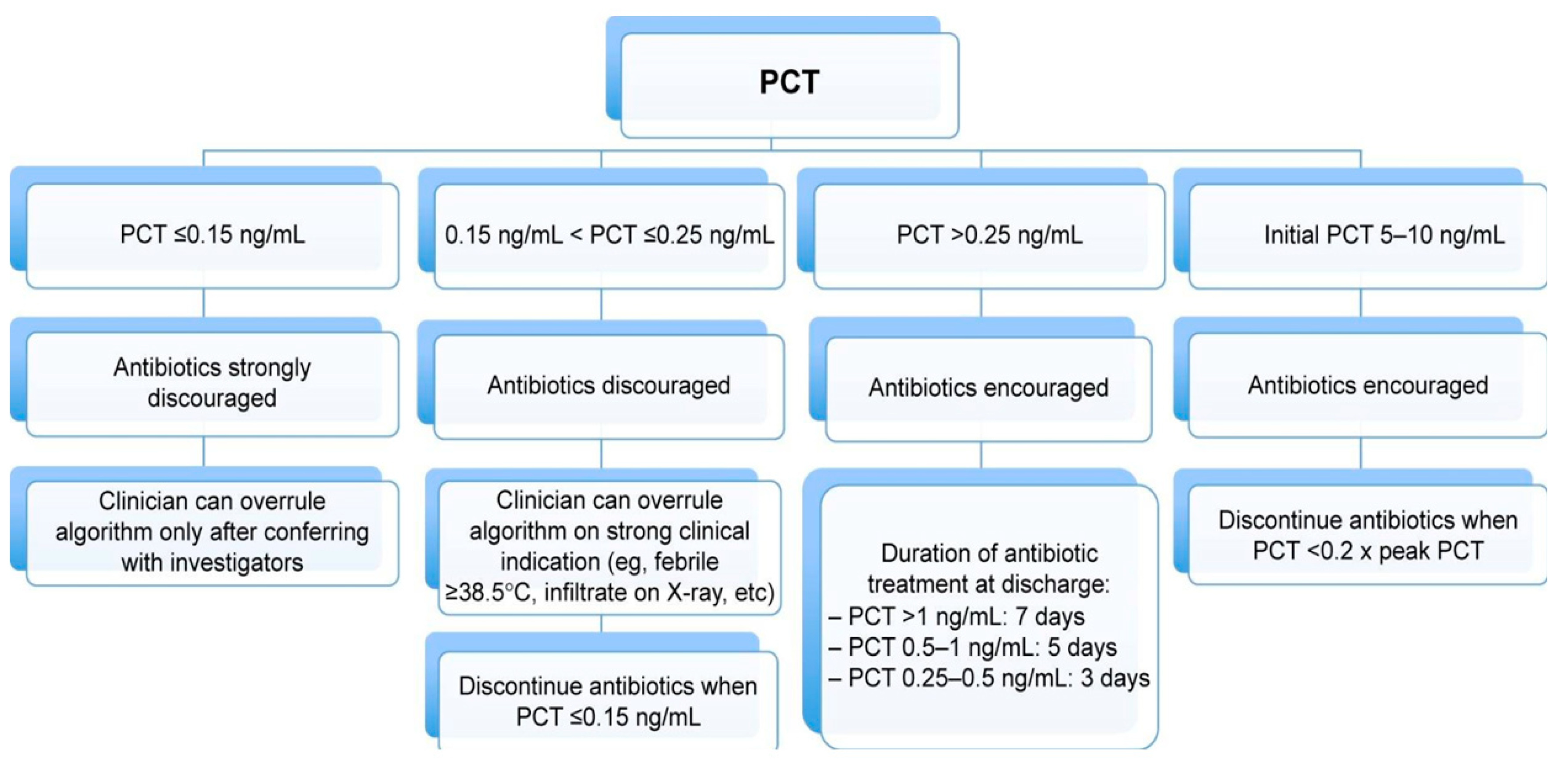
3.1. Procalcitonin for Initiating Antibiotics in Critically ill Patients
3.2. Procalcitonin for Antibiotic Reduction
4. Phenotypes of Lung Inflammation and How to Use This for Improved Management
5. Omics: Clinical Phenotypes and Advanced Bioinformatics—How to Integrate
5.1. Study Population—The Art of Selection
5.2. The Importance of Validation
6. Wrap up: Biomarkers of Lung Injury
Funding
Conflicts of Interest
References
- Jensen, J.U.S.; Itenov, T.S.; Thormar, K.M.; Hein, L.; Mohr, T.T.; Andersen, M.H.; Løken, J.; Tousi, H.; Lundgren, B.; Boesen, H.C.; et al. Prediction of non-recovery from ventilator-demanding acute respiratory failure, ARDS and death using lung damage biomarkers: Data from a 1200-patient critical care randomized trial. Ann. Intensiv. Care 2016, 6, 114. [Google Scholar] [CrossRef] [PubMed]
- Eisner, M.D.; Parsons, P.; Matthay, M.; Ware, L.; Greene, K. Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax 2003, 58, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, A.; Mudway, I.; Svensson, M.; Hagenbjork-Gustafsson, A.; Thomasson, L.; Helleday, R.; Dumont, X.; Forsberg, B.; Nordberg, G.; Bernard, A. Clara cell protein as a biomarker for ozone-induced lung injury in humans. Eur. Respir. J. 2003, 22, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Holm, F.S.; Sivapalan, P.; Seersholm, N.; Itenov, T.S.; Christensen, P.H.; Jensen, J.S. Acute Lung Injury in Critically Ill Patients: Actin-Scavenger Gelsolin Signals Prolonged Respiratory Failure. Shock 2018. [Google Scholar] [CrossRef] [PubMed]
- Ambade, V.N.; Sontakke, A.N.; Barthwal, M.; Tyagi, R.; Basannar, D.R. Diagnostic Utility of Biomarkers in COPD. Respir. Care 2015, 60, 1729–1742. [Google Scholar] [CrossRef]
- Spoorenberg, S.M.; Vestjens, G.T.; Rijkers, B.; Meek, C.H. van Moorsel, J.C.; Grutters, W.J. Bos, and Group Ovidius Study. Ykl-40, Ccl18 and Sp-D Predict Mortality in Patients Hospitalized with Community-Acquired Pneumonia. Respirology 2017, 22, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Fujishima, T.; Koba, H.; Murakami, S.; Kurokawa, K.; Shibuya, Y.; Shiratori, M.; Kuroki, Y.; Abe, S. Serum Surfactant Proteins a and D as Prognostic Factors in Idiopathic Pulmonary Fibrosis and Their Relationship to Disease Extent. Am. J. Respir. Crit. Care Med. 2000, 162, 1109–1114. [Google Scholar] [CrossRef]
- Sato, H.; Callister, M.; Mumby, S.; Quinlan, G.; Welsh, K.; Dubois, R.; Evans, T.; Quinlan, G. KL-6 levels are elevated in plasma from patients with acute respiratory distress syndrome. Eur. Respir. J. 2004, 23, 142–145. [Google Scholar] [CrossRef]
