Reliability of Instantaneous Wave-Free Ratio (iFR) for the Evaluation of Left Main Coronary Artery Lesions
Abstract
1. Introduction
2. Methods
2.1. Patient Population
2.2. Coronary Angiography and Intracoronary Pressure Measurement
2.3. Statistical Analysis
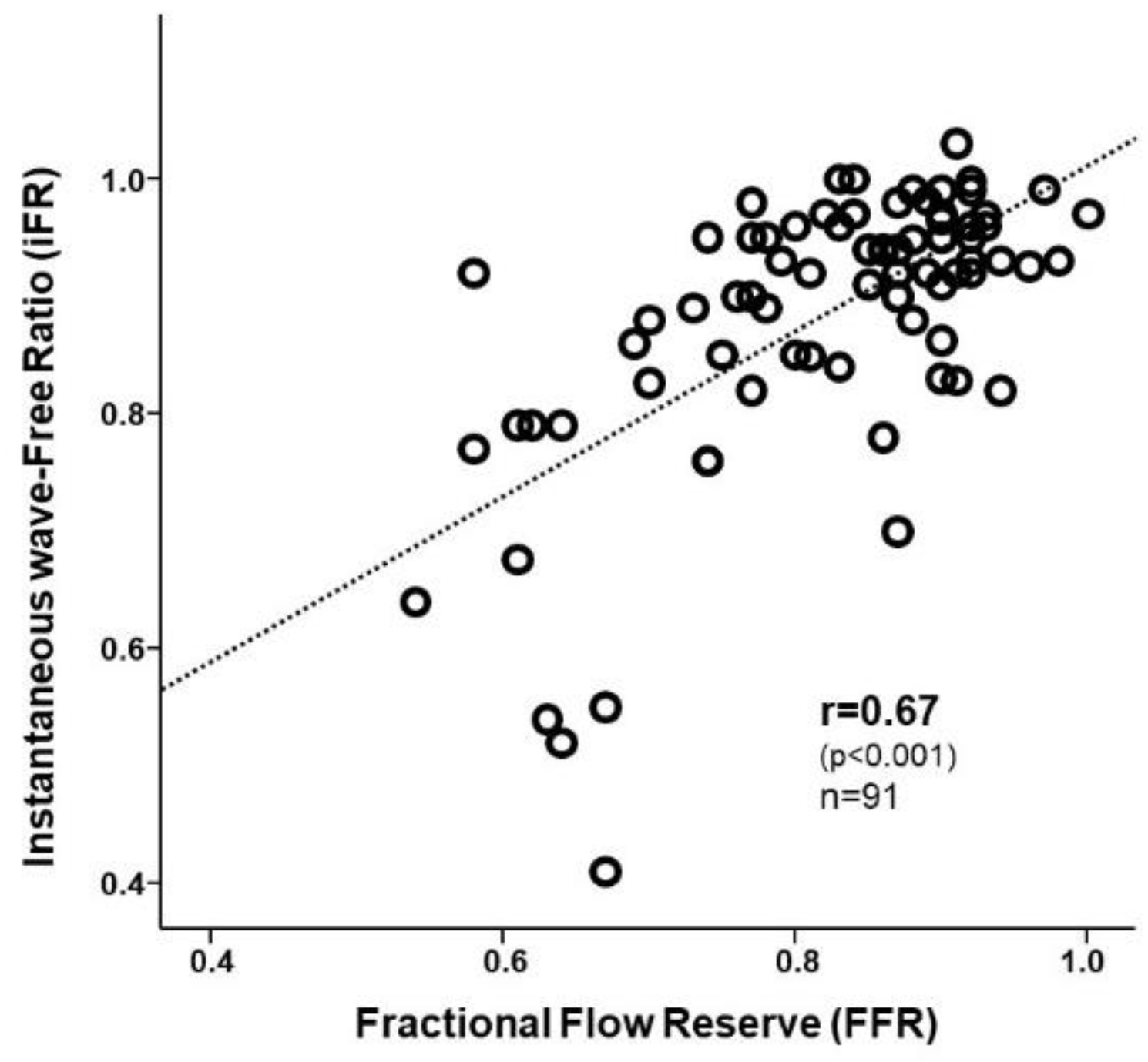
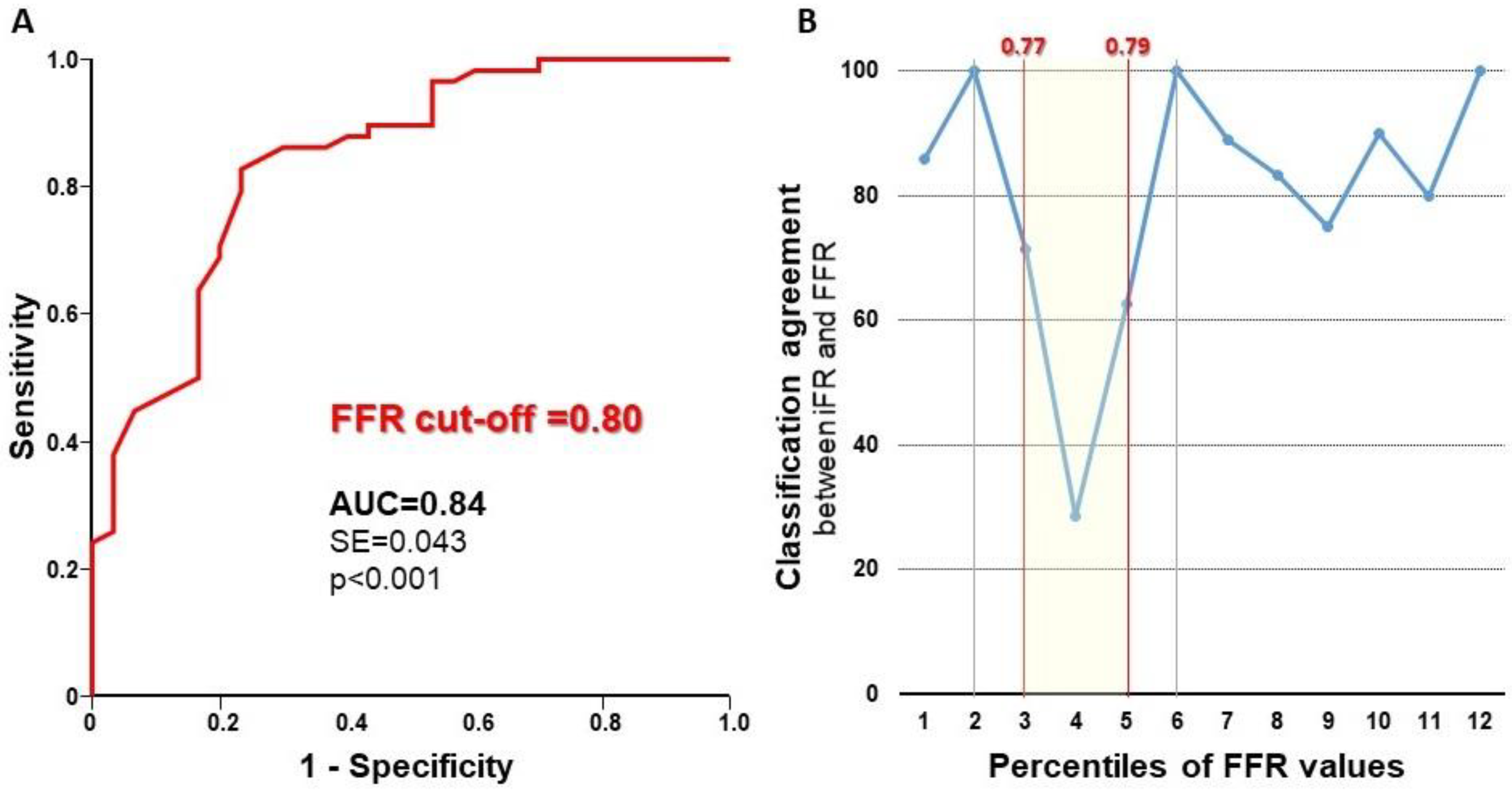
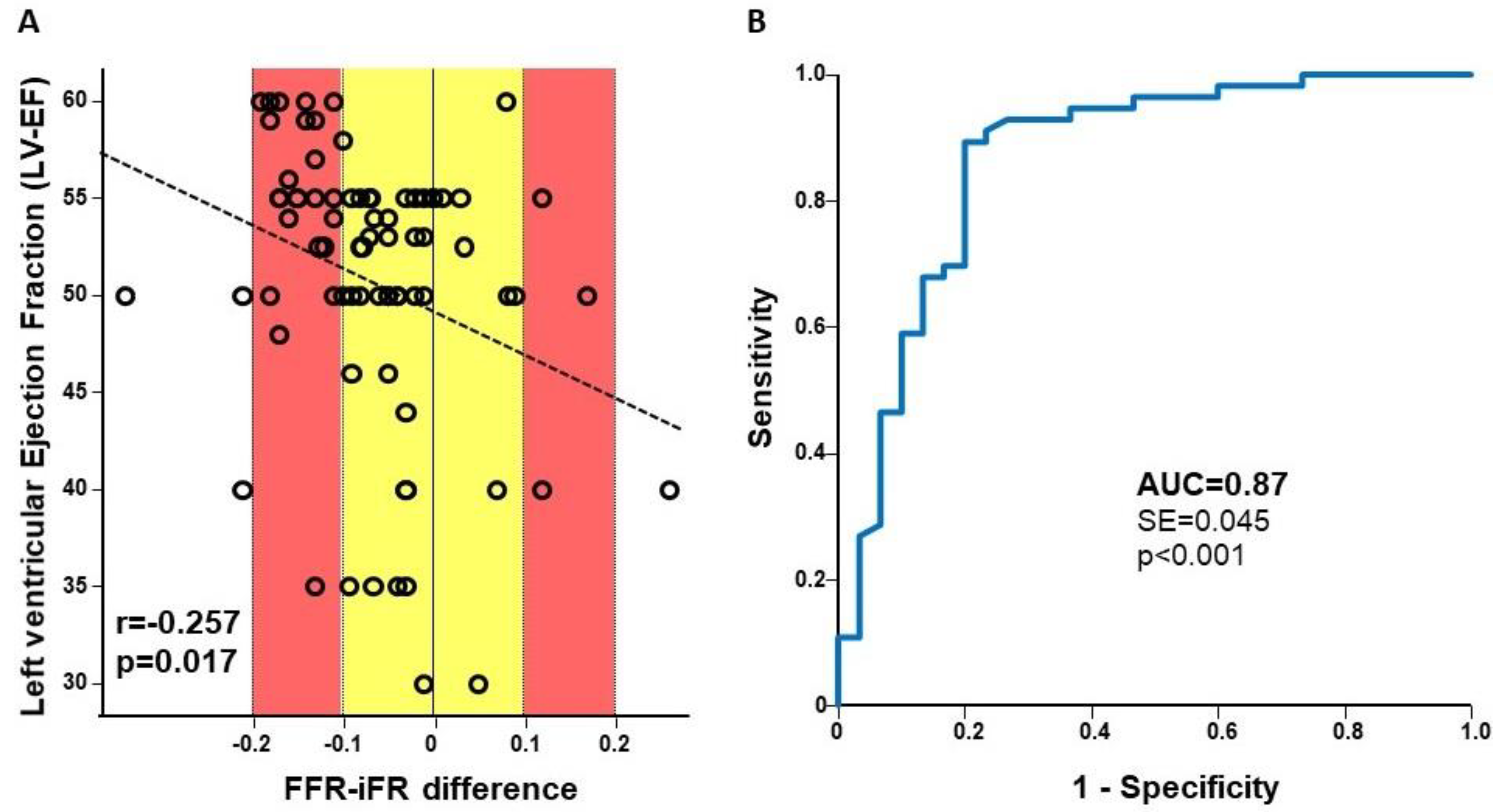
3. Results
3.1. Baseline Characteristics
3.2. Diagnostic Characteristics
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Topol, E.J.; Nissen, S.E. Our preoccupation with coronary luminology. The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation 1995, 92, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
