Postoperative Imaging and Tumor Marker Surveillance in Resected Pancreatic Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Statistical Analysis
3. Results
3.1. Clinicopathological Features and Adjuvant Treatment
3.2. The Follow-Up and the Treatment Outcome of Four Surveillance Groups
3.3. Salvage Treatment for Recurrent Patients
3.4. Prognostic Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kalser, M.H.; Ellenberg, S.S. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch. Surg. 1985, 120, 899–903. [Google Scholar] [PubMed]
- Klinkenbijl, J.H.; Jeekel, J.; Sahmoud, T.; van Pel, R.; Couvreur, M.L.; Veenhof, C.H.; Arnaud, J.P.; Gonzalez, D.G.; de Wit, L.T.; Hennipman, A.; et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: Phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann. Surg. 1999, 230, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Smeenk, H.G.; van Eijck, C.H.; Hop, W.C.; Erdmann, J.; Tran, K.C.; Debois, M.; van Cutsem, E.; van Dekken, H.; Klinkenbijl, J.H.; Jeekel, J. Long-term survival and metastatic pattern of pancreatic and periampullary cancer after adjuvant chemoradiation or observation: Long-term results of EORTC trial 40891. Ann. Surg. 2007, 246, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Oettle, H.; Neuhaus, P.; Hochhaus, A.; Hartmann, J.T.; Gellert, K.; Ridwelski, K.; Niedergethmann, M.; Zulke, C.; Fahlke, J.; Arning, M.B.; et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: The CONKO-001 randomized trial. JAMA 2013, 310, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Stocken, D.D.; Friess, H.; Bassi, C.; Dunn, J.A.; Hickey, H.; Beger, H.; Fernandez-Cruz, L.; Dervenis, C.; Lacaine, F.; et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N. Engl. J. Med. 2004, 350, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Uesaka, K.; Boku, N.; Fukutomi, A.; Okamura, Y.; Konishi, M.; Matsumoto, I.; Kaneoka, Y.; Shimizu, Y.; Nakamori, S.; Sakamoto, H.; et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: A phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 2016, 388, 248–257. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O’Reilly, D.A.; Cunningham, D.; Wadsley, J.; et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef]
- Conroy, T.; Hammel, P.; Hebbar, M.; Abdelghani, M.B.; Wei, A.C.C.; Raoul, J.L.; Chone, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. Unicancer GI PRODIGE 24/CCTG PA.6 trial: A multicenter international randomized phase III trial of adjuvant mFOLFIRINOX versus gemcitabine (gem) in patients with resected pancreatic ductal adenocarcinomas. J. Clin. Oncol. 2018, 36, LBA4001. [Google Scholar] [CrossRef]
- Seubert, B.; Grunwald, B.; Kobuch, J.; Cui, H.; Schelter, F.; Schaten, S.; Siveke, J.T.; Lim, N.H.; Nagase, H.; Simonavicius, N.; et al. Tissue inhibitor of metalloproteinases (TIMP)-1 creates a premetastatic niche in the liver through SDF-1/CXCR4-dependent neutrophil recruitment in mice. Hepatology 2015, 61, 238–248. [Google Scholar] [CrossRef]
- Grunwald, B.; Harant, V.; Schaten, S.; Fruhschutz, M.; Spallek, R.; Hochst, B.; Stutzer, K.; Berchtold, S.; Erkan, M.; Prokopchuk, O.; et al. Pancreatic Premalignant Lesions Secrete Tissue Inhibitor of Metalloproteinases-1, Which Activates Hepatic Stellate Cells Via CD63 Signaling to Create a Premetastatic Niche in the Liver. Gastroenterology 2016, 151, 1011.e1017–1024.e1017. [Google Scholar] [CrossRef]
- Rhim, A.D.; Mirek, E.T.; Aiello, N.M.; Maitra, A.; Bailey, J.M.; McAllister, F.; Reichert, M.; Beatty, G.L.; Rustgi, A.K.; Vonderheide, R.H.; et al. EMT and dissemination precede pancreatic tumor formation. Cell 2012, 148, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Feig, C.; Gopinathan, A.; Neesse, A.; Chan, D.S.; Cook, N.; Tuveson, D.A. The pancreas cancer microenvironment. Clin. Cancer Res. 2012, 18, 4266–4276. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology. Available online: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf (accessed on 29 April 2019).