- Jabaudon, M.; Blondonnet, R.; Roszyk, L.; Bouvier, D.; Audard, J.; Clairefond, G.; Fournier, M.; Marceau, G.; Dechelotte, P.; Pereira, B.; et al. Soluble Receptor for Advanced Glycation End-Products Predicts Impaired Alveolar Fluid Clearance in Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2015, 192, 191–199. [Google Scholar] [CrossRef]
- Papiris, S.A.; Tomos, I.P.; Karakatsani, A.; Spathis, A.; Korbila, I.; Analitis, A.; Kolilekas, L.; Kagouridis, K.; Loukides, S.; Karakitsos, P.; et al. High levels of IL-6 and IL-8 characterize early-on idiopathic pulmonary fibrosis acute exacerbations. Cytokine 2018, 102, 168–172. [Google Scholar] [CrossRef]
- Zinter, M.S.; Orwoll, B.E.; Spicer, A.C.; Alkhouli, M.F.; Calfee, C.S.; Matthay, M.A.; Sapru, A. Incorporating Inflammation into Mortality Risk in Pediatric Acute Respiratory Distress Syndrome. Crit. Care Med. 2017, 45, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Hermans, C.; Petrek, M.; Kolek, V.; Weynand, B.; Pieters, T.; Lambert, M.; Bernard, A. Serum Clara cell protein (CC16), a marker of the integrity of the air-blood barrier in sarcoidosis. Eur. Respir. J. 2001, 18, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Stenberg, H.; Wadelius, E.; Moitra, S.; Åberg, I.; Ankerst, J.; Diamant, Z.; Bjermer, L.; Tufvesson, E. Club Cell Protein (Cc16) in Plasma, Bronchial Brushes, Bal and Urine Following an Inhaled Allergen Challenge in Allergic Asthmatics. Biomarkers 2018, 23, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhang, W.; Wang, L.; Tian, F. Diagnostic and Prognostic Values of Club Cell Protein 16 (Cc16) in Critical Care Patients with Acute Respiratory Distress Syndrome. J. Clin. Lab. Anal. 2018, 32, e22262. [Google Scholar] [CrossRef] [PubMed]
- Zemans, R.L.; Jacobson, S.; Keene, J.; Kechris, K.; Miller, B.E.; Tal-Singer, R.; Bowler, R.P. Multiple biomarkers predict disease severity, progression and mortality in COPD. Respir. Res. 2017, 18, 117. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, D.J.; Mehl, R.; Izumo, S.; Nadal-Ginard, B.; Yin, H.L. Muscle is the major source of plasma gelsolin. J. Biol. Chem. 1988, 263, 8239–8243. [Google Scholar] [PubMed]
- Haddad, J.G.; Harper, K.D.; Guoth, M.; Pietra, G.G.; Sanger, J.W. Angiopathic Consequences of Saturating the Plasma Scavenger System for Actin. Proc Natl Acad Sci. USA 1990, 87, 1381–1385. [Google Scholar] [CrossRef] [PubMed]
- Epstein, F.H.; Lee, W.M.; Galbraith, R.M. The Extracellular Actin-Scavenger System and Actin Toxicity. N. Engl. J. Med. 1992, 326, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Orwoll, B.E.; Spicer, A.C.; Zinter, M.S.; Alkhouli, M.F.; Khemani, R.G.; Flori, H.R.; Neuhaus, J.M.; Calfee, C.S.; Matthay, M.A.; Sapru, A. Elevated soluble thrombomodulin is associated with organ failure and mortality in children with acute respiratory distress syndrome (ARDS): A prospective observational cohort study. Crit. Care 2015, 19, 435. [Google Scholar] [CrossRef]