- Marcus, M.L.; Skorton, D.J.; Johnson, M.R.; Collins, S.M.; Harrison, D.G.; Kerber, R.E. Visual estimation of precent diameter coronary stenosis: “A battered gold standard”. J. Am. Coll. Cardiol. 1988, 11, 882–885. [Google Scholar] [CrossRef]
- Leesar, M.A.; Mintz, G.S. Hemodynamic and intravascular ultrasound assessment of an ambiguous left main coronary artery stenosis. Catheter. Cardiovasc. Interv. 2007, 70, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Abizaid, A.S.; Mintz, G.S.; Abizaid, A.; Mehran, R.; Lansky, A.J.; Pichard, A.D.; Satler, L.F.; Wu, H.; Kent, K.M.; Leon, M.B. One-year follow-up after intravascular ultrasound assessment of moderate left main coronary artery disease in patients with ambiguous angiograms. J. Am. Coll. Cardiol. 1999, 34, 707–715. [Google Scholar] [CrossRef]
- Cameron, A.; Kemp, H.G.; Fisher, L.D.; Gosselin, A.; Judkins, M.P.; Kennedy, J.W.; Lesperance, J.; Mudd, J.G.; Ryan, T.J.; Silverman, J.F.; et al. Left main coronary artery stenosis: Angiographic determination. Circulation 1983, 68, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chen, T.; Ecabert, O.; Prummer, S.; Ostermeier, M.; Comaniciu, D. Image-based device tracking for the co-registration of angiography and intravascular ultrasound images. Med. Image Comput. Comput. Assist. Interv. 2011, 14, 161–168. [Google Scholar]
- De Bruyne, B.; Pijls, N.H.; Kalesan, B.; Barbato, E.; Tonino, P.A.; Piroth, Z.; Jagic, N.; Möbius-Winkler, S.; Rioufol, G.; Witt, N.; et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N. Engl. J. Med. 2012, 367, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Bech, G.J.; De Bruyne, B.; Pijls, N.H.; de Muinck, E.D.; Hoorntje, J.C.; Escaned, J.; Stella, P.R.; Boersma, E.; Bartunek, J.; Koolen, J.J.; et al. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: A randomized trial. Circulation 2001, 103, 2928–2934. [Google Scholar] [CrossRef]
- De Bruyne, B. FAME II: FFR Pinpoints Stable CAD Patients who Fare Worse with OMT. Available online: http://theheart.org/article/1398627.do (accessed on 18 June 2019).
