Effects of a Lifestyle Intervention in Routine Care on Prenatal Dietary Behavior—Findings from the Cluster-Randomized GeliS Trial
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Study Participants
2.3. The Lifestyle Intervention Program
2.4. Study Outcomes
2.5. Data Collection and Processing
2.6. Statistical Analysis
3. Results
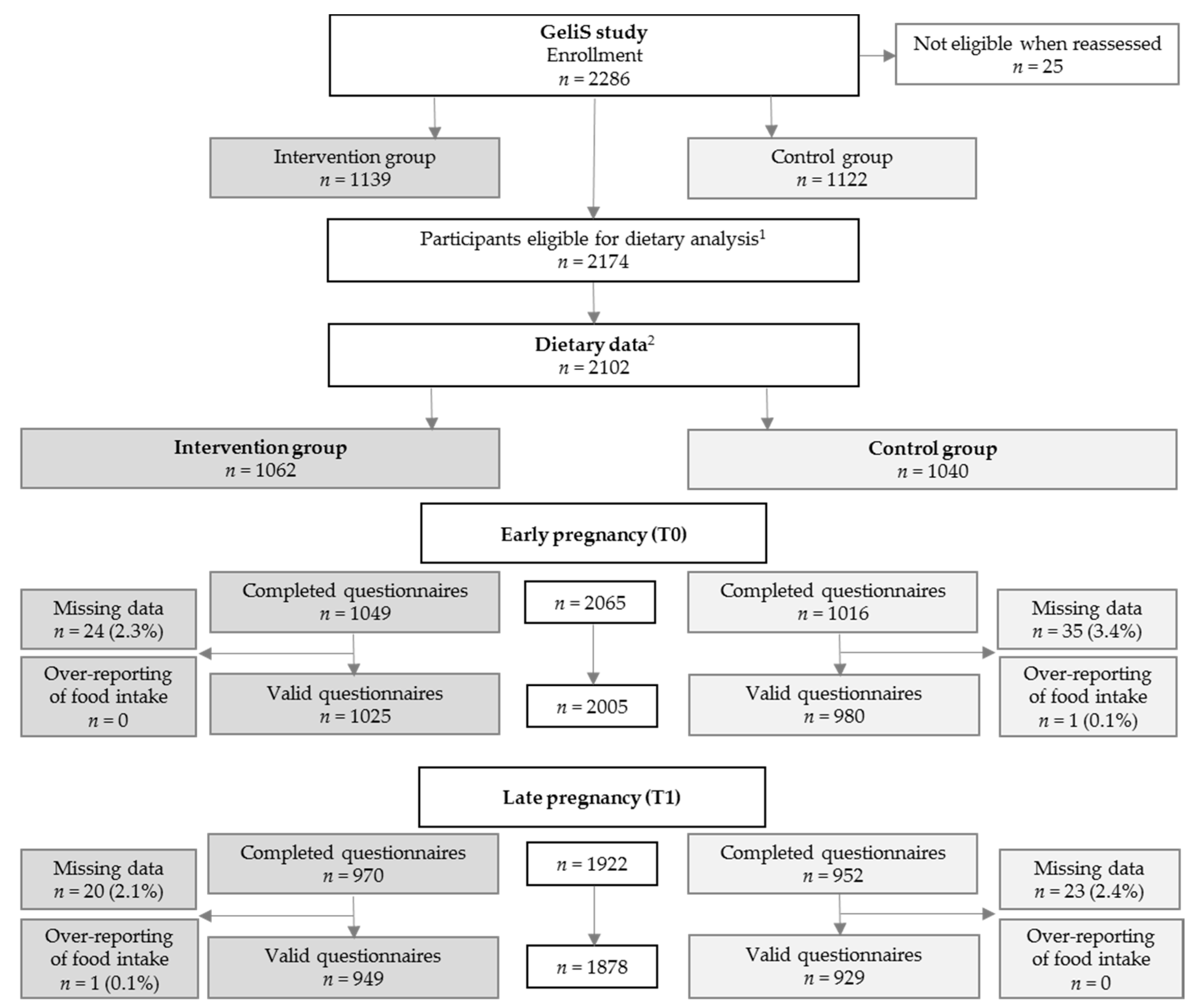
3.1. Study Participants and Baseline Characteristics
3.2. Food Intake
3.3. Energy and Macronutrient Intake
3.4. Healthy Eating Index
3.5. Dietary Behavior and Gestational Weight Gain
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sebire, N.J.; Jolly, M.; Harris, J.P.; Wadsworth, J.; Joffe, M.; Beard, R.W.; Regan, L.; Robinson, S. Maternal obesity and pregnancy outcome: A study of 287,213 pregnancies in London. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.; Boyle, J.A.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; Rode, L.; et al. Association of gestational weight gain with maternal and infant outcomes: A systematic review and meta-analysis. JAMA 2017, 317, 2207–2225. [Google Scholar] [CrossRef] [PubMed]
- Brunner, S.; Stecher, L.; Ziebarth, S.; Nehring, I.; Rifas-Shiman, S.L.; Sommer, C.; Hauner, H.; von Kries, R. Excessive gestational weight gain prior to glucose screening and the risk of gestational diabetes: A meta-analysis. Diabetologia 2015, 58, 2229–2237. [Google Scholar] [CrossRef] [PubMed]
- Mamun, A.A.; Mannan, M.; Doi, S.A.R. Gestational weight gain in relation to offspring obesity over the life course: A systematic review and bias-adjusted meta-analysis. Obes. Rev. 2014, 15, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Han, S.; Zhu, J.; Sun, X.; Ji, C.; Guo, X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: A systematic review and meta-analysis. PLoS ONE 2013, 8, e61627. [Google Scholar] [CrossRef] [PubMed]
- Nehring, I.; Schmoll, S.; Beyerlein, A.; Hauner, H.; von Kries, R. Gestational weight gain and long-term postpartum weight retention: A meta-analysis. Am. J. Clin. Nutr. 2011, 94, 1225–1231. [Google Scholar] [CrossRef]
- Weight Gain During Pregnancy. Reexamining the Guidelines; Rasmussen, K.M., Yaktine, A.L., Eds.; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.L.; Boyle, J.A.; Harrison, C.L.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; et al. Gestational weight gain across continents and ethnicity: Systematic review and meta-analysis of maternal and infant outcomes in more than one million women. BMC Med. 2018, 16, 153. [Google Scholar] [CrossRef]