- Khorana, A.A.; Mangu, P.B.; Berlin, J.; Engebretson, A.; Hong, T.S.; Maitra, A.; Mohile, S.G.; Mumber, M.; Schulick, R.; Shapiro, M.; et al. Potentially curable pancreatic cancer: American society of clinical oncology clinical practice guideline update. J. Clin. Oncol. 2017, 35, 2324–2328. [Google Scholar] [CrossRef] [PubMed]
- Ducreux, M.; Cuhna, A.S.; Caramella, C.; Hollebecque, A.; Burtin, P.; Goéré, D.; Seufferlein, T.; Haustermans, K.; Van Laethem, J.L.; Conroy, T.; et al. ESMO Guidelines Committee. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26, v56–v68. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, D.; Fou, L.; Hasler, E.; Hawkins, J.; O’Connell, S.; Pelone, F.; Callaway, M.; Campbell, F.; Capel, M.; Charnley, R.; et al. Diagnosis and management of pancreatic cancer in adults: A summary of guidelines from the UK National Institute for Health and Care Excellence. Pancreatology 2018, 18, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Daamen, L.A.; Groot, V.P.; Intven, M.P.W.; Besselink, M.G.; Busch, O.R.; Koerkamp, B.G.; Mohammad, N.H.; Hermans, J.J.; van Laarhoven, H.W.M.; Nuyttens, J.J.; et al. Dutch Pancreatic Cancer Group. Postoperative surveillance of pancreatic cancer patients. Eur. J. Surg. Oncol. 2019, 1. [Google Scholar]
- Sheffield, K.M.; Crowell, K.T.; Lin, Y.L.; Djukom, C.; Goodwin, J.S.; Riall, T.S. Surveillance of pancreatic cancer patients after surgical resection. Ann. Surg. Oncol. 2012, 19, 1670–1677. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, C.W.; Fleming, J.B.; Lee, J.E.; Wang, X.; Pisters, P.W.; Vauthey, J.N.; Varadhachary, G.; Wolff, R.A.; Katz, M.H. Yield of clinical and radiographic surveillance in patients with resected pancreatic adenocarcinoma following multimodal therapy. HPB (Oxford) 2012, 14, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Daamen, L.A.; Groot, V.P.; Heerkens, H.D.; Intven, M.P.W.; van Santvoort, H.C.; Molenaar, I.Q. Systematic review on the role of serum tumor markers in the detection of recurrent pancreatic cancer. HPB (Oxford) 2018, 20, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Balaj, C.; Ayav, A.; Oliver, A.; Jausset, F.; Sellal, C.; Claudon, M.; Laurent, V. CT imaging of early local recurrence of pancreatic adenocarcinoma following pancreaticoduodenectomy. Abdom. Radiol. (NY) 2016, 41, 273–282. [Google Scholar] [CrossRef]
- Tzeng, C.W.; Abbott, D.E.; Cantor, S.B.; Fleming, J.B.; Lee, J.E.; Pisters, P.W.; Varadhachary, G.R.; Abbruzzese, J.L.; Wolff, R.A.; Ahmad, S.A.; et al. Frequency and intensity of postoperative surveillance after curative treatment of pancreatic cancer: A cost-effectiveness analysis. Ann. Surg. Oncol. 2013, 20, 2197–2203. [Google Scholar] [CrossRef] [PubMed]