- Johansen, M.; Johansson, P.I.; Ostrowski, S.R.; Bestle, M.H.; Hein, L.; Jensen, A.L.G.; Søe-Jensen, P.; Andersen, M.H.; Steensen, M.; Mohr, T.; et al. Profound Endothelial Damage Predicts Impending Organ Failure and Death in Sepsis. Semin. Thromb. Hemost. 2015, 41, 016–025. [Google Scholar] [CrossRef]
- Jensen, J.U.; Hein, L.; Lundgren, B.; Bestle, M.H.; Mohr, T.T.; Andersen, M.H.; Thornberg, K.J.; Løken, J.; Steensen, M.; Fox, Z.; et al. Procalcitonin-Guided Interventions against Infections to Increase Early Appropriate Antibiotics and Improve Survival in the Intensive Care Unit: A Randomized Trial. Crit. Care Med. 2011, 39, 2048–2058. [Google Scholar] [CrossRef] [PubMed]
- Briel, M.; Schuetz, P.; Mueller, B.; Young, J.; Schild, U.; Nusbaumer, C.; Périat, P.; Bucher, H.C.; Christ-Crain, M. Procalcitonin-Guided Antibiotic Use Vs a Standard Approach for Acute Respiratory Tract Infections in Primary Care. Arch. Intern. Med. 2008, 168, 2000–2007. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, P.; Christ-Crain, M.; Thomann, R.; Falconnier, C.; Wolbers, M.; Widmer, I.; Neidert, S.; Fricker, T.; Blum, C.; Schild, U.; et al. Effect of Procalcitonin-Based Guidelines Vs Standard Guidelines on Antibiotic Use in Lower Respiratory Tract Infections: The Prohosp Randomized Controlled Trial. JAMA 2009, 302, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Corti, C.; Fally, M.; Fabricius-Bjerre, A.; Mortensen, K.; Jensen, B.N.; Andreassen, H.F.; Porsbjerg, C.; Knudsen, J.D.; Jensen, J.-U. Point-of-care procalcitonin test to reduce antibiotic exposure in patients hospitalized with acute exacerbation of COPD. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Meier, M.A.; Branche, A.; Neeser, O.L.; Wirz, Y.; Haubitz, S.; Bouadma, L.; Wolff, M.; Luyt, C.E.; Chastre, J.; Tubach, F.; et al. Procalcitonin-Guided Antibiotic Treatment in Patients with Positive Blood Cultures: A Patient-Level Meta-Analysis of Randomized Trials. Clin. Infect. Dis. 2018, 69, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Bouadma, L.; Luyt, C.E.; Tubach, F.; Cracco, C.; Alvarez, A.; Schwebel, C.; Schortgen, F.; Lasocki, S.; Veber, B.; Dehoux, M.; et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): A multicentre randomised controlled trial. Lancet 2010, 375, 463–474. [Google Scholar] [CrossRef]
- de Jong, E.; van Oers, J.A.; Beishuizen, A.; Vos, P.; Vermeijden, W.J.; Haas, L.E.; Loef, B.G.; Dormans, T.; van Melsen, G.C.; Kluiters, Y.C.; et al. Efficacy and Safety of Procalcitonin Guidance in Reducing the Duration of Antibiotic Treatment in Critically Ill Patients: A Randomised, Controlled, Open-Label Trial. Lancet Infect. Dis. 2016, 16, 819–827. [Google Scholar] [CrossRef]
