Low Vitamin K Status Is Associated with Increased Elastin Degradation in Chronic Obstructive Pulmonary Disease
Abstract
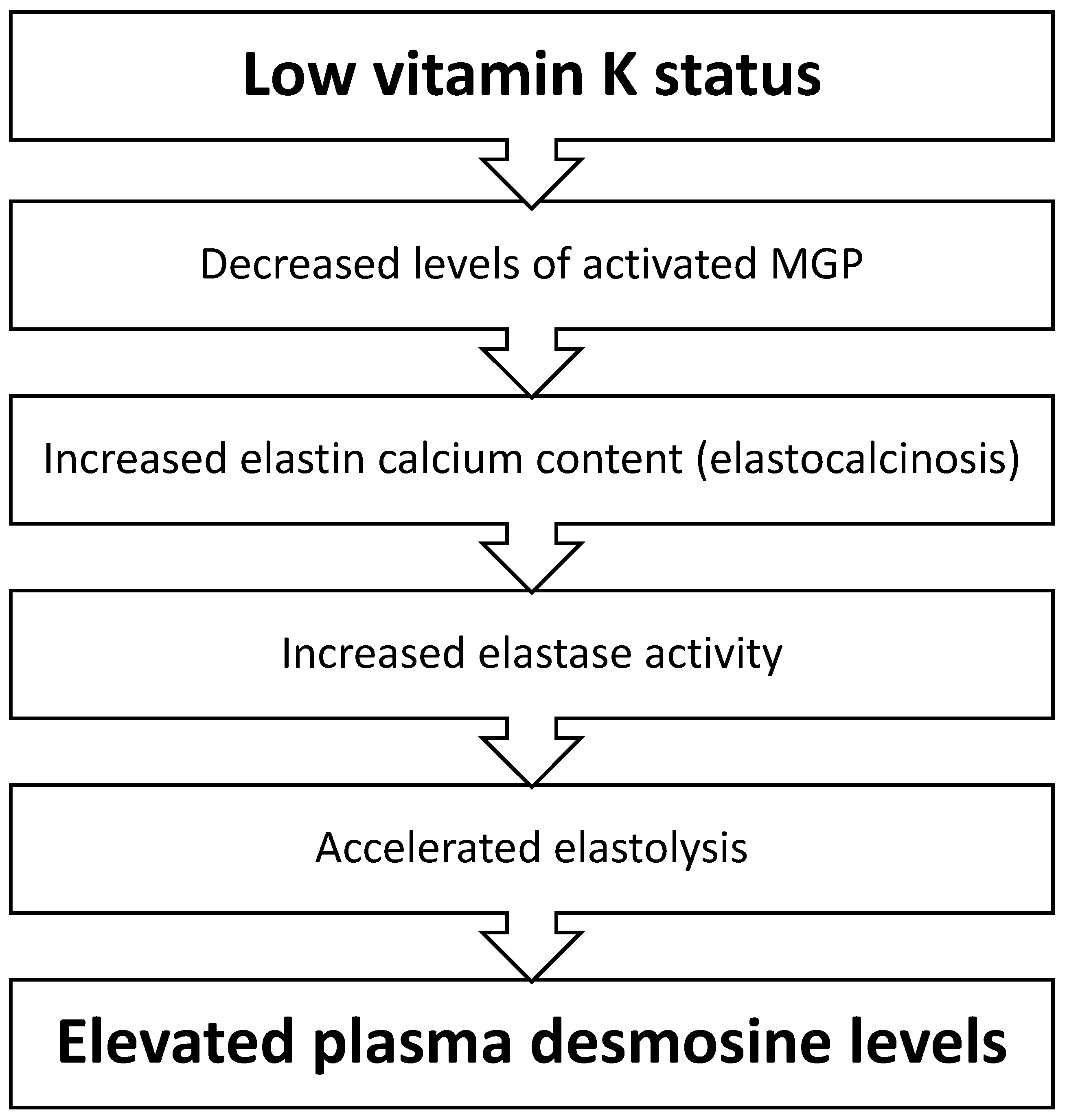
1. Introduction
2. Experimental Section
2.1. Study Population and Design
2.2. Assessments and Follow-up
2.3. Quantification of Vitamin K Status
2.4. Quantification of Elastin Degradation
2.5. Statistical Analysis
3. Results
3.1. ICE-Age Cohort
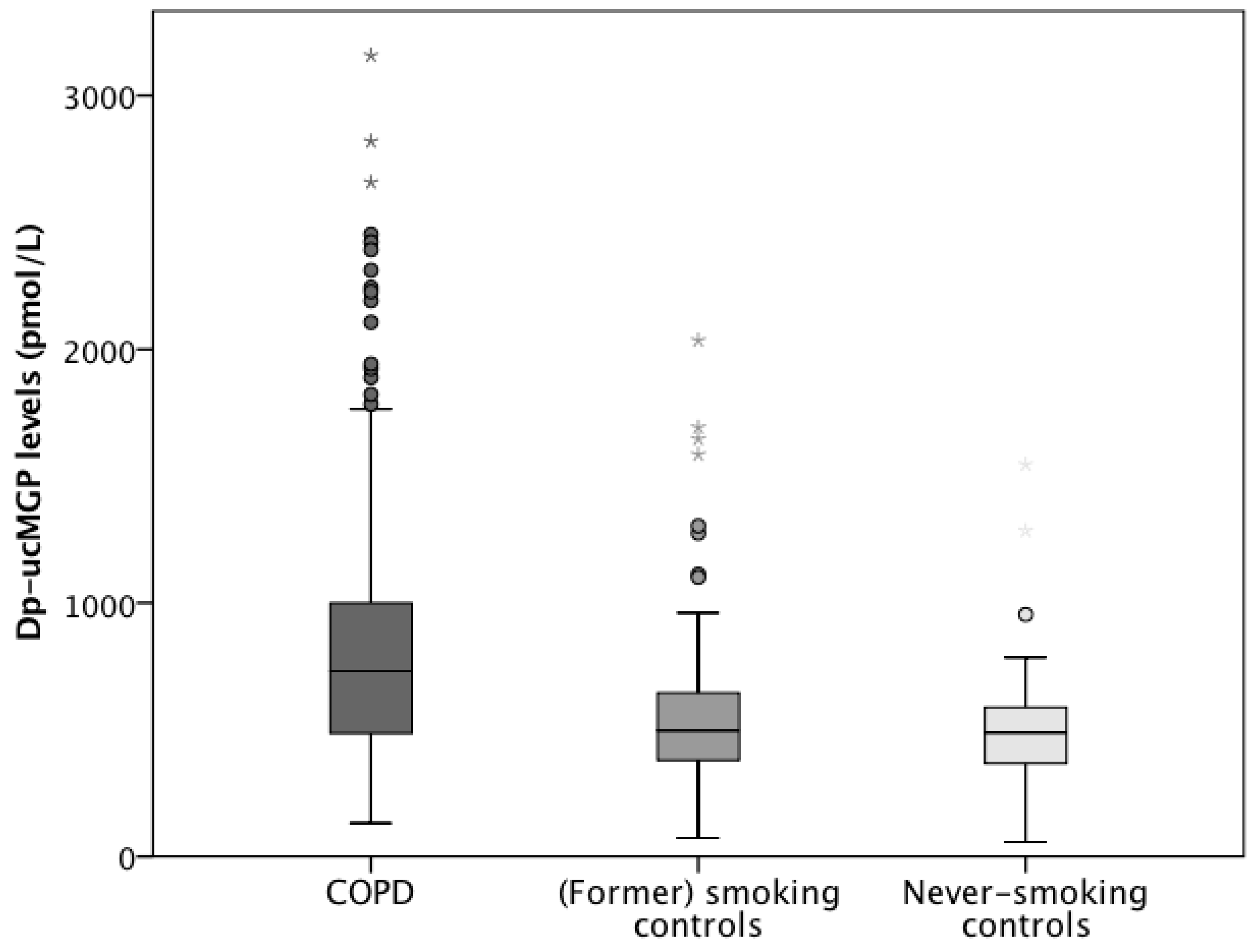
3.1.1. Vitamin K Status in the ICE-Age Cohort
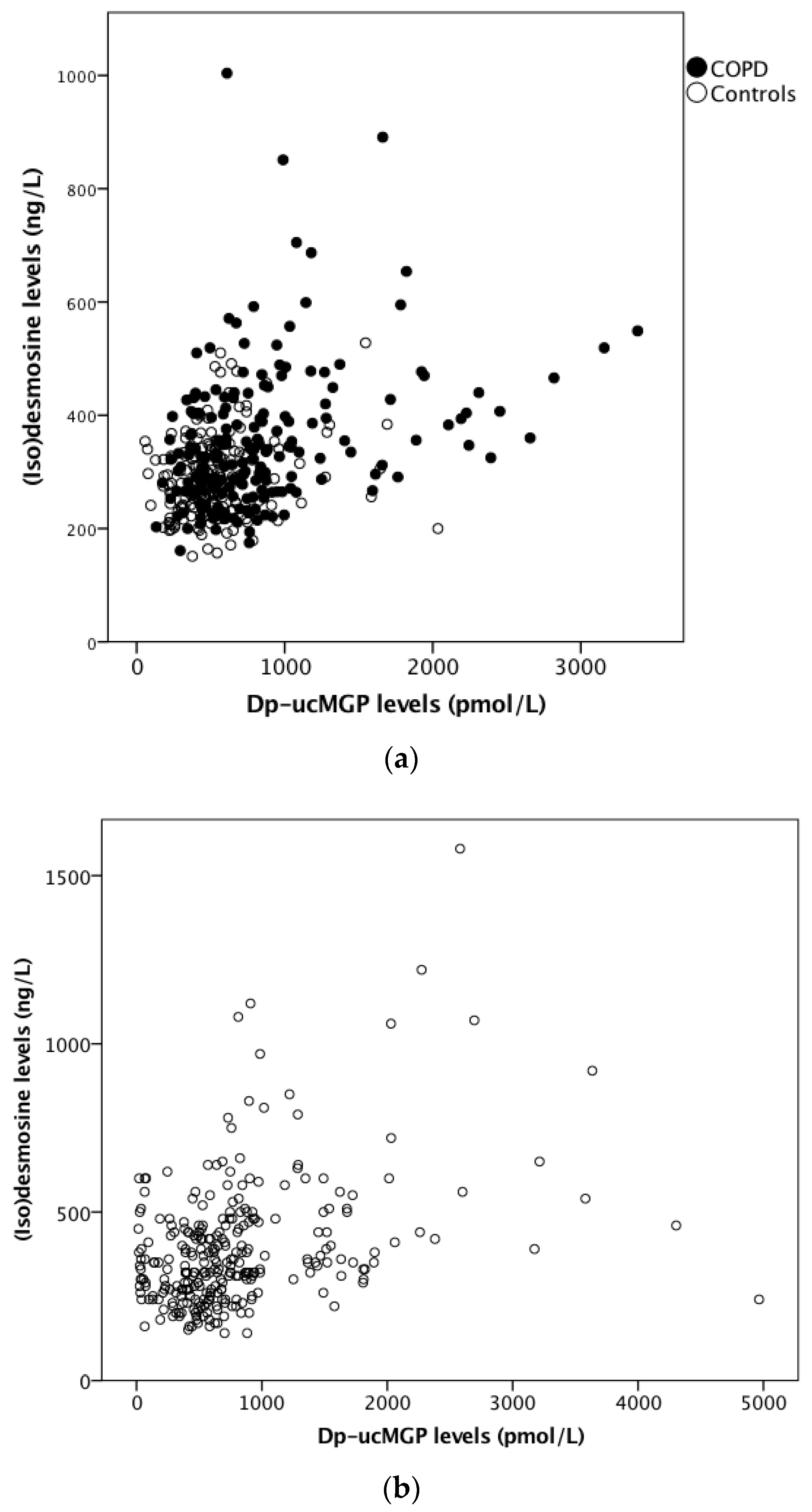
3.1.2. Elastin Degradation in the ICE-Age Cohort
3.2. Leuven COPD Cohort
3.2.1. Vitamin K Status in the Leuven COPD Cohort
3.2.2. Elastin Degradation in the Leuven COPD Cohort
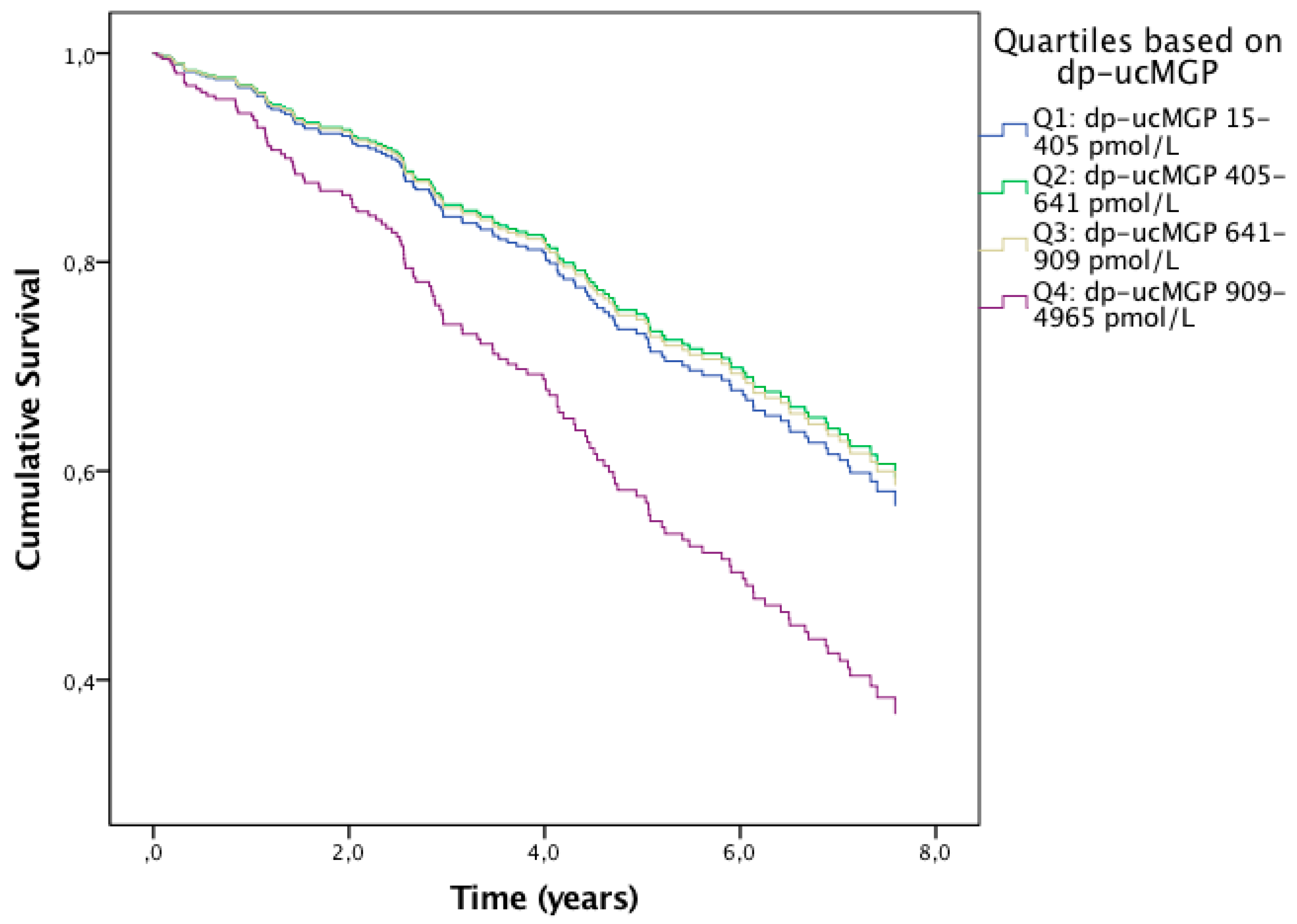
3.2.3. Effects of Vitamin K Status on Mortality in the Leuven COPD Cohort
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2017, 195, 557–582. [Google Scholar] [CrossRef] [PubMed]
- Caram, L.M.; Amaral, R.A.; Ferrari, R.; Tanni, S.E.; Correa, C.R.; Paiva, S.A.; Godoy, I. Serum Vitamin A and Inflammatory Markers in Individuals with and without Chronic Obstructive Pulmonary Disease. Mediat. Inflamm. 2015, 2015, 862086. [Google Scholar] [CrossRef] [PubMed]
- Lehouck, A.; Mathieu, C.; Carremans, C.; Baeke, F.; Verhaegen, J.; Van Eldere, J.; Decallonne, B.; Bouillon, R.; Decramer, M.; Janssens, W. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: A randomized trial. Ann. Intern. Med. 2012, 156, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; James, W.Y.; Hooper, R.L.; Barnes, N.C.; Jolliffe, D.A.; Greiller, C.L.; Islam, K.; McLaughlin, D.; Bhowmik, A.; Timms, P.M.; et al. Vitamin D3 supplementation in patients with chronic obstructive pulmonary disease (ViDiCO): A multicentre, double-blind, randomised controlled trial. Lancet Respir. Med. 2015, 3, 120–130. [Google Scholar] [CrossRef]
- Gröber, U.; Reichrath, J.; Holick, M.F.; Kisters, K. Vitamin K: An old vitamin in a new perspective. Dermatoendocrinology 2015, 6, e968490. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Uitto, J.; Reutelingsperger, C.P. Vitamin K-dependent carboxylation of matrix Gla-protein: A crucial switch to control ectopic mineralization. Trends Mol. Med. 2013, 19, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Price, P.A.; Toroian, D.; Lim, J.E. Mineralization by inhibitor exclusion: The calcification of collagen with fetuin. J. Biol. Chem. 2009, 284, 17092–17101. [Google Scholar] [CrossRef]