- Tjaden, C.; Michalski, C.W.; Strobel, O.; Giese, N.; Hennche, A.K.; Buchler, M.W.; Hackert, T. Clinical Impact of Structured Follow-up After Pancreatic Surgery. Pancreas 2016, 45, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Ettrich, T.J.; Schulte, L.A.; Eitel, N.; Ettrich, K.; Berger, A.W.; Perkhofer, L.; Seufferlein, T. Surveillance after resection of pancreatic ductal adenocarcinoma with curative intent—A multicenter survey in Germany and review of the literature. Z. Gastroenterol. 2017, 55, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Pietrasz, D.; Pecuchet, N.; Garlan, F.; Didelot, A.; Dubreuil, O.; Doat, S.; Imbert-Bismut, F.; Karoui, M.; Vaillant, J.C.; Taly, V.; et al. Plasma Circulating Tumor DNA in Pancreatic Cancer Patients is a Prognostic Marker. Clin. Cancer Res. 2017, 23, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, S.; Zhang, B.; Ni, Q.; Yu, X.; Xu, J. Circulating biomarkers for early diagnosis of pancreatic cancer: Facts and hopes. Am. J. Cancer Res. 2018, 8, 332–353. [Google Scholar] [PubMed]
- Groot, V.P.; Mosier, S.; Javed, A.A.; Teinor, J.A.; Gemenetzis, G.; Ding, D.; Haley, L.M.; Yu, J.; Burkhart, R.A.; Hasanain, A.; et al. Circulating Tumor DNA as a Clinical Test in Resected Pancreatic Cancer. Clin. Cancer Res. 2019, 29. Clincanres.0197.2019. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouche, O.; Guimbaud, R.; Becouarn, Y.; Adenis, A.; Raoul, J.L.; Gourgou-Bourgade, S.; de la Fouchardiere, C.; et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef]
- Dhir, M.; Zenati, M.S.; Hamad, A.; Singhi, A.D.; Bahary, N.; Hogg, M.E.; Zeh, H.J., 3rd; Zureikat, A.H. FOLFIRINOX Versus Gemcitabine/Nab-Paclitaxel for Neoadjuvant Treatment of Resectable and Borderline Resectable Pancreatic Head Adenocarcinoma. Ann. Surg. Oncol. 2018, 25, 1896–1903. [Google Scholar] [CrossRef]
- Goldstein, D.; El-Maraghi, R.H.; Hammel, P.; Heinemann, V.; Kunzmann, V.; Sastre, J.; Scheithauer, W.; Siena, S.; Tabernero, J.; Teixeira, L.; et al. nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: Long-term survival from a phase III trial. J. Natl. Cancer Inst. 2015, 107, dju413. [Google Scholar] [CrossRef]
- Wang-Gillam, A.; Li, C.P.; Bodoky, G.; Dean, A.; Shan, Y.S.; Jameson, G.; Macarulla, T.; Lee, K.H.; Cunningham, D.; Blanc, J.F.; et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): A global, randomised, open-label, phase 3 trial. Lancet 2016, 387, 545–557. [Google Scholar] [CrossRef]