- Schuetz, P.; Wirz, Y.; Sager, R.; Christ-Crain, M.; Stolz, D.; Tamm, M.; Bouadma, L.; Luyt, C.E.; Wolff, M.; Chastre, J.; et al. Effect of Procalcitonin-Guided Antibiotic Treatment on Mortality in Acute Respiratory Infections: A Patient Level Meta-Analysis. Lancet Infect. Dis. 2018, 18, 95–107. [Google Scholar] [CrossRef]
- Siva, R.; Green, R.H.; Brightling, C.E.; Shelley, M.; Hargadon, B.; McKenna, S.; Monteiro, W.; Berry, M.; Parker, D.; Wardlaw, A.J.; et al. Eosinophilic airway inflammation and exacerbations of COPD: A randomised controlled trial. Eur. Respir. J. 2007, 29, 906–913. [Google Scholar] [CrossRef]
- Singh, D.; Kolsum, U.; Brightling, C.E.; Locantore, N.; Agusti, A.; Tal-Singer, R. Eosinophilic inflammation in COPD: Prevalence and clinical characteristics. Eur. Respir. J. 2014, 44, 1697–1700. [Google Scholar] [CrossRef]
- George, L.; Brightling, C.E. Eosinophilic Airway Inflammation: Role in Asthma and Chronic Obstructive Pulmonary Disease. Ther. Adv. Chronic. Dis. 2016, 7, 34–51. [Google Scholar] [CrossRef] [PubMed]
- De Groot, J.C.; Storm, H.; Amelink, M.; De Nijs, S.B.; Eichhorn, E.; Reitsma, B.H.; Bel, E.H.; Brinke, A.T. Clinical profile of patients with adult-onset eosinophilic asthma. ERJ Open Res. 2016, 2. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.H.; Lamb, A.; Chase, R.; Singh, D.; Parker, M.M.; Saferali, A.; Vestbo, J.; Tal-Singer, R.; Castaldi, P.J.; Silverman, E.K.; et al. Blood Eosinophil Count Thresholds and Exacerbations in Patients with Chronic Obstructive Pulmonary Disease. J. Allergy Clin. Immunol. 2018, 141, 2037–2047. [Google Scholar] [CrossRef] [PubMed]
- Vedel-Krogh, S.; Nielsen, S.F.; Lange, P.; Vestbo, J.; Nordestgaard, B.G. Blood Eosinophils and Exacerbations in Chronic Obstructive Pulmonary Disease. The Copenhagen General Population Study. Am. J. Respir. Crit. Care Med. 2016, 193, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Beeh, K.M.; Beier, J.; Kornmann, O.; Mander, A.; Buhl, R. Long-term repeatability of induced sputum cells and inflammatory markers in stable, moderately severe COPD. Chest 2003, 123, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Bafadhel, M.; McKenna, S.; Terry, S.; Mistry, V.; Pancholi, M.; Venge, P.; Lomas, D.A.; Barer, M.R.; Johnston, S.L.; Pavord, I.D.; et al. Blood Eosinophils to Direct Corticosteroid Treatment of Exacerbations of Chronic Obstructive Pulmonary Disease: A Randomized Placebo-Controlled Trial. Am. J. Respir Crit. Care Med. 2012, 186, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Sivapalan, P.; Lapperre, T.S.; Janner, J.; Laub, R.R.; Moberg, M.; Bech, C.S.; Eklöf, J.; Holm, F.S.; Armbruster, K.; Sivapalan, P.; et al. Eosinophil-Guided Corticosteroid Therapy in Patients Admitted to Hospital with Copd Exacerbation (Cortico-Cop): A Multicentre, Randomised, Controlled, Open-Label, Non-Inferiority Trial. Lancet Respir. Med. 2019, 7, 699–709. [Google Scholar] [CrossRef]