- Bech, G.J.; Droste, H.; Pijls, N.H.; De Bruyne, B.; Bonnier, J.J.; Michels, H.R.; Peels, K.H.; Koolen, J.J. Value of fractional flow reserve in making decisions about bypass surgery for equivocal left main coronary artery disease. Heart 2001, 86, 547–552. [Google Scholar] [CrossRef]
- Jiménez-Navarro, M.; Hernández-García, J.M.; Alonso-Briales, J.H.; Kühlmorgen, B.; Gómez-Doblas, J.J.; García-Pinilla, J.M.; López-Salguero, R.; Galván Ede, T. Should we treat patients with moderately severe stenosis of the left main coronary artery and negative FFR results? J. Invasive Cardiol. 2004, 16, 398–400. [Google Scholar]
- Jasti, V.; Ivan, E.; Yalamanchili, V.; Wongpraparut, N.; Leesar, M.A. Correlations between fractional flow reserve and intravascular ultrasound in patients with an ambiguous left main coronary artery stenosis. Circulation 2004, 110, 2831–2836. [Google Scholar] [CrossRef] [PubMed]
- Suemaru, S.; Iwasaki, K.; Yamamoto, K.; Kusachi, S.; Hina, K.; Hirohata, S.; Hirota, M.; Murakami, M.; Kamikawa, S.; Murakami, T.; et al. Coronary pressure measurement to determine treatment strategy for equivocal left main coronary artery lesions. Heart Vessels 2005, 20, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Legutko, J.; Dudek, D.; Rzeszutko, L.; Wizimirski, M.; Dubiel, J.S. Fractional flow reserve assessment to determine the indications for myocardial revascularisation in patients with borderline stenosis of the left main coronary artery. Kardiol. Pol. 2005, 63, 499–506. [Google Scholar] [PubMed]
- Lindstaedt, M.; Yazar, A.; Germing, A.; Fritz, M.K.; Holland-Letz, T.; Mügge, A.; Bojara, W. Clinical outcome in patients with intermediate or equivocal left main coronary artery disease after deferral of surgical revascularization on the basis of fractional flow reserve measurements. Am. Heart J. 2006, 152, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Courtis, J.; Rodés-Cabau, J.; Larose, E.; Potvin, J.M.; Déry, J.P.; Larochellière, R.D.; Côté, M.; Cousterousse, O.; Nguyen, C.M.; Proulx, G.; et al. Usefulness of coronary fractional flow reserve measurements in guiding clinical decisions in intermediate or equivocal left main coronary stenosis. Am. J. Cardiol. 2009, 103, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Hamilos, M.; Muller, O.; Cuisset, T.; Ntalianis, A.; Chlouverakis, G.; Sarno, G.; Nelis, O.; Bartunek, J.; Vanderheyden, M.; Wyffels, E.; et al. Long-term clinical outcome after fractional flow reserve-guided treatment in patients with angiographically equivocal left main coronary artery stenosis. Circulation 2009, 120, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Escaned, J.; Malik, I.S.; Mikhail, G.W.; Foale, R.A.; Mila, R.; Tarkin, J.; Petraco, R.; Broyd, C.; Jabbour, R.; et al. Development and validation of a new adenosine-independent index of stenosis severity from coronary wave-intensity analysis results of the ADVISE (ADenosine Vasodilator Independent Stenosis Evaluation) study. J. Am. Coll. Cardiol. 2012, 59, 1392–1402. [Google Scholar] [CrossRef]
- Indolfi, C.; Mongiardo, A.; Spaccarotella, C.; Torella, D.; Caiazzo, G.; Polimeni, A.; Sorrentino, S.; Micieli, M.; Sabatino, J.; Curcio, A.; et al. The instantaneous wave-free ratio (iFR) for evaluation of non-culprit lesions in patients with acute coronary syndrome and multivessel disease. Int. J. Cardiol. 2015, 178, 46–54. [Google Scholar] [CrossRef]