- Wang-Gillam, A.; Hubner, R.A.; Siveke, J.T.; Von Hoff, D.D.; Belanger, B.; de Jong, F.A.; Mirakhur, B.; Chen, L.T. NAPOLI-1 phase 3 study of liposomal irinotecan in metastatic pancreatic cancer: Final overall survival analysis and characteristics of long-term survivors. Eur. J. Cancer 2019, 108, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Diab, M.; Azmi, A.; Mohammad, R.; Philip, P.A. Pharmacotherapeutic strategies for treating pancreatic cancer: Advances and challenges. Expert. Opin. Pharmacother. 2019, 20, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Loosen, S.H.; Neumann, U.P.; Trautwein, C.; Roderburg, C.; Luedde, T. Current and future biomarkers for pancreatic adenocarcinoma. Tumour Biol. 2017, 39, 1010428317692231. [Google Scholar] [CrossRef] [PubMed]
- Gaianigo, N.; Melisi, D.; Carbone, C. EMT and Treatment Resistance in Pancreatic Cancer. Cancers (Basel) 2017, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.H.; Hsu, M.C.; Chen, L.T.; Hung, W.C.; Pan, M.R. Alteration of Epigenetic Modifiers in Pancreatic Cancer and Its Clinical Implication. J. Clin. Med. 2019, 8, 903. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Kuo, Y.H.; Tien, Y.W.; Hsu, C.; Hsu, C.H.; Kuo, S.H.; Cheng, A.L. Inferior survival of advanced pancreatic cancer patients who received gemcitabine-based chemotherapy but did not participate in clinical trials. Oncology 2011, 81, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Barhli, A.; Cros, J.; Bartholin, L.; Neuzillet, C. Prognostic stratification of resected pancreatic ductal adenocarcinoma: Past, present, and future. Dig. Liver Dis. 2018, 50, 979–990. [Google Scholar] [CrossRef]
- Sohal, D.P.S.; Tullio, K.; Khorana, A.A. Do patients with pancreatic body or tail cancer benefit from adjuvant therapy? A cohort study. Surg. Oncol. 2018, 27, 245–250. [Google Scholar] [CrossRef]
- Birnbaum, D.J.; Bertucci, F.; Finetti, P.; Birnbaum, D.; Mamessier, E. Head and Body/Tail Pancreatic Carcinomas Are Not the Same Tumors. Cancers (Basel) 2019, 11, 497. [Google Scholar] [CrossRef]
- Motoi, F.; Murakami, Y.; Okada, K.I.; Matsumoto, I.; Uemura, K.; Satoi, S.; Sho, M.; Honda, G.; Fukumoto, T.; Yanagimoto, H.; et al. Sustained Elevation of Postoperative Serum Level of Carbohydrate Antigen 19-9 is High-Risk Stigmata for Primary Hepatic Recurrence in Patients with Curatively Resected Pancreatic Adenocarcinoma. World J. Surg. 2019, 43, 634–641. [Google Scholar] [CrossRef]
- Rieser, C.J.; Zenati, M.; Hamad, A.; Al Abbas, A.I.; Bahary, N.; Zureikat, A.H.; Zeh, H.J., 3rd; Hogg, M.E. CA19-9 on Postoperative Surveillance in Pancreatic Ductal Adenocarcinoma: Predicting Recurrence and Changing Prognosis over Time. Ann. Surg. Oncol. 2018, 25, 3483–3491. [Google Scholar] [CrossRef] [PubMed]