- Pavord, I.D.; Lettis, S.; Anzueto, A.; Barnes, N. Blood eosinophil count and pneumonia risk in patients with chronic obstructive pulmonary disease: A patient-level meta-analysis. Lancet Respir. Med. 2016, 4, 731–741. [Google Scholar] [CrossRef]
- Itenov, T.S.; Murray, D.D.; Jensen, J.U.S. Sepsis: Personalized Medicine Utilizing ‘Omic’ Technologies—A Paradigm Shift? Health 2018, 6, 111. [Google Scholar] [CrossRef]
- Kucuksezer, U.C.; Ozdemir, C.; Akdis, M.; Akdis, C.A. Precision/Personalized Medicine in Allergic Diseases and Asthma. Arch. Immunol. Ther. Exp. 2018, 66, 431–442. [Google Scholar] [CrossRef]
- Canonica, G.W.; Ferrando, M.; Baiardini, I.; Puggioni, F.; Racca, F.; Passalacqua, G.; Heffler, E. Passalacqua, and E. Heffler. “Asthma: Personalized and Precision Medicine. Curr. Opin. Allergy Clin. Immunol. 2018, 18, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Ogino, S.; Nishihara, R.; VanderWeele, T.J.; Wang, M.; Nishi, A.; Lochhead, P.; Qian, Z.R.; Zhang, X.; Wu, K.; Nan, H.; et al. Review Article: The Role of Molecular Pathological Epidemiology in the Study of Neoplastic and Non-Neoplastic Diseases in the Era of Precision Medicine. Epidemiology 2016, 27, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Kan, M.; Shumyatcher, M.; Himes, B.E. Using omics approaches to understand pulmonary diseases. Respir. Res. 2017, 18, 149. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.J.; Calfee, C.S. Novel translational approaches to the search for precision therapies for acute respiratory distress syndrome. Lancet Respir. Med. 2017, 5, 512–523. [Google Scholar] [CrossRef]
- Rogers, A.J.; Matthay, M.A. Applying metabolomics to uncover novel biology in ARDS. Am. J. Physiol. Cell. Mol. Physiol. 2014, 306, L957–L961. [Google Scholar] [CrossRef] [PubMed]
- Bime, C.; Pouladi, N.; Sammani, S.; Batai, K.; Casanova, N.; Zhou, T.; Kempf, C.L.; Sun, X.; Camp, S.M.; Wang, T.; et al. Genome-Wide Association Study in African Americans with Acute Respiratory Distress Syndrome Identifies the Selectin P Ligand Gene as a Risk Factor. Am. J. Respir Crit. Care Med. 2018, 197, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, T.E.; Azad, T.D.; Donato, M.; Haynes, W.A.; Perumal, T.M.; Henao, R.; Bermejo-Martin, J.F.; Almansa, R.; Tamayo, E.; Howrylak, J.A.; et al. Unsupervised Analysis of Transcriptomics in Bacterial Sepsis across Multiple Datasets Reveals Three Robust Clusters. Crit. Care Med. 2018, 46, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Scicluna, B.P.; Van Vught, L.A.; Zwinderman, A.H.; Wiewel, M.A.; Davenport, E.E.; Burnham, K.L.; Nürnberg, P.; Schultz, M.J.; Horn, J.; Cremer, O.L.; et al. Classification of patients with sepsis according to blood genomic endotype: A prospective cohort study. Lancet Respir. Med. 2017, 5, 816–826. [Google Scholar] [CrossRef]