- Basalyga, D.M.; Simionescu, D.T.; Xiong, W.; Baxter, B.T.; Starcher, B.C.; Vyavahare, N.R. Elastin degradation and calcification in an abdominal aorta injury model: Role of matrix metalloproteinases. Circulation 2004, 110, 3480–3487. [Google Scholar] [CrossRef]
- Bouvet, C.; Moreau, S.; Blanchette, J.; de Blois, D.; Moreau, P. Sequential activation of matrix metalloproteinase 9 and transforming growth factor beta in arterial elastocalcinosis. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 856–862. [Google Scholar] [CrossRef]
- Lee, J.S.; Basalyga, D.M.; Simionescu, A.; Isenburg, J.C.; Simionescu, D.T.; Vyavahare, N.R. Elastin calcification in the rat subdermal model is accompanied by up-regulation of degradative and osteogenic cellular responses. Am. J. Pathol. 2006, 168, 490–498. [Google Scholar] [CrossRef]
- Fraser, J.D.; Price, P.A. Lung, heart, and kidney express high levels of mRNA for the vitamin K-dependent matrix Gla protein. Implications for the possible functions of matrix Gla protein and for the tissue distribution of the gamma-carboxylase. J. Biol. Chem. 1988, 263, 11033–11036. [Google Scholar] [PubMed]
- Mithieux, S.M.; Weiss, A.S. Elastin. Adv. Protein Chem. 2005, 70, 437–461. [Google Scholar] [PubMed]
- Rabinovich, R.A.; Miller, B.E.; Wrobel, K.; Ranjit, K.; Williams, M.C.; Drost, E.; Edwards, L.D.; Lomas, D.A.; Rennard, S.I.; Agustí, A.; et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Circulating desmosine levels do not predict emphysema progression but are associated with cardiovascular risk and mortality in COPD. Eur. Respir. J. 2016, 47, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Rutten, E.P.; Gopal, P.; Wouters, E.F.; Franssen, F.M.; Hageman, G.J.; Vanfleteren, L.E.; Spruit, M.A.; Reynaert, N.L. Various Mechanistic Pathways Representing the Aging Process Are Altered in COPD. Chest 2016, 149, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Burgel, P.R.; Paillasseur, J.L.; Peene, B.; Dusser, D.; Roche, N.; Coolen, J.; Troosters, T.; Decramer, M.; Janssens, W. Two distinct chronic obstructive pulmonary disease (COPD) phenotypes are associated with high risk of mortality. PLoS ONE. 2012, 7, e51048. [Google Scholar] [CrossRef]
- Schurgers, L.J.; Vermeer, C. Determination of phylloquinone and menaquinones in food. Effect of food matrix on circulating vitamin K concentrations. Pathophysiol. Haemost. Thromb. 2000, 30, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Cranenburg, E.C.; Vermeer, C.; Koos, R.; Boumans, M.L.; Hackeng, T.M.; Bouwman, F.G.; Kwaijtaal, M.; Brandenburg, V.M.; Ketteler, M.; Schurgers, L.J. The circulating inactive form of matrix Gla Protein (ucMGP) as a biomarker for cardiovascular calcification. J Vasc. Res. 2008, 45, 427–436. [Google Scholar] [CrossRef]