- Davies, J.E.; Sen, S.; Dehbi, H.M.; Al-Lamee, R.; Petraco, R.; Nijjer, S.S.; Bhindi, R.; Lehman, S.J.; Walters, D.; Sapontis, J.; et al. Use of the instantaneous wave-free ratio or fractional flow reserve in PCI. N. Engl. J. Med. 2017, 376, 1824–1834. [Google Scholar] [CrossRef]
- Götberg, M.; Christiansen, E.H.; Gudmundsdottir, I.J.; Sandhall, L.; Danielewicz, M.; Jakobsen, L.; Olsson, S.E.; Öhagen, P.; Olsson, H.; Omerovic, E.; et al. Instantaneous wave-free ratio versus fractional flow reserve to guide PCI. N. Engl. J. Med. 2017, 376, 1813–1823. [Google Scholar]
- De Rosa, S.; Polimeni, A.; Petraco, R.; Davies, J.E.; Indolfi, C. Diagnostic performance of the instantaneous wave-free ratio: Comparison with fractional flow reserve. Circ. Cardiovasc. Interv. 2018, 11, e004613. [Google Scholar] [CrossRef] [PubMed]
- Berry, C.; van ’t Veer, M.; Witt, N.; Kala, P.; Bocek, O.; Pyxaras, S.A.; McClure, J.D.; Fearon, W.F.; Barbato, E.; Tonino, P.A.; et al. VERIFY (VERification of instantaneous wave-free ratio and fractional flow reserve for the assessment of coronary artery stenosis severity in everyday practice): A multicenter study in consecutive patients. J. Am. Coll. Cardiol. 2013, 61, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Jeremias, A.; Maehara, A.; Généreux, P.; Asrress, K.N.; Berry, C.; De Bruyne, B.; Davies, J.E.; Escaned, J.; Fearon, W.F.; Gould, K.L.; et al. Multicenter core laboratory comparison of the instantaneous wave-free ratio and resting Pd/Pa with fractional flow reserve: The RESOLVE study. J. Am. Coll. Cardiol. 2014, 63, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Petraco, R.; Dehbi, H.M.; Howard, J.P.; Shun-Shin, M.J.; Sen, S.; Nijjer, S.S.; Mayet, J.; Davies, J.E.; Francis, D.P. Effects of disease severity distribution on the performance of quantitative diagnostic methods and proposal of a novel ‘V-plot’ methodology to display accuracy values. Open Heart 2018, 5, e000663. [Google Scholar] [CrossRef] [PubMed]
- Fearon, W.F.; Yong, A.S.; Lenders, G.; Toth, G.G.; Dao, C.; Daniels, D.V.; Pijls, N.H.J.; De Bruyne, B. The impact of downstream coronary stenosis on fractional flow reserve assessment of intermediate left main coronary artery disease: Human validation. JACC Cardiovasc. Interv. 2015, 8, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Tonino, P.A.; De Bruyne, B.; Pijls, N.H.; Siebert, U.; Ikeno, F.; van’ t Veer, M.; Klauss, V.; Manoharan, G.; Engstrøm, T.; Oldroyd, K.G.; et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N. Engl. J. Med. 2009, 360, 213–224. [Google Scholar] [CrossRef]
- Pijls, N.H.; Van Gelder, B.; Van der Voort, P.; Peels, K.; Bracke, F.A.; Bonnier, H.J.; el Gamal, M.I. Fractional flow reserve. A useful index to evaluate the influence of an epicardial coronary stenosis on myocardial blood flow. Circulation 1995, 92, 3183–3193. [Google Scholar] [CrossRef]
- De Rosa, S.; Polimeni, A.; Sabatino, J.; Indolfi, C. Long-term outcomes of coronary artery bypass grafting versus stent-PCI for unprotected left main disease: A meta-analysis. BMC Cardiovasc. Disord. 2017, 17, 240. [Google Scholar] [CrossRef]