- Johnson, J.L.; Farr, S.L.; Dietz, P.M.; Sharma, A.J.; Barfield, W.D.; Robbins, C.L. Trends in gestational weight gain: The Pregnancy Risk Assessment Monitoring System, 2000–2009. Am. J. Obstet. Gynecol. 2015, 212, 806. [Google Scholar] [CrossRef]
- Muktabhant, B.; Lawrie, T.A.; Lumbiganon, P.; Laopaiboon, M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Shieh, C.; Cullen, D.L.; Pike, C.; Pressler, S.J. Intervention strategies for preventing excessive gestational weight gain: Systematic review and meta-analysis. Obes. Rev. 2018, 19, 1093–1109. [Google Scholar] [CrossRef]
- Skouteris, H.; Hartley-Clark, L.; McCabe, M.; Milgrom, J.; Kent, B.; Herring, S.J.; Gale, J. Preventing excessive gestational weight gain: A systematic review of interventions. Obes. Rev. 2010, 11, 757–768. [Google Scholar] [CrossRef] [PubMed]
- International Weight Management in Pregnancy (i-WIP) Collaborative Group. Effect of diet and physical activity based interventions in pregnancy on gestational weight gain and pregnancy outcomes: Meta-analysis of individual participant data from randomised trials. BMJ 2017, 358. [Google Scholar] [CrossRef]
- Dodd, J.M.; Turnbull, D.; McPhee, A.J.; Deussen, A.R.; Grivell, R.M.; Yelland, L.N.; Crowther, C.A.; Wittert, G.; Owens, J.A.; Robinson, J.S. Antenatal lifestyle advice for women who are overweight or obese: LIMIT randomised trial. BMJ 2014, 348. [Google Scholar] [CrossRef] [PubMed]
- Poston, L.; Bell, R.; Croker, H.; Flynn, A.C.; Godfrey, K.M.; Goff, L.; Hayes, L.; Khazaezadeh, N.; Nelson, S.M.; Oteng-Ntim, E.; et al. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): A multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2015, 3, 767–777. [Google Scholar] [CrossRef]
- Dodd, J.M.; Cramp, C.; Sui, Z.; Yelland, L.N.; Deussen, A.R.; Grivell, R.M.; Moran, L.J.; Crowther, C.A.; Turnbull, D.; McPhee, A.J.; et al. The effects of antenatal dietary and lifestyle advice for women who are overweight or obese on maternal diet and physical activity: The LIMIT randomised trial. BMC Med. 2014, 12, 161. [Google Scholar] [CrossRef] [PubMed]