- Ma, S.; Lin, Y.Y.; Turino, G.M. Measurements of desmosine and isodesmosine by mass spectrometry in COPD. Chest 2007, 131, 1363–1371. [Google Scholar] [CrossRef]
- Ma, S.; Turino, G.M.; Hayashi, T.; Yanuma, H.; Usuki, T.; Lin, Y.Y. Stable deuterium internal standard for the isotope-dilution LC-MS/MS analysis of elastin degradation. Anal. Biochem. 2013, 440, 158–165. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Tammeling, G.J.; Cotes, J.E.; Pedersen, O.F.; Peslin, R.; Yernault, J.C. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur. Respir. J. 1993, 16, 5–40. [Google Scholar] [CrossRef]
- Cranenburg, E.C.; Koos, R.; Schurgers, L.J.; Magdeleyns, E.J.; Schoonbrood, T.H.; Landewé, R.B.; Brandenburg, V.M.; Bekers, O.; Vermeer, C. Characterisation and potential diagnostic value of circulating matrix Gla protein (MGP) species. Thromb. Haemost. 2010, 104, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Casaburi, R.; Celli, B.; Crapo, J.; Criner, G.; Croxton, T.; Gaw, A.; Jones, P.; Kline-Leidy, N.; Lomas, D.A.; Merrill, D.; et al. The COPD Biomarker Qualification Consortium (CBQC). COPD 2013, 10, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Maclay, J.D.; McAllister, D.A.; Rabinovich, R.; Haq, I.; Maxwell, S.; Hartland, S.; Connell, M.; Murchison, J.T.; van Beek, E.J.; Gray, R.D.; et al. Systemic elastin degradation in chronic obstructive pulmonary disease. Thorax 2012, 67, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Lin, Y.Y.; Cantor, J.O.; Chapman, K.R.; Sandhaus, R.A.; Fries, M.; Edelman, J.M.; McElvaney, G.; Turino, G.M. The Effect of Alpha-1 Proteinase Inhibitor on Biomarkers of Elastin Degradation in Alpha-1 Antitrypsin Deficiency: An Analysis of the RAPID/RAPID Extension Trials. Chronic Obstr. Pulm. Dis. 2017, 4, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Nimptsch, K.; Rohrmann, S.; Kaaks, R.; Linseisen, J. Dietary vitamin K intake in relation to cancer incidence and mortality: Results from the Heidelberg cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Heidelberg). Am. J. Clin. Nutr. 2010, 91, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Hanson, C.; Sayles, H.; Rutten, E.P.; Wouters, E.F.; MacNee, W.; Calverley, P.; Meza, J.L.; Rennard, S. The association between dietary intake and phenotypical characteristics of COPD in the ECLIPSE cohort. J. COPD F. 2014, 1, 115–124. [Google Scholar] [CrossRef][Green Version]