- Sperti, C.; Pasquali, C.; Bissoli, S.; Chierichetti, F.; Liessi, G.; Pedrazzoli, S. Tumor relapse after pancreatic cancer resection is detected earlier by 18-FDG PET than by CT. J. Gastrointest. Surg. 2010, 14, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Reitz, D.; Gerger, A.; Seidel, J.; Kornprat, P.; Samonigg, H.; Stotz, M.; Szkandera, J.; Pichler, M. Combination of tumour markers CEA and CA19-9 improves the prognostic prediction in patients with pancreatic cancer. J. Clin. Pathol. 2015, 68, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.X.; Liu, L.; Xiang, J.F.; Wang, W.Q.; Qi, Z.H.; Wu, C.T.; Liu, C.; Long, J.; Xu, J.; Ni, Q.X.; et al. Postoperative serum CEA and CA125 levels are supplementary to perioperative CA19-9 levels in predicting operative outcomes of pancreatic ductal adenocarcinoma. Surgery 2017, 161, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Simeone, D.M.; Ji, B.; Banerjee, M.; Arumugam, T.; Li, D.; Anderson, M.A.; Bamberger, A.M.; Greenson, J.; Brand, R.E.; Ramachandran, V.; et al. CEACAM1, a novel serum biomarker for pancreatic cancer. Pancreas 2007, 34, 436–443. [Google Scholar] [CrossRef]
- Beauchemin, N.; Arabzadeh, A. Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer Metastasis Rev. 2013, 32, 643–671. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, F.; Wicklein, D.; Horst, J.; Sundermann, P.; Maar, H.; Streichert, T.; Tachezy, M.; Izbicki, J.R.; Bockhorn, M.; Schumacher, U. Carcinoembryonic antigen-related cell adhesion molecules (CEACAM) 1, 5 and 6 as biomarkers in pancreatic cancer. PLoS ONE 2014, 9, e113023. [Google Scholar] [CrossRef]
- Koopmann, J.; Fedarko, N.S.; Jain, A.; Maitra, A.; Iacobuzio-Donahue, C.; Rahman, A.; Hruban, R.H.; Yeo, C.J.; Goggins, M. Evaluation of osteopontin as biomarker for pancreatic adenocarcinoma. Cancer Epidemiol. Biomarkers Prev. 2004, 13, 487–491. [Google Scholar] [CrossRef]
- Kuhlmann, K.F.; van Till, J.W.; Boermeester, M.A.; de Reuver, P.R.; Tzvetanova, I.D.; Offerhaus, G.J.; Ten Kate, F.J.; Busch, O.R.; van Gulik, T.M.; Gouma, D.J.; et al. Evaluation of matrix metalloproteinase 7 in plasma and pancreatic juice as a biomarker for pancreatic cancer. Cancer Epidemiol. Biomarkers Prev. 2007, 16, 886–891. [Google Scholar] [CrossRef]
- Zhou, W.; Sokoll, L.J.; Bruzek, D.J.; Zhang, L.; Velculescu, V.E.; Goldin, S.B.; Hruban, R.H.; Kern, S.E.; Hamilton, S.R.; Chan, D.W.; et al. Identifying markers for pancreatic cancer by gene expression analysis. Cancer Epidemiol. Biomarkers Prev. 1998, 7, 109–112. [Google Scholar]