- Escaned, J.; Collet, C.; Ryan, N.; De Maria, G.L.; Walsh, S.; Sabate, M.; Davies, J.; Lesiak, M.; Moreno, R.; Cruz-Gonzalez, I.; et al. Clinical outcomes of state-of-the-art percutaneous coronary revascularization in patients with de novo three vessel disease: 1-year results of the SYNTAX II study. Eur. Heart J. 2017, 38, 3124–3134. [Google Scholar] [CrossRef]
- Yong, A.S.; Daniels, D.; De Bruyne, B.; Kim, H.S.; Ikeno, F.; Lyons, J.; Pijls, N.H.; Fearon, W.F. Fractional flow reserve assessment of left main stenosis in the presence of downstream coronary stenoses. Circ. Cardiovasc Interv. 2013, 6, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Kikuta, Y.; Cook, C.M.; Sharp, A.S.P.; Salinas, P.; Kawase, Y.; Shiono, Y.; Giavarini, A.; Nakayama, M.; De Rosa, S.; Sen, S.; et al. Pre-angioplasty instantaneous wave-free ratio pullback predicts hemodynamic outcome in humans with coronary artery disease: Primary results of the international multicenter iFR GRADIENT registry. JACC Cardiovasc. Interv. 2018, 11, 757–767. [Google Scholar] [CrossRef] [PubMed]

| Clinical Profile | |
| Age (years) | 65.7 ± 9.6 |
| Sex (male/female) | 67/13 |
| Heart rate (bpm) | 73.3 ± 13.3 |
| BP (systolic, mmHg) | 129.0 ± 29.0 |
| BP (diastolic, mmHg) | 77.1 ± 18.5 |
| LV-EF (%) | 50.5 ± 7.4 |
| Hypertension | 84.6% |
| Smoker | 29.5% |
| Diabetes | 23.1% |
| Hypercholesterolemia | 44.9% |
| Pharmacological Therapy | |
| ASA | 100% |
| DAPT | 97% |
| ACE-I | 79% |
| ARB | 10% |
| CCB | 11% |
| βB | 93% |
| OHA | 10% |
| Promethazine | 0% |
| Ondasetron | 0% |
| Clinical Indication | |
| Stable Angina | 38 (47.5%) |
| Unstable Angina/NSTEMI | 35 (43.7%) |
| STEMI | 7 (8.8%) |
| Lesion Location | |
| Ostial LMCA | 17(18.7%) |
| LMCA shaft | 10 (11%) |
| LMCA bifurcation | 64 (70.3%) |
| Pressure Wire Positioning | |
| LAD | 68 (74.7%) |
| LCx | 22 (24.2%) |
| RIM | 1 (1.1%) |
| Predictor Variable | Wald Coefficient | p Value |
|---|---|---|
| Hypertension | 0.686 | 0.407 |
| Diabetes | 0.372 | 0.220 |
| LVEF | 4.216 | 0.040 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Rosa, S.; Polimeni, A.; De Velli, G.; Conte, M.; Sorrentino, S.; Spaccarotella, C.; Mongiardo, A.; Sabatino, J.; Contarini, M.; Todaro, D.; et al. Reliability of Instantaneous Wave-Free Ratio (iFR) for the Evaluation of Left Main Coronary Artery Lesions. J. Clin. Med. 2019, 8, 1143. https://doi.org/10.3390/jcm8081143
De Rosa S, Polimeni A, De Velli G, Conte M, Sorrentino S, Spaccarotella C, Mongiardo A, Sabatino J, Contarini M, Todaro D, et al. Reliability of Instantaneous Wave-Free Ratio (iFR) for the Evaluation of Left Main Coronary Artery Lesions. Journal of Clinical Medicine. 2019; 8(8):1143. https://doi.org/10.3390/jcm8081143
Chicago/Turabian StyleDe Rosa, Salvatore, Alberto Polimeni, Giovanni De Velli, Micaela Conte, Sabato Sorrentino, Carmen Spaccarotella, Annalisa Mongiardo, Jolanda Sabatino, Marco Contarini, Daniel Todaro, and et al. 2019. "Reliability of Instantaneous Wave-Free Ratio (iFR) for the Evaluation of Left Main Coronary Artery Lesions" Journal of Clinical Medicine 8, no. 8: 1143. https://doi.org/10.3390/jcm8081143
APA StyleDe Rosa, S., Polimeni, A., De Velli, G., Conte, M., Sorrentino, S., Spaccarotella, C., Mongiardo, A., Sabatino, J., Contarini, M., Todaro, D., & Indolfi, C. (2019). Reliability of Instantaneous Wave-Free Ratio (iFR) for the Evaluation of Left Main Coronary Artery Lesions. Journal of Clinical Medicine, 8(8), 1143. https://doi.org/10.3390/jcm8081143