- Wong, H.R.; Cvijanovich, N.; Lin, R.; Allen, G.L.; Thomas, N.J.; Willson, D.F.; Freishtat, R.J.; Anas, N.; Meyer, K.; Checchia, P.A.; et al. Identification of Pediatric Septic Shock Subclasses Based on Genome-Wide Expression Profiling. BMC Med. 2009, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Viswan, A.; Ghosh, P.; Gupta, D.; Azim, A.; Sinha, N. Distinct Metabolic Endotype Mirroring Acute Respiratory Distress Syndrome (ARDS) Subphenotype and its Heterogeneous Biology. Sci. Rep. 2019, 9, 2108. [Google Scholar] [CrossRef] [PubMed]
- Viswan, A.; Singh, C.; Rai, R.K.; Azim, A.; Sinha, N.; Baronia, A.K. Metabolomics based predictive biomarker model of ARDS: A systemic measure of clinical hypoxemia. PLoS ONE 2017, 12, e0187545. [Google Scholar] [CrossRef] [PubMed]
- Brazma, A.; Hingamp, P.; Quackenbush, J.; Sherlock, G.; Spellman, P.; Stoeckert, C.; Aach, J.; Ansorge, W.; Ball, C.A.; Causton, H.C.; et al. Minimum Information About a Microarray Experiment (Miame)-toward Standards for Microarray Data. Nat. Genet. 2001, 29, 365. [Google Scholar] [CrossRef] [PubMed]
- Sansone, S.A.; Fan, T.; Goodacre, R.; Griffin, J.L.; Hardy, N.W.; Kaddurah-Daouk, R.; Kristal, B.S.; Lindon, J.; Mendes, P.; Morrison, N.; et al. The Metabolomics Standards Initiative. Nat. Biotechnol. 2007, 25, 846. [Google Scholar] [PubMed]
- Orchard, S.; Salwinski, L.; Kerrien, S.; Montecchi-Palazzi, L.; Oesterheld, M.; Stümpflen, V.; Ceol, A.; Chatr-Aryamontri, A.; Armstrong, J.; Woollard, P.; et al. The Minimum Information Required for Reporting a Molecular Interaction Experiment (Mimix). Nat. Biotechnol. 2007, 25, 894. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.F.; Paton, N.W.; Lilley, K.S.; Binz, P.A.; Julian, R.K., Jr.; Jones, A.R.; Zhu, W.; Apweiler, R.; Aebersold, R.; Deutsch, E.W.; et al. The Minimum Information about a Proteomics Experiment (Miape). Nat. Biotechnol. 2007, 25, 887. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, E.W.; Ball, C.A.; Berman, J.J.; Bova, G.S.; Brazma, A.; Bumgarner, R.E.; Campbell, D.; Causton, H.C.; Christiansen, J.H.; Daian, F.; et al. Minimum Information Specification for in Situ Hybridization and Immunohistochemistry Experiments (Misfishie). Nat. Biotechnol. 2008, 26, 305. [Google Scholar] [CrossRef] [PubMed]
- Field, D.; Garrity, G.; Gray, T.; Morrison, N.; Selengut, J.; Sterk, P.; Tatusova, T.; Thomson, N.; Allen, M.J.; Angiuoli, S.V.; et al. The Minimum Information about a Genome Sequence (Migs) Specification. Nat. Biotechnol. 2008, 26, 541–547. [Google Scholar] [CrossRef]