- Firpo, M.A.; Gay, D.Z.; Granger, S.R.; Scaife, C.L.; DiSario, J.A.; Boucher, K.M.; Mulvihill, S.J. Improved diagnosis of pancreatic adenocarcinoma using haptoglobin and serum amyloid A in a panel screen. World J. Surg. 2009, 33, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Poruk, K.E.; Firpo, M.A.; Adler, D.G.; Mulvihill, S.J. Screening for pancreatic cancer: Why, how, and who? Ann. Surg. 2013, 257, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Nordby, T.; Hugenschmidt, H.; Fagerland, M.W.; Ikdahl, T.; Buanes, T.; Labori, K.J. Follow-up after curative surgery for pancreatic ductal adenocarcinoma: Asymptomatic recurrence is associated with improved survival. Eur. J. Surg. Oncol. 2013, 39, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Guo, J.C.; Yeh, K.H.; Tien, Y.W.; Cheng, A.L.; Kuo, S.H. Association of radiotherapy with favorable prognosis in daily clinical practice for treatment of locally advanced and metastatic pancreatic cancer. J. Gastroenterol. Hepatol. 2016, 31, 2004–2012. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Ahn, S.; Cho, I.K.; Lee, J.; Kim, J.; Hwang, J.H. Management of recurrent pancreatic cancer after surgical resection: A protocol for systematic review, evidence mapping and meta-analysis. BMJ Open 2018, 8, e017249. [Google Scholar] [CrossRef] [PubMed]
- Groot, V.P.; van Santvoort, H.C.; Rombouts, S.J.; Hagendoorn, J.; Borel Rinkes, I.H.; van Vulpen, M.; Herman, J.M.; Wolfgang, C.L.; Besselink, M.G.; Molenaar, I.Q. Systematic review on the treatment of isolated local recurrence of pancreatic cancer after surgery; re-resection, chemoradiotherapy and SBRT. HPB (Oxford) 2017, 19, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Jiang, R.; Liang, F.; Yu, G.; Long, J.; Zhao, J. Definitive chemoradiotherapy and salvage chemotherapy for patients with isolated locoregional recurrence after radical resection of primary pancreatic cancer. Cancer Manag. Res. 2019, 11, 5065–5073. [Google Scholar] [CrossRef]
- Sohal, D.P.S.; Kennedy, E.B.; Khorana, A.; Copur, M.S.; Crane, C.H.; Garrido-Laguna, I.; Krishnamurthi, S.; Moravek, C.; O’Reilly, E.M.; Philip, P.A.; et al. Metastatic Pancreatic Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 2545–2556. [Google Scholar] [CrossRef]
- Khorana, A.A.; McKernin, S.E.; Berlin, J.; Hong, T.S.; Maitra, A.; Moravek, C.; Mumber, M.; Schulick, R.; Zeh, H.J.; Katz, M.H.G. Potentially Curable Pancreatic Adenocarcinoma: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2019, JCO1900946. [Google Scholar] [CrossRef]
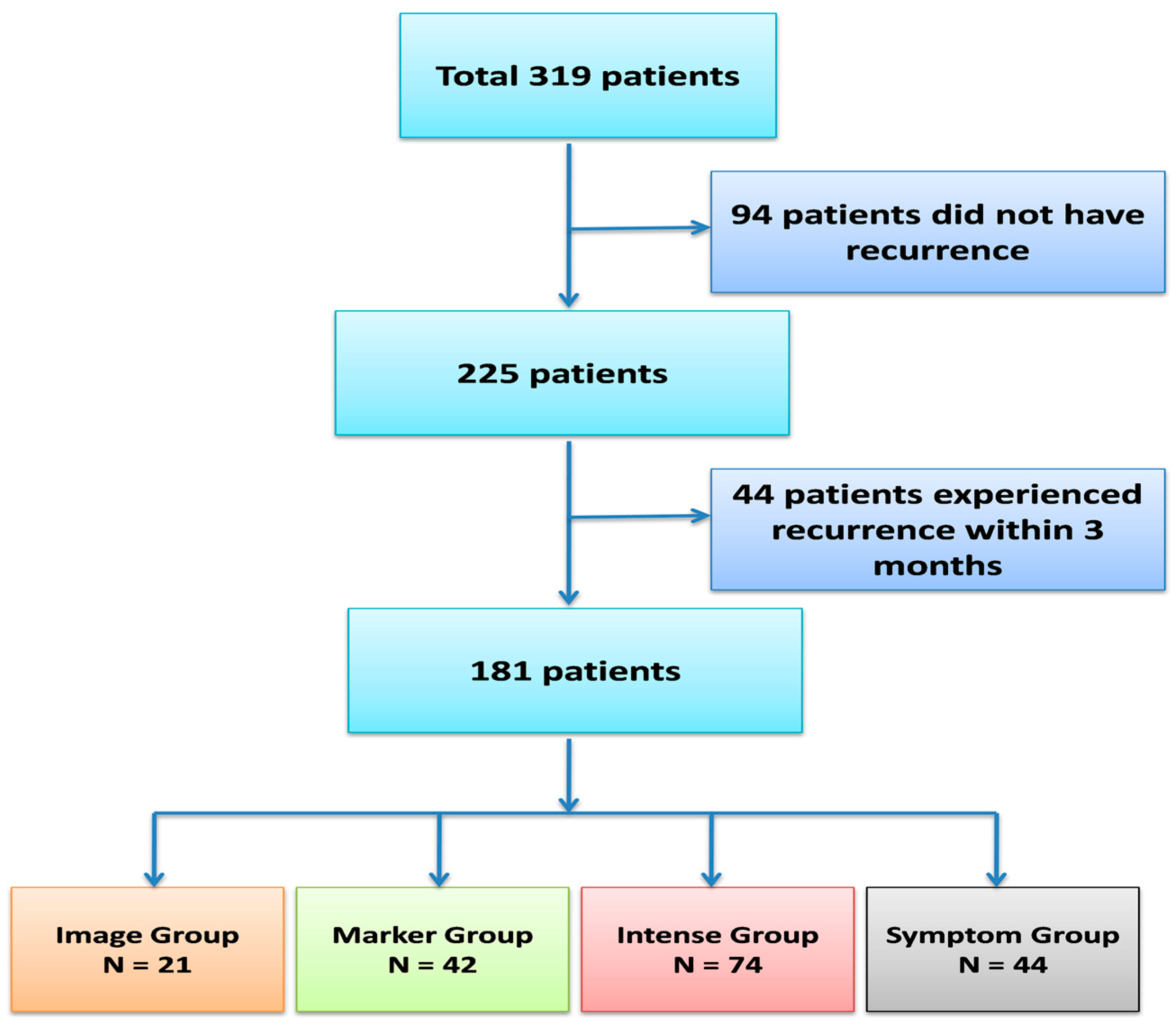



| 8 | Symptom Group (n = 44) | Imaging Group (n = 21) | Marker Group (n = 42) | Intense Group (n = 74) | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |||