- Kinnunen, T.I.; Aittasalo, M.; Koponen, P.; Ojala, K.; Mansikkamäki, K.; Weiderpass, E.; Fogelholm, M.; Luoto, R. Feasibility of a controlled trial aiming to prevent excessive pregnancy-related weight gain in primary health care. BMC Pregnancy Childbirth 2008, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.L.; Lombard, C.B.; Strauss, B.J.; Teede, H.J. Optimizing healthy gestational weight gain in women at high risk of gestational diabetes: A randomized controlled trial. Obesity 2013, 21, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Daley, A.J.; Jolly, K.; Jebb, S.A.; Roalfe, A.K.; Mackillop, L.; Lewis, A.L.; Clifford, S.; Kenyon, S.; MacArthur, C.; Aveyard, P. Effectiveness of regular weighing, weight target setting and feedback by community midwives within routine antenatal care in preventing excessive gestational weight gain: Randomised controlled trial. BMC Obes. 2015, 3, 7. [Google Scholar] [CrossRef]
- Rauh, K.; Gabriel, E.; Kerschbaum, E.; Schuster, T.; von Kries, R.; Amann-Gassner, U.; Hauner, H. Safety and efficacy of a lifestyle intervention for pregnant women to prevent excessive maternal weight gain: A cluster-randomized controlled trial. BMC Pregnancy Childbirth 2013, 13, 151. [Google Scholar] [CrossRef]
- Kunath, J.; Günther, J.; Rauh, K.; Hoffmann, J.; Stecher, L.; Rosenfeld, E.; Kick, L.; Ulm, K.; Hauner, H. Effects of a lifestyle intervention during pregnancy to prevent excessive gestational weight gain in routine care—The cluster-randomised GeliS trial. BMC Med. 2019, 17, 5. [Google Scholar] [CrossRef]
- Rauh, K.; Kunath, J.; Rosenfeld, E.; Kick, L.; Ulm, K.; Hauner, H. Healthy living in pregnancy: A cluster-randomized controlled trial to prevent excessive gestational weight gain–Rationale and design of the GeliS study. BMC Pregnancy Childbirth 2014, 14, 119. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Bauer, C.-P.; Bung, P.; Cremer, M.; Flothkötter, M.; Hellmers, C.; Kersting, M.; Krawinkel, M.; Przyrembel, H.; Rasenack, R.; et al. Ernährung in der Schwangerschaft—Teil 2. Handlungsempfehlungen des Netzwerks “Gesund ins Leben—Netzwerk Junge Familie”. Dtsch. Med. Wochenschr. 2012, 137, 1366–1372. [Google Scholar] [PubMed]
- Haftenberger, M.; Heuer, T.; Heidemann, C.; Kube, F.; Krems, C.; Mensink, G.B.M. Relative validation of a food frequency questionnaire for national health and nutrition monitoring. Nutr. J. 2010, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Mensink, G.B.M.; Robert Koch Institute (RKI), Berlin, Germany. Personal communication, 2018.
- Max Rubner-Institut (MRI); Federal Research Institute of Nutrition, Food (Bundesforschungsinstitut für Ernährung und Lebensmittel), Karlsruhe, Germany; Verzehrsmengen ausgewählter Lebensmittel aus der Nationalen Verzehrsstudie II (NVS II). Personal communication, 2018.