- Piscaer, I.; Wouters, E.F.; Vermeer, C.; Janssens, W.; Franssen, F.M.; Janssen, R. Vitamin K deficiency: The linking pin between COPD and cardiovascular diseases? Respir Res. 2017, 18, 189. [Google Scholar] [CrossRef]
- Mayer, O., Jr.; Seidlerová, J.; Bruthans, J.; Filipovský, J.; Timoracká, K.; Vaněk, J.; Cerná, L.; Wohlfahrt, P.; Cífková, R.; Theuwissen, E.; et al. Desphospho-uncarboxylated matrix Gla-protein is associated with mortality risk in patients with chronic stable vascular disease. Atherosclerosis 2014, 235, 162–168. [Google Scholar] [CrossRef]
- Schlieper, G.; Westenfeld, R.; Krüger, T.; Cranenburg, E.C.; Magdeleyns, E.J.; Brandenburg, V.M.; Djuric, Z.; Damjanovic, T.; Ketteler, M.; Vermeer, C.; et al. Circulating nonphosphorylated carboxylated matrix gla protein predicts survival in ESRD. J. Am. Soc. Nephrol. 2011, 22, 387–395. [Google Scholar] [CrossRef]
- Piscaer, I.; Kalkman, G.A.; Fleuren, H.W.; Janssens, W.; Wouters, E.F.; Franssen, F.M.; Janssen, R. Use of Vitamin K Antagonists Is Associated with Increased Mortality in Chronic Obstructive Pulmonary Disease [abstract]. Am. J. Respir. Crit. Care Med. 2018, 197, A4234. [Google Scholar]
- Noth, I.; Anstrom, K.J.; Calvert, S.B.; de Andrade, J.; Flaherty, K.R.; Glazer, C.; Kaner, R.J.; Olman, M.A. Idiopathic Pulmonary Fibrosis Clinical Research Network (IPFnet). A placebo-controlled randomized trial of warfarin in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2012, 186, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Tomassetti, S.; Ruy, J.H.; Gurioli, C.; Ravaglia, C.; Buccioli, M.; Tantalocco, P.; Decker, P.A.; Cavazza, A.; Dubini, A.; Agnoletti, V.; et al. The effect of anticoagulant therapy for idiopathic pulmonary fibrosis in real life practice. Sarcoidosis Vasc. Diffus. Lung Dis. 2013, 30, 121–127. [Google Scholar]
- Kreuter, M.; Wijsenbeek, M.S.; Vasakova, M.; Spagnolo, P.; Kolb, M.; Costabel, U.; Weycker, D.; Kirchgaessler, K.U.; Maher, T.M. Unfavourable effects of medically indicated oral anticoagulants on survival in idiopathic pulmonary fibrosis. Eur. Respir. J. 2016, 47, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- King, C.S.; Freiheit, E.; Brown, A.W.; Shlobin, O.A.; Venuto, D.; Nathan, S.D. Effects of Anticoagulation on Survival in Interstitial Lung Disease: An Analysis of the Pulmonary Fibrosis Foundation (PFF) Registry. Am. J. Respir. Crit. Care Med. 2019, 296, A2642. [Google Scholar]