| Age | Median | 72.5 | 70.3 | 65.1 | 66.0 | 0.069 ¶ | ||||
| Gender | Male | 24 | 54.5% | 14 | 66.7% | 29 | 69.0% | 44 | 59.5% | 0.518 |
| Female | 20 | 45.5% | 7 | 33.3% | 13 | 31.0% | 30 | 40.5% | ||
| ECOG PS | 0 | 3 | 6.8% | 3 | 14.3% | 7 | 16.7% | 13 | 17.6% | 0.511 |
| 1 | 34 | 77.3% | 14 | 66.7% | 32 | 76.2% | 55 | 74.3% | ||
| 2 | 7 | 15.9% | 4 | 19.0% | 3 | 7.1% | 5 | 6.8% | ||
| 3 | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 1 | 1.4% | ||
| Primary site | Head | 37 | 84.1% | 15 | 71.4% | 33 | 78.6% | 55 | 74.3% | 0.900 |
| Body | 2 | 4.5% | 2 | 9.5% | 3 | 7.1% | 5 | 6.8% | ||
| Tail | 5 | 11.4% | 4 | 19.5% | 6 | 14.3% | 14 | 18.9% | ||
| Section margin | Negative | 33 | 75.0% | 15 | 71.4% | 36 | 85.7% | 59 | 79.7% | 0.510 |
| Positive | 11 | 25.0% | 6 | 28.6% | 6 | 14.3% | 15 | 20.3% | ||
| pT | T1 | 4 | 9.1% | 2 | 9.5% | 1 | 2.4% | 0 | 0.0% | 0.198 |
| T2 | 6 | 13.6% | 3 | 14.3% | 5 | 11.9% | 9 | 12.2% | ||
| T3 | 34 | 77.3% | 16 | 76.2% | 36 | 85.7% | 65 | 87.8% | ||
| T4 | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | ||
| pN | N0 | 24 | 54.5% | 10 | 47.6% | 23 | 54.8% | 34 | 45.9% | 0.736 |
| N1 | 20 | 45.5% | 11 | 52.4% | 19 | 45.2% | 40 | 54.1% | ||
| Differentiation | Grade 1 | 9 | 20.5% | 4 | 19.0% | 10 | 23.8% | 10 | 13.5% | 0.764 |
| Grade 2 | 30 | 68.2% | 16 | 76.2% | 29 | 69.0% | 55 | 74.3% | ||
| Grade 3 | 5 | 11.4% | 1 | 4.8% | 3 | 7.1% | 9 | 12.2% | ||
| Adjuvant C/T | No | 40 | 90.9% | 18 | 85.7% | 27 | 64.3% | 41 | 55.4% | <0.001 * |
| Yes | 4 | 9.1% | 3 | 14.3% | 15 | 35.7% | 33 | 44.6% | ||
| Pattern of first R | Distant | 27 | 61.4% | 14 | 66.7% | 34 | 81.0% | 56 | 75.7% | 0.175 |
| Local | 17 | 38.6% | 7 | 33.3% | 8 | 19.0% | 18 | 24.3% | ||
| Treatment after R | No | 24 | 54.5% | 8 | 38.1% | 17 | 40.5% | 24 | 32.4% | 0.130 |
| Yes | 20 | 45.5% | 13 | 61.9% | 25 | 59.5% | 50 | 67.6% | ||
| CA199 post OP (U/mL) | Median | 19.5 | 37.85 | 24.8 | 38.4 | 0.149 ¶ | ||||
| Elevated | 7 | 41.2% | 5 | 55.6% | 17 | 44.7% | 37 | 50.7% | 0.827 | |
| Normal | 10 | 58.8% | 4 | 44.4% | 21 | 55.3% | 36 | 49.3% | ||
| CEA post OP (ng/mL) | Median | 2.45 | 0.91 | 1.26 | 1.52 | 0.042 ¶ * | ||||
| Elevated | 2 | 11.1% | 0 | 0.0% | 1 | 2.9% | 6 | 9.2% | 0.539 | |
| Normal | 16 | 88.9% | 6 | 100.0% | 33 | 97.1% | 59 | 90.8% | ||
| CA199 when R (U/mL) | Median | 209.71 | 1011.95 | 501 | 374.4 | 0.521¶ | ||||
| Elevated | 27 | 81.8% | 9 | 75.0% | 30 | 81.1% | 55 | 78.6% | 0.950 | |
| Normal | 6 | 18.2% | 3 | 25.0% | 7 | 18.9% | 15 | 21.4% | ||
| CEA when R (ng/mL) | Median | 3.0 | 2.89 | 2.45 | 2.3 | 0.435 ¶ | ||||