- Meltzer, H.M.; Brantsaeter, A.L.; Ydersbond, T.A.; Alexander, J.; Haugen, M. Methodological challenges when monitoring the diet of pregnant women in a large study: Experiences from the Norwegian Mother and Child Cohort Study (MoBa). Matern. Child Nutr. 2008, 4, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, D.-A. Entwicklung eines Index zur Bewertung der Ernährungsqualität in der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1); German (“Development of a Dietary Quality Index in the German Health Examination Survey for Adults”); Robert Koch Institute: Berlin, Germany, 2017. [Google Scholar]
- Donner, A.; Klar, N. Design and Analysis of Cluster Randomization Trials in Health Research; Wiley & Sons: Chichester, UK, 2000. [Google Scholar]
- Guelinckx, I.; Devlieger, R.; Mullie, P.; Vansant, G. Effect of lifestyle intervention on dietary habits, physical activity, and gestational weight gain in obese pregnant women: A randomized controlled trial. Am. J. Clin. Nutr. 2010, 91, 373–380. [Google Scholar] [CrossRef]
- Kinnunen, T.I.; Puhkala, J.; Raitanen, J.; Ahonen, S.; Aittasalo, M.; Virtanen, S.M.; Luoto, R. Effects of dietary counselling on food habits and dietary intake of Finnish pregnant women at increased risk for gestational diabetes—A secondary analysis of a cluster-randomized controlled trial. Matern. Child Nutr. 2014, 10, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Hui, A.; Back, L.; Ludwig, S.; Gardiner, P.; Sevenhuysen, G.; Dean, H.; Sellers, E.; McGavock, J.; Morris, M.; Bruce, S.; et al. Lifestyle intervention on diet and exercise reduced excessive gestational weight gain in pregnant women under a randomised controlled trial. BJOG 2012, 119, 70–77. [Google Scholar] [CrossRef]
- Jing, W.; Huang, Y.; Liu, X.; Luo, B.; Yang, Y.; Liao, S. The effect of a personalized intervention on weight gain and physical activity among pregnant women in China. Int. J. Gynaecol. Obstet. 2015, 129, 138–141. [Google Scholar] [CrossRef]
- Donazar-Ezcurra, M.; Lopez-Del Burgo, C.; Martinez-Gonzalez, M.A.; Basterra-Gortari, F.J.; de Irala, J.; Bes-Rastrollo, M. Soft drink consumption and gestational diabetes risk in the SUN project. Clin. Nutr. 2018, 37, 638–645. [Google Scholar] [CrossRef]
- Grundt, J.H.; Eide, G.E.; Brantsaeter, A.-L.; Haugen, M.; Markestad, T. Is consumption of sugar-sweetened soft drinks during pregnancy associated with birth weight? Matern. Child Nutr. 2017, 13. [Google Scholar] [CrossRef]
- Jen, V.; Erler, N.S.; Tielemans, M.J.; Braun, K.V.; Jaddoe, V.W.; Franco, O.H.; Voortman, T. Mothers’ intake of sugar-containing beverages during pregnancy and body composition of their children during childhood: The Generation R Study. Am. J. Clin. Nutr. 2017, 105, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Valkama, A.; Koivusalo, S.; Lindström, J.; Meinilä, J.; Kautiainen, H.; Stach-Lempinen, B.; Rönö, K.; Klemetti, M.; Pöyhönen-Alho, M.; Tiitinen, A.; et al. The effect of dietary counselling on food intakes in pregnant women at risk for gestational diabetes: A secondary analysis of a randomised controlled trial RADIEL. Eur. J. Clin. Nutr. 2016, 70, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Northstone, K.; Emmett, P.; Rogers, I. Dietary patterns in pregnancy and associations with socio-demographic and lifestyle factors. Eur. J. Clin. Nutr. 2007, 62, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Sagedal, L.R.; Øverby, N.C.; Bere, E.; Torstveit, M.K.; Lohne-Seiler, H.; Småstuen, M.; Hillesund, E.R.; Henriksen, T.; Vistad, I. Lifestyle intervention to limit gestational weight gain: The Norwegian Fit for Delivery randomised controlled trial. BJOG 2017, 124, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Stuebe, A.M.; Oken, E.; Gillman, M.W. Associations of diet and physical activity during pregnancy with risk for excessive gestational weight gain. Am. J. Obstet. Gynecol. 2009, 201, 58. [Google Scholar] [CrossRef] [PubMed]
- Bärebring, L.; Brembeck, P.; Löf, M.; Brekke, H.K.; Winkvist, A.; Augustin, H. Food intake and gestational weight gain in Swedish women. SpringerPlus 2016, 5, 377. [Google Scholar] [CrossRef]
- Tielemans, M.J.; Garcia, A.H.; Peralta Santos, A.; Bramer, W.M.; Luksa, N.; Luvizotto, M.J.; Moreira, E.; Topi, G.; de Jonge, E.A.L.; Visser, T.L.; et al. Macronutrient composition and gestational weight gain: A systematic review. Am. J. Clin. Nutr. 2016, 103, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Jebeile, H.; Mijatovic, J.; Louie, J.C.Y.; Prvan, T.; Brand-Miller, J.C. A systematic review and metaanalysis of energy intake and weight gain in pregnancy. Am. J. Obstet. Gynecol. 2016, 214, 465–483. [Google Scholar] [CrossRef]
- Maslova, E.; Halldorsson, T.I.; Astrup, A.; Olsen, S.F. Dietary protein-to-carbohydrate ratio and added sugar as determinants of excessive gestational weight gain: A prospective cohort study. BMJ Open 2015, 5, e005839. [Google Scholar] [CrossRef]
- Renault, K.M.; Carlsen, E.M.; Nørgaard, K.; Nilas, L.; Pryds, O.; Secher, N.J.; Olsen, S.F.; Halldorsson, T.I. Intake of Sweets, Snacks and Soft Drinks Predicts Weight Gain in Obese Pregnant Women: Detailed Analysis of the Results of a Randomised Controlled Trial. PLoS ONE 2015, 10, e0133041. [Google Scholar] [CrossRef]
| Intervention (n = 1062) | Control (n = 1040) | Total (n = 2102) | |
|---|---|---|---|
| Prepregnancy age (years) | 30.1 ± 4.3 | 30.3 ± 4.6 | 30.2 ± 4.5 |
| Prepregnancy weight (kg) | 68.4 ± 13.1 | 67.9 ± 13.7 | 68.2 ± 13.4 |
| Prepregnancy BMI (kg/m2) | 24.4 ± 4.4 | 24.3 ± 4.6 | 24.4 ± 4.5 |
| Prepregnancy BMI category (n (%)) | |||
| BMI 18.5–24.9 kg/m2 | 685/1062 (64.5%) | 687/1040 (66.1%) | 1372/2102 (65.3%) |
| BMI 25.0–29.9 kg/m2 | 253/1062 (23.8%) | 225/1040 (21.6%) | 478/2102 (22.7%) |
| BMI 30.0–40.0 kg/m2 | 124/1062 (11.7%) | 128/1040 (12.3%) | 252/2102 (12.0%) |
| Educational level | |||
| General secondary school | 157/1061 (14.8%) | 173/1036 (16.7%) | 330/2097 (15.7%) |
| Intermediate secondary school | 454/1061 (42.8%) | 430/1036 (41.5%) | 884/2097 (42.2%) |
| High school | 450/1061 (42.4%) | 433/2096 (41.8%) | 883/2097 (42.1%) |
| Country of birth (n (%)) | |||
| Germany | 933/1061 (87.9%) | 930/1037 (89.7%) | 1863/2098 (88.8%) |
| Others | 128/1061 (12.1%) | 107/1037 (10.3%) | 235/2098 (11.2%) |