- Thomas, T.; Gilbert, J.; Meyer, F. Metagenomics—A guide from sampling to data analysis. Microb. Inform. Exp. 2012, 2, 3. [Google Scholar] [CrossRef]
- Calfee, C.S.; Delucchi, K.; Parsons, P.E.; Thompson, B.T.; Ware, L.B.; Matthay, M.A.; Nhlbi Ards Network. Subphenotypes in Acute Respiratory Distress Syndrome: Latent Class Analysis of Data from Two Randomised Controlled Trials. Lancet Respir. Med. 2014, 2, 611–620. [Google Scholar] [CrossRef]

| Pathophysiological Entity for Biomarker | Biomarker | Established and Validated | Clinical Use Potential | Implemented Broadly | Included in This Review |
|---|---|---|---|---|---|
| Alveolar damage (Pneumocytes type I and II) | SPD | Yes | Risk stratification in mechanically ventilated patients | No | Yes |
| s-RAGE | (yes) | ? | No | Yes | |
| KL-6 | (yes) | ? | No | Yes | |
| FGF-7 | No | No | No | No | |
| Airway (conductive) damage | CC16 | Yes | Possibly not in acute lung injury | No | Yes |
| Endothelial | VEGF | Yes | + | No | No |
| Gelsolin | (yes) * | ? | No | Yes | |
| sTM | (yes) | - | No | No | |
| Syndecan-1 | No | - | No | No | |
| Inflammation/Infection | PCT | Yes | Antibiotic reduction | Yes | Yes |
| Eosinophilic granulocyte | Yes | Reduction of corticosteroid use | Yes | Yes | |
| IL-1β | Yes | No | No | No | |
| TNFα | Yes | No | No | No | |
| Mitochondrial DNA | No | Yes—possibly | No | No |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murray, D.D.; Itenov, T.S.; Sivapalan, P.; Eklöf, J.V.; Holm, F.S.; Schuetz, P.; Jensen, J.U. Biomarkers of Acute Lung Injury The Individualized Approach: for Phenotyping, Risk Stratification and Treatment Surveillance. J. Clin. Med. 2019, 8, 1163. https://doi.org/10.3390/jcm8081163
Murray DD, Itenov TS, Sivapalan P, Eklöf JV, Holm FS, Schuetz P, Jensen JU. Biomarkers of Acute Lung Injury The Individualized Approach: for Phenotyping, Risk Stratification and Treatment Surveillance. Journal of Clinical Medicine. 2019; 8(8):1163. https://doi.org/10.3390/jcm8081163
Chicago/Turabian StyleMurray, Daniel D., Theis Skovsgaard Itenov, Pradeesh Sivapalan, Josefin Viktoria Eklöf, Freja Stæhr Holm, Philipp Schuetz, and Jens Ulrik Jensen. 2019. "Biomarkers of Acute Lung Injury The Individualized Approach: for Phenotyping, Risk Stratification and Treatment Surveillance" Journal of Clinical Medicine 8, no. 8: 1163. https://doi.org/10.3390/jcm8081163
APA StyleMurray, D. D., Itenov, T. S., Sivapalan, P., Eklöf, J. V., Holm, F. S., Schuetz, P., & Jensen, J. U. (2019). Biomarkers of Acute Lung Injury The Individualized Approach: for Phenotyping, Risk Stratification and Treatment Surveillance. Journal of Clinical Medicine, 8(8), 1163. https://doi.org/10.3390/jcm8081163