- Eurlings, I.M.; Dentener, M.A.; Cleutjens, J.P.; Peutz, C.J.; Rohde, G.G.; Wouters, E.F.; Reynaert, N.L. Similar matrix alterations in alveolar and small airway walls of COPD patients. BMC Pulm. Med. 2014, 14, 90. [Google Scholar] [CrossRef]
- Heulens, N.; Korf, H.; Cielen, N.; De Smidt, E.; Maes, K.; Gysemans, C.; Verbeken, E.; Gayan-Ramirez, G.; Mathieu, C.; Janssens, W. Vitamin D deficiency exacerbates COPD-like characteristics in the lungs of cigarette smoke-exposed mice. Respir. Res. 2015, 16, 110. [Google Scholar] [CrossRef]
| ICE-Age Cohort | COPD | (Former) Smoking Controls | Never-Smoking Controls |
|---|---|---|---|
| Subjects, n | 192 | 124 | 62 |
| Age, years | 61.9 ± 7.1 | 61.3 ± 5.9 | 60.1 ± 7.7 |
| Male, n (%) | 113 (58.9) | 63 (50.8) | 23 (37.1) |
| Smoking status | |||
| Never-smokers, n (%) | 3 (1.6) | 62 (100) | |
| Former smokers, n (%) | 124 (64.6) | 105 (84.7) | |
| Current smokers, n (%) | 65 (33.9) | 19 (15.3) | |
| Smoking pack years | 49.2 ± 29.7 | 18.1 ± 14.9 | 0.0 ± 0.2 |
| BMI, kg/m2 | 26.7 ± 5.3 | 27.3 ± 3.4 | 26.0 ± 3.0 |
| VKAs | |||
| No VKAs, n (%) | 174 (90.6) | 120 (96.8) | 61 (98.4) |
| VKA use, n (%) | 14 (7.3) | 3 (2.4) | 0 (0) |
| VKA use unknown, n (%) | 4 (2.1) | 1 (0.8) | 1 (1.6) |
| COPD stage | |||
| COPD GOLD I, n (%) | 1 (0.5) | ||
| COPD GOLD II, n (%) | 95 (49.5) | ||
| COPD GOLD III, n (%) | 77 (40.1) | ||
| COPD GOLD IV, n (%) | 19 (9.9) | ||
| MRC, points | 3.0 ± 1.0 | ||
| Lung function values | |||
| FEV1, L | 1.39 ± 0.52 | 3.36 ± 0.70 | 3.31 ± 0.79 |
| Percent of predictive normal value | 49.3 ± 15.7 | 118.3 ± 13.7 | 122.1 ± 16.0 |
| FVC, L | 3.41 ± 0.92 | 4.30 ± 0.90 | 4.16 ± 0.98 |
| Percent of predictive normal value | 97.5 ± 20.5 | 122.9 ± 14.9 | 126.4 ± 17.1 |
| FVC IN, L | 3.48 ± 0.92 | 4.43 ± 0.91 | 4.25 ± 0.99 |
| Percent of predictive normal value | 95.4 ± 19.3 | 121.9 ± 14.1 | 124.2 ± 16.0 |
| FEV1/FVC, % | 41.0 ± 11.5 | 78.3 ± 4.2 | 79.5 ± 5.2 |
| DLCO, mmol/min/kPa | 4.59 ± 1.83 | 8.03 ± 1.89 | 7.82 ± 2.22 |
| Percent of predictive normal value | 53.4 ± 19.1 | 92.3 ± 15.9 | 94.0 ± 13.5 |
| KCO, mmol/min/kPa | 0.96 ± 0.31 | 1.43 ± 0.24 | 1.50 ± 22 |
| Percent of predictive normal value | 67.7 ± 23.1 | 100.8 ± 15.2 | 102.1 ± 16.9 |
| ITGV, L | 4.61 ± 1.28 | 3.12 ± 0.72 | 3.06 ± 0.76 |
| Percent of predictive normal value | 144.1 ± 34.0 | 99.4 ± 17.0 | 100.7 ± 20.4 |