| Nulliparous (n (%)) | 661/1062 (62.2%) | 557/1039 (53.6%) | 1218/2101 (58.0%) |
| Living with a partner (n (%)) | 1023/1058 (96.7%) | 988/1037 (95.3%) | 2011/2095 (96.0%) |
| Full-time employed | 568/1048 (54.2%) | 514/1031 (49.9%) | 1082/2079 (52.0%) |
| Time Point | Intervention Group | Control Group | Adjusted Effect Size 1 (95% CI) | Adjusted p Value 1 | |||
|---|---|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | ||||
| Beverage consumption (mL/day) | T0 | 1025 | 3414.37 ± 2176.67 | 979 | 3434.05 ± 2176.93 | ||
| T1 | 949 | 3029.63 ± 1883.61 | 928 | 3060.04 ± 1965.45 | –34.46(–226.08,157.15) | 0.724 | |
| Time effect | p < 0.001 2 | p < 0.001 2 | |||||
| Caffeinated beverages (mL/day) | T0 | 1024 | 118.02 ± 172.24 | 979 | 142.91 ± 204.40 | ||
| T1 | 949 | 134.59 ± 152.65 | 927 | 148.00 ± 177.60 | –6.38(–12.85,0.10) | 0.054 | |
| Time effect | p = 0.005 2 | p = 0.283 2 | |||||
| Soft drinks (mL/day) | T0 | 1025 | 207.74 ± 600.99 | 979 | 247.50 ± 676.18 | ||
| T1 | 949 | 155.45 ± 439.32 | 927 | 235.36 ± 641.33 | –57.03(–86.24,–27.83) | <0.001 | |
| Time effect | p = 0.004 2 | p = 0.276 2 | |||||
| Alcoholic drinks (mL/day) | T0 | 1023 | 11.13 ±40.88 | 974 | 14.01 ± 49.85 | ||
| T1 | 947 | 13.80 ± 39.28 | 922 | 15.62 ± 46.99 | –1.31(–5.36,2.75) | 0.527 | |
| Time effect | p = 0.055 2 | p = 0.416 2 | |||||
| Vegetables (g/day) | T0 | 1022 | 178.63 ± 146.58 | 980 | 171.73 ± 151.76 | ||
| T1 | 949 | 201.04 ± 159.94 | 927 | 175.72 ± 160.22 | 19.83(2.75,36.91) | 0.023 | |
| Time effect | p < 0.001 2 | p = 0.295 2 | |||||
| Fruit (g/day) | T0 | 1022 | 328.57 ± 324.63 | 980 | 296.36 ± 304.94 | ||
| T1 | 948 | 315.50 ± 298.02 | 928 | 291.61 ± 271.70 | 4.73(–6.33,15.79) | 0.402 | |
| Time effect | p =0.243 2 | p = 0.761 2 | |||||
| Nuts (g/day) | T0 | 1021 | 2.54 ± 5.78 | 975 | 1.86 ± 4.05 | ||
| T1 | 946 | 2.98 ± 6.00 | 925 | 2.60 ± 5.46 | 0.08(–0.19,0.35) | 0.563 | |
| Time effect | p =0.018 2 | p < 0.001 2 | |||||
| Cereal (g/day) | T0 | 1025 | 121.76 ± 83.11 | 979 | 116.06 ± 80.79 | ||
| T1 | 949 | 115.01 ± 69.49 | 929 | 112.87 ± 70.66 | –0.81(–7.75,6.13) | 0.818 | |
| Time effect | p =0.014 2 | p = 0.291 2 | |||||
| Side dishes (g/day) | T0 | 1024 | 142.98 ± 80.74 | 980 | 144.57 ± 96.03 | ||
| T1 | 949 | 141.50 ± 78.85 | 929 | 139.47 ± 94.80 | 1.83(–3.78,7.44) | 0.523 | |
| Time effect | p =0.378 2 | p = 0.128 2 | |||||
| Dairy products (g/day) | T0 | 1025 | 327.66 ± 277.66 | 979 | 333.17 ± 384.98 | ||
| T1 | 949 | 397.52 ± 335.81 | 929 | 372.16 ± 365.10 | 24.56(–5.13,54.26) | 0.105 | |
| Time effect | p < 0.001 2 | p = 0.002 2 | |||||
| Cheese (g/day) | T0 | 1021 | 26.58 ± 34.55 | 975 | 26.77 ± 36.41 | ||
| T1 | 944 | 25.76 ± 28.49 | 923 | 25.42 ± 29.30 | –0.68(–4.24,2.89) | 0.709 | |
| Time effect | p = 0.566 2 | p = 0.983 2 | |||||
| Eggs (g/day) | T0 | 1019 | 14.20 ± 15.60 | 973 | 15.03 ± 17.99 | ||
| T1 | 945 | 14.20 ± 21.86 | 926 | 13.85 ± 15.67 | 0.28(–1.60, 2.15) | 0.774 | |
| Time effect | p = 0.966 2 | p = 0.096 2 | |||||
| Fat spread (g/day) | T0 | 1016 | 5.04 ± 6.57 | 977 | 5.14 ± 6.21 | ||
| T1 | 946 | 5.39 ± 6.05 | 922 | 5.50 ± 5.77 | –0.15(–0.67,0.37) | 0.561 | |