| Elevated | 12 | 40.0% | 2 | 22.2% | 10 | 33.3% | 15 | 23.4% | 0.366 | |
| Normal | 18 | 60.0% | 7 | 77.8% | 20 | 66.7% | 49 | 76.6% | ||
| Symptom Group | Imaging Group | Marker Group | Intense Group | p-Value | |
|---|---|---|---|---|---|
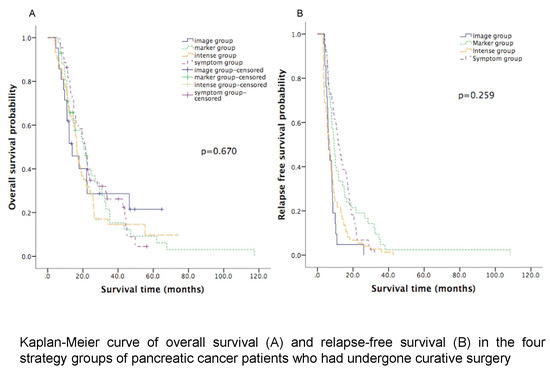
| Median OS (months) | 21.4 (95% CI: 17.9 to 25.0) | 13.9 (95% CI: 6.5 to 21.4) | 20.5 (95% CI: 13.5 to 27.5) | 16.5 (95% CI: 14.9 to 18.1) | 0.670 |
| Median RFS (months) | 11.7 (95% CI: 8.9 to 14.5) | 6.3 (95% CI: 4.6 to 7.9) | 9.3 (95% CI: 7.5 to 11.1) | 6.9 (95% CI: 5.2 to 8.6) | 0.259 |
| Median PROS (months) | 6.9 (95% CI: 2.9 to 10.9) | 7.5 (95% CI: 1.2 to 13.9) | 5.0 (95% CI: 3.0 to 7.0) | 7.8 (95% CI: 5.6 to 10.0) | 0.953 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Hazard Ratio (HR) | 95.0% CI for HR | P-Value | Hazard Ratio (HR) | 95.0% CI for HR | P-Value | |
| Adjuvant chemotherapy Yes vs. No | 1.003 | 0.696–1.445 | 0.988 | 0.925 | 0.596–1.436 | 0.728 |
| ECOG PS 0 vs. ≥1 | 0.626 | 0.381–1.028 | 0.064 | 0.516 | 0.295–0.903 | 0.020 * |
| Primary site Non-tail vs. tail | 0.930 | 0.599–1.445 | 0.747 | 0.599 | 0.360–0.997 | 0.049 * |
| Section margin Positive vs. Negative | 1.230 | 0.817–1.852 | 0.322 | 1.128 | 0.736–1.729 | 0.581 |
| T stage T1/T2 vs. T3 | 0.732 | 0.471–1.139 | 0.167 | 0.623 | 0.387–1.002 | 0.051 |
| N stage N1 vs. N0 | 1.406 | 1.010–1.956 | 0.043 * | 1.368 | 0.961–1.947 | 0.082 |
| Differentiation Grade 1/2 vs. Grade 3 | 0.595 | 0.353–1.002 | 0.051 | 0.553 | 0.322–0.950 | 0.032 * |
| Treatment after recurrence Yes vs. No | 0.958 | 0.682–1.347 | 0.806 | 0.996 | 0.675–1.469 | 0.983 |
| Follow up strategy | 0.985 | 0.831–1.167 | 0.862 | 0.996 | 0.837–1.184 | 0.960 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Guo, J.-C.; Yang, S.-H.; Tien, Y.-W.; Kuo, S.-H. Postoperative Imaging and Tumor Marker Surveillance in Resected Pancreatic Cancer. J. Clin. Med. 2019, 8, 1115. https://doi.org/10.3390/jcm8081115
Wu H, Guo J-C, Yang S-H, Tien Y-W, Kuo S-H. Postoperative Imaging and Tumor Marker Surveillance in Resected Pancreatic Cancer. Journal of Clinical Medicine. 2019; 8(8):1115. https://doi.org/10.3390/jcm8081115
Chicago/Turabian StyleWu, Hsu, Jhe-Cyuan Guo, Shih-Hung Yang, Yu-Wen Tien, and Sung-Hsin Kuo. 2019. "Postoperative Imaging and Tumor Marker Surveillance in Resected Pancreatic Cancer" Journal of Clinical Medicine 8, no. 8: 1115. https://doi.org/10.3390/jcm8081115
APA StyleWu, H., Guo, J.-C., Yang, S.-H., Tien, Y.-W., & Kuo, S.-H. (2019). Postoperative Imaging and Tumor Marker Surveillance in Resected Pancreatic Cancer. Journal of Clinical Medicine, 8(8), 1115. https://doi.org/10.3390/jcm8081115