| RV, L | 3.51 ± 1.12 | 2.11 ± 0.44 | 1.95 ± 0.41 |
| Percent of predictive normal value | 159.2 ± 46.8 | 96.5 ± 17.1 | 91.6 ± 18.8 |
| Leuven COPD Cohort | COPD Stage | |||
|---|---|---|---|---|
| GOLD I | GOLD II | GOLD III | GOLD IV | |
| Subjects, n | 69 | 74 | 75 | 72 |
| Age, years | 64.1 ± 7.1 | 67.0 ± 71 | 67.6 ± 8.6 | 64.1 ± 8.1 |
| Male, n (%) | 52 (75.4) | 55 (74.3) | 59 (78.7) | 56 (77.8) |
| Smoking pack years | 44.3 ± 22.7 | 51.0 ± 24.4 | 50.2 ± 25.7 | 51.5 ± 28.3 |
| BMI, kg/m2 | 25.9 ± 3.5 | 26.1 ± 4.8 | 25.2 ± 5.5 | 23.4 ± 5.6 |
| VKA use, n (%) | 5 (7.2) | 10 (13.5) | 7 (9.3) | 5 (6.9) |
| MRC, points | 1.6 ± 0.8 | 2.5 ± 1.0 | 3.1 ± 1.1 | 3.4 ± 1.2 |
| Aortic calcification score, points | 0.79 ± 0.87 | 1.20 ± 0.88 | 1.45 ± 0.98 | 1.50 ± 1.05 |
| Lung function values | ||||
| FEV1, L | 2.68 ± 0.57 | 1.72 ± 0.41 | 1.13 ± 0.22 | 0.69 ± 0.14 |
| Percent of predictive normal value | 90.4 ± 8.6 | 61.4 ± 7.5 | 39.9 ± 5.2 | 24.3 ± 4.1 |
| FVC, L | 4.26 ± 0.91 | 3.35 ± 0.77 | 2.91 ± 0.65 | 2.31 ± 0.71 |
| Percent of predictive normal value | 114.3 ± 13.3 | 94.1 ± 16.2 | 81.5 ± 14.0 | 64.4 ± 15.0 |
| FEV1/FVC, % | 63.2 ± 5.1 | 52.0 ± 8.1 | 39.6 ± 7.4 | 31.4 ± 6.5 |
| VC, L | 4.24 ± 0.90 | 3.38 ± 0.80 | 2.99 ± 0.65 | 2.41 ± 0.70 |
| Percent of predictive normal value | 109.5 ± 13.1 | 91.7 ± 15.7 | 81.0 ± 13.8 | 64.4 ± 14.6 |
| DLCO, mmol/min/kPa | 6.63 ± 2.22 | 4.89 ± 1.57 | 3.89 ± 1.40 | 3.04 ± 0.88 |
| Percent of predictive normal value | 74.6 ± 20.5 | 57.4 ± 15.7 | 46.1 ± 15.6 | 35.8 ± 9.8 |
| KCO, mmol/min/kPa | 1.12 ± 0.30 | 1.04 ± 0.30 | 0.91 ± 0.30 | 0.88 ± 0.28 |
| Percent of predictive normal value | 81.3 ± 21.2 | 76.0 ± 22.1 | 67.1 ± 21.4 | 63.9 ± 20.0 |
| ITGV, L | 4.09 ± 0.81 | 4.41 ± 1.07 | 5.22 ± 1.21 | 6.10 ± 1.45 |
| Percent of predictive normal value | 121.5 ± 19.3 | 132.4 ± 28.8 | 156.2 ± 33.5 | 183.1 ± 36.9 |
| RV, L | 2.72 ± 0.67 | 3.17 ± 0.80 | 3.89 ± 1.00 | 4.95 ± 1.29 |
| Percent of predictive normal value | 115.6 ± 23.1 | 134.7 ± 31.7 | 166.0 ± 41.7 | 212.7 ± 50.3 |
| TLC, L | 6.95 ± 1.26 | 6.55 ± 1.31 | 6.88 ± 1.32 | 7.36 ± 1.56 |
| Percent of predictive normal value | 108.0 ± 11.0 | 104.8 ± 16.6 | 110.4 ± 18.1 | 118.3 ± 20.2 |
| Airway resistance, kPa/L/sec | 0.34 ± 0.12 | 0.47 ± 0.14 | 0.57 ± 0.14 | 0.77 ± 0.20 |
| Percent of predictive normal value | 152.4 ± 54.8 | 214.5 ± 64.2 | 260.8 ± 63.7 | 349.4 ± 91.7 |
| Specific airway conductance, 1/kPa*sec | 0.78 ± 0.24 | 0.51 ± 0.16 | 0.35 ± 0.11 | 0.23 ± 0.07 |