| Time effect | p = 0.129 2 | p = 0.032 2 | |||||
| Fish (g/day) | T0 | 1025 | 12.78 ± 16.10 | 979 | 12.74 ± 13.86 | ||
| T1 | 947 | 14.51 ± 12.13 | 926 | 12.49 ± 12.52 | 1.82(0.68, 2.96) | 0.002 | |
| Time effect | p = 0.001 2 | p = 0.729 2 | |||||
| Meat and meat products (g/day) | T0 | 1024 | 82.14 ± 57.35 | 980 | 80.31 ± 56.53 | ||
| T1 | 949 | 85.49 ± 51.48 | 929 | 84.79 ± 52.00 | –0.93(–5.46, 3.61) | 0.689 | |
| Time effect | p = 0.113 2 | p = 0.010 2 | |||||
| Sweets and snacks (g/day) | T0 | 1025 | 70.14 ± 55.15 | 980 | 71.40 ± 68.25 | ||
| T1 | 949 | 88.76 ± 70.00 | 929 | 89.85 ± 65.62 | –0.99(–8.19, 6.21) | 0.788 | |
| Time effect | p < 0.001 2 | p < 0.001 2 | |||||
| Fast food (g/day) | T0 | 1024 | 46.80 ± 37.12 | 980 | 48.13 ± 35.77 | ||
| T1 | 949 | 43.14 ± 31.22 | 929 | 44.52 ± 30.46 | –1.56(–3.84,0.72) | 0.180 | |
| Time effect | p = 0.001 2 | p = 0.002 2 | |||||
| Time Point | Intervention Group | Control Group | Adjusted Effect Size 1 (95% CI) | Adjusted p Value 1 | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| Whole grain bread | T0 | 953/1019 | 93.5% | 911/975 | 93.4% | ||
| T1 | 918/943 | 97.3% | 866/922 | 93.9% | 2.95(1.49,5.87) | 0.002 | |
| Time effect | p < 0.001 2 | p = 0.740 2 | |||||
| Low-fat milk/yoghurt | T0 | 725/1006 | 72.1% | 675/965 | 69.9% | ||
| T1 | 692/943 | 73.4% | 638/915 | 69.7% | 1.28(1.12,1.47) | <0.001 | |
| Time effect | p = 0.369 2 | p = 0.662 2 | |||||
| Low-fat cheese/sausage | T0 | 531/976 | 54.4% | 496/939 | 52.8% | ||
| T1 | 517/923 | 56.0% | 465/902 | 51.6% | 1.27(1.20,1.35) | <0.001 | |
| Time effect | p = 0.289 2 | p = 0.199 2 | |||||
| Sugar in coffee/tea | T0 | 382/993 | 38.5% | 390/950 | 41.1% | ||
| T1 | 335/911 | 36.8% | 359/891 | 40.3% | 0.86(0.72,1.03) | 0.098 | |
| Time effect | p = 0.119 2 | p = 0.811 2 | |||||
| Rapeseed oil and olive oil (for meat and fish) | T0 | 474/888 | 53.4% | 477/833 | 57.3% | ||
| T1 | 478/786 | 60.8% | 447/796 | 56.2% | 1.72(1.57,1.89) | <0.001 | |
| Time effect | p < 0.001 2 | p = 0.553 2 | |||||
| Rapeseed oil and olive oil (for vegetables) | T0 | 507/860 | 59.0% | 497/840 | 59.2% | ||
| T1 | 514/780 | 65.9% | 475/793 | 59.9% | 1.55(1.32,1.82) | <0.001 | |
| Time effect | p < 0.001 2 | p = 0.825 2 | |||||
| Cooking at least 5 times per week | T0 | 586/1022 | 57.3% | 545/975 | 55.9% | ||
| T1 | 604/940 | 64.3% | 575/924 | 62.2% | 1.20(1.00,1.43) | 0.050 | |
| Time effect | p < 0.001 2 | p < 0.001 2 | |||||
| Vegetarian | T0 | 67/1019 | 6.6% | 68/969 | 7.0% | ||
| T1 | 57/938 | 6.1% | 49/917 | 5.3% | 1.45(1.10,1.92) | 0.008 | |
| Time effect | p = 0.321 2 | p = 0.029 2 | |||||
| Time Point | Intervention Group | Control Group | Adjusted Effect Size 1 (95% CI) | Adjusted p Value 1 | |||
|---|---|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | ||||
| Energy (kcal/day) | T0 | 940 | 1974.22 ± 618.56 | 880 | 1944.66 ± 661.60 | ||
| T1 | 892 | 2000.39 ± 606.90 | 862 | 2010.79 ± 646.59 | –17.67(–115.71,80.36) | 0.724 | |
| Time effect | p = 0.137 2 | p < 0.001 2 | |||||
| Carbohydrates (E%) | T0 | 940 | 57.13 ± 8.07 | 880 | 57.18 ± 8.13 | ||