| Percent of predictive normal value | 91.2 ± 28.4 | 60.0 ± 19.1 | 41.1 ± 13.1 | 26.7 ± 8.2 |
| Mortality | ||||
| Died during follow-up, n (%) | 16 (23.2) | 27 (36.5) | 44 (58.7) | 47 (65.3) |
| Mortality unknown, n (%) | 4 (5.8) | 0 (0) | 2 (2.7) | 1 (1.4) |
| Follow-up, years | 5.9 ± 2.0 | 6.1 ± 2.1 | 4.9 ± 2.7 | 4.3 ± 2.8 |
| dp-ucMGP | pDES | |||||
| ICE-Age Cohort | Ratio | 95% CI | p-Value | Ratio | 95% CI | p-Value |
| VKA use, yes | 198.6% | 138.7 to 274.0% | p < 0.0005 | 4.2% | −8.1 to 18.1% | p = 0.522 |
| Presence of COPD, yes | 36.8% | 21.5 to 53.7% | p < 0.0005 | 15.6% | 8.3 to 23.4% | p < 0.0005 |
| Gender, female | −8.9% | −17.4 to 0.5% | p = 0.064 | 5.3% | −0.3 to 11.2% | p = 0.064 |
| Age, per year | 2.3% | 1.6 to 3.0% | p < 0.0005 | 2.1% | 1.7 to 2.5% | p < 0.0005 |
| BMI, per kg/m2 | 3.6% | 2.5 to 4.7% | p < 0.0005 | −0.1% | −0.7 to 0.5% | p = 0.794 |
| Smoking packyears, per year | 0.1% | −0.1 to 0.3% | p = 0.489 | 0.1% | 0.0 to 0.2% | p = 0.132 |
| Dp−ucMGP, per 10% | - | - | - | 1.0% | 0.4 to 1.5% | p = 0.001 |
| Lung function parameters | ||||||
| FEV1, per 10 pp | −3.0% | −6.8 to 1.0% | p = 0.173 | 1.0% | −1.0 to 4.1% | p = 0.323 |
| FVC, per 10 pp | −1.0% | −3.9 to 2.0% | p = 0.533 | 1.0% | −2.0 to 3.0% | p = 0.585 |
| FVC IN, per 10 pp | −1.0% | −3.9 to 2.0% | p = 0.547 | 0.0% | −2.0 to 3.0% | p = 0.667 |
| FEV1/FVC, per 10 pp | −1.0% | −1.0 to 6.6% | p = 0.199 | 1.0% | −3.0 to 5.0% | p = 0.607 |
| DLCO, per 10 pp | −4.9% | −8.6 to −2.0% | p = 0.005 | −3.9% | −5.8 to −1.0% | p = 0.004 |
| KCO, per 10 pp | −3.0% | −5.8 to 2.0% | p = 0.076 | −3.0% | −4.9 to −1.0% | p = 0.004 |
| RV, per 10 pp | 0.1% | −1.0 to 2.0% | p = 0.430 | 0.0% | −1.0 to 1.0% | p = 0.428 |
| ITGV, per 10 pp | 0.0% | −2.0 to 2.0% | p = 0.850 | 0.0% | −2.0 to 1.0% | p = 0.723 |
| dp-ucMGP | pDES | |||||
| Leuven COPD Cohort | Ratio | 95% CI | p-Value | Ratio | 95% CI | p-Value |
| VKA use, yes | 141.1% | 62.9 to 256.4% | p < 0.0005 | 5.4% | −9.3 to 22.5% | p = 0.485 |
| Gender, female | 14.3% | −13.1 to 50.4% | p = 0.336 | 5.0% | −5.4 to 16.6% | p = 0.360 |
| Age, per year | 1.3% | −0.2 to 2.8% | p = 0.079 | 3.0% | 2.5 to 3.7% | p < 0.0005 |
| BMI, per kg/m2 | 1.9% | −0.4 to 4.3% | p = 0.109 | −0.5% | −1.3 to 0.4% | p = 0.294 |
| Smoking packyears, per year | 0.0% | −0.4 to 0.5% | p = 0.845 | 0.2% | 0.0 to 0.3% | p = 0.065 |
| Dp-ucMGP, per 10% | - | - | - | 0.6% | 0.2 to 1.1% | p = 0.004 |
| Lung function parameters | ||||||
| FEV1, per 10 pp | 2.0% | −3.0 to 6.2% | p = 0.472 | 2.0%, | 0.0 to 4.1%, | p = 0.027 |
| FVC, per 10 pp | 0.0% | −4.9 to 5.1% | p = 0.925 | 1.0% | −1.0 to 3.0% | p = 0.296 |
| FEV1/FVC, per 10 pp | 5.1% | −3.9 to 13.9% | p = 0.272 | 5.1%, | 2.0 to 9.4% | p = 0.001 |
| VC, per 10 pp | 0.0% | −4.9 to 5.1% | p = 0.989 | 1.0% | −1.0 to 3.0% | p = 0.441 |
| DLCO, per 10 pp | −4.9% | −10.4 to 1.0% | p = 0.075 | −2.0% | −3.9 to 0.0% | p = 0.084 |
| KCO, per 10 pp | −4.9% | −10.4 to 1.0% | p = 0.075 | −2.0% | −3.9 to −0.02% | p = 0.048 |
| ITGV, per 10 pp | −3.9% | −6.8 to −1.0% | p = 0.023 | −2.0% | −3.0 to −1.0% | p = 0.002 |
| RV, per 10 pp | −3.0% | −4.9 to −1.0% | p = 0.012 | −1.0% | −2.0 to −1.0% | p = 0.001 |
| TLC, per 10 pp | −9.5% | −15.6 to −3.0 % | p = 0.004 | −4.9% | −6.8 to −2.0% | p = 0.001 |
| Airway resistance, per 10 pp | 0.0% | −2.0 to 1.0% | p = 0.472 | 0.0% | −1.0 to 0.1% | p = 0.114 |
| Specific airway conductance, per 10 pp | 3.0% | −1.0 to 7.3% | p = 0.139 | 2.0% | 0.0 to 3.0% | p = 0.009 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piscaer, I.; van den Ouweland, J.M.W.; Vermeersch, K.; Reynaert, N.L.; Franssen, F.M.E.; Keene, S.; Wouters, E.F.M.; Janssens, W.; Vermeer, C.; Janssen, R. Low Vitamin K Status Is Associated with Increased Elastin Degradation in Chronic Obstructive Pulmonary Disease. J. Clin. Med. 2019, 8, 1116. https://doi.org/10.3390/jcm8081116
Piscaer I, van den Ouweland JMW, Vermeersch K, Reynaert NL, Franssen FME, Keene S, Wouters EFM, Janssens W, Vermeer C, Janssen R. Low Vitamin K Status Is Associated with Increased Elastin Degradation in Chronic Obstructive Pulmonary Disease. Journal of Clinical Medicine. 2019; 8(8):1116. https://doi.org/10.3390/jcm8081116
Chicago/Turabian StylePiscaer, Ianthe, Jody M. W. van den Ouweland, Kristina Vermeersch, Niki L. Reynaert, Frits M. E. Franssen, Spencer Keene, Emiel F. M. Wouters, Wim Janssens, Cees Vermeer, and Rob Janssen. 2019. "Low Vitamin K Status Is Associated with Increased Elastin Degradation in Chronic Obstructive Pulmonary Disease" Journal of Clinical Medicine 8, no. 8: 1116. https://doi.org/10.3390/jcm8081116
APA StylePiscaer, I., van den Ouweland, J. M. W., Vermeersch, K., Reynaert, N. L., Franssen, F. M. E., Keene, S., Wouters, E. F. M., Janssens, W., Vermeer, C., & Janssen, R. (2019). Low Vitamin K Status Is Associated with Increased Elastin Degradation in Chronic Obstructive Pulmonary Disease. Journal of Clinical Medicine, 8(8), 1116. https://doi.org/10.3390/jcm8081116