| T1 | 892 | 54.66 ± 7.01 | 862 | 55.46 ± 7.41 | –0.31(–1.50,0.88) | 0.606 | |
| Time effect | p < 0.001 2 | p < 0.001 2 | |||||
| Saccharose (g/day) | T0 | 940 | 54.84 ± 30.72 | 880 | 54.48 ± 33.47 | ||
| T1 | 892 | 56.08 ± 27.93 | 862 | 57.82 ± 31.18 | –0.78(–4.79,3.23) | 0.702 | |
| Time effect | p = 0.206 2 | p = 0.001 2 | |||||
| Protein (E%) | T0 | 940 | 16.14 ± 3.11 | 880 | 16.16 ± 3.38 | ||
| T1 | 892 | 16.55 ± 3.03 | 862 | 15.95 ± 2.99 | 0.44(0.08,0.81) | 0.018 | |
| Time effect | p < 0.001 2 | p = 0.171 2 | |||||
| Fat (E%) | T0 | 940 | 26.65 ± 6.41 | 880 | 26.49 ± 6.37 | ||
| T1 | 892 | 28.77 ± 5.78 | 862 | 28.55 ± 6.00 | –0.07(–1.01,0.87) | 0.888 | |
| Time effect | p < 0.001 2 | p < 0.001 2 | |||||
| Saturated fat (g/day) | T0 | 940 | 26.55 ± 11.66 | 880 | 26.01 ± 12.07 | ||
| T1 | 892 | 29.72 ± 12.92 | 862 | 29.58 ± 12.96 | –0.45(–2.38,1.48) | 0.647 | |
| Time effect | p < 0.001 2 | p < 0.001 2 | |||||
| Saturated fat (E%) | T0 | 940 | 12.61 ± 3.46 | 880 | 12.55 ± 3.44 | ||
| T1 | 892 | 13.77 ± 3.17 | 862 | 13.69 ± 3.31 | –0.05(–0.54,0.44) | 0.845 | |
| Time effect | p < 0.001 2 | p < 0.001 2 | |||||
| MUFA (g/day) | T0 | 940 | 17.44 ± 7.48 | 880 | 16.95 ± 7.60 | ||
| T1 | 892 | 19.19 ± 8.11 | 862 | 19.22 ± 8.16 | –0.43(–1.52,0.66) | 0.441 | |
| Time effect | p < 0.001 2 | p < 0.001 2 | |||||
| PUFA (g/day) | T0 | 940 | 6.24 ± 2.43 | 880 | 6.08 ± 2.43 | ||
| T1 | 892 | 6.40 ± 2.38 | 862 | 6.35 ± 2.44 | –0.08(–0.33,0.17) | 0.517 | |
| Time effect | p = 0.039 2 | p < 0.001 2 | |||||
| Cholesterol (mg/day) | T0 | 940 | 227.32 ± 97.71 | 880 | 226.11 ± 108.11 | ||
| T1 | 892 | 241.21 ± 125.34 | 862 | 237.59 ± 106.56 | 2.19(–10.74,15.13) | 0.740 | |
| Time effect | p < 0.001 2 | p < 0.001 2 | |||||
| Fiber (g/day) | T0 | 940 | 24.82 ± 10.60 | 880 | 23.36 ± 10.54 | ||
| T1 | 892 | 24.72 ± 10.10 | 862 | 23.25 ± 9.86 | 0.72(–0.46,1.90) | 0.232 | |
| Time effect | p = 0.814 2 | p = 0.996 2 | |||||
| Alcohol (g/day) | T0 | 940 | 0.20 ± 0.91 | 880 | 0.44 ± 2.32 | ||
| T1 | 892 | 0.07 ± 0.24 | 862 | 0.11 ± 0.43 | –0.03(–0.06,0.00) | 0.068 | |
| Time effect | p < 0.001 2 | p < 0.001 2 | |||||
| Healthy Eating Index | T0 | 1025 | 58.81 ± 8.60 | 980 | 57.54 ± 8.93 | ||
| T1 | 949 | 59.33 ± 8.21 | 929 | 57.60 ± 8.52 | 1.05(–0.42,2.53) | 0.162 | |
| Time effect | p < 0.001 2 | p < 0.001 2 | |||||
| Intervention Group | Control Group | Adjusted Effect Size 1 (95% CI) | Adjusted p Value 1 | Interaction p Value | |||
|---|---|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | ||||
| Prepregnancy age category | 0.001 2 | ||||||
| Age 18–25 years | 121 | 58.18 ± 7.84 | 131 | 53.78 ± 7.76 | 3.39 (1.36,5.43) | 0.001 | |
| Age 26–35 years | 723 | 59.31 ± 8.13 | 669 | 57.84 ± 8.37 | 0.96 (–0.60,2.53) | 0.228 | |
| Age 36–43 years | 105 | 60.80 ± 8.99 | 127 | 60.31 ± 8.82 | –0.08 (–2.00,1.84) | 0.934 | |
| Prepregnancy BMI category | 0.011 3 | ||||||
| BMI 18.5–24.9 kg/m2 | 612 | 60.06 ± 8.15 | 612 | 58.15 ± 8.46 | 1.45 (–0.12,3.03) | 0.069 | |
| BMI 25.0–29.9 kg/m2 | 228 | 58.70 ± 8.22 | 197 | 56.85 ± 8.90 | 0.89 (–1.20,2.98) | 0.403 | |
| BMI 30.0–40.0 kg/m2 | 109 | 56.55 ± 7.90 | 120 | 56.03 ± 7.95 | –0.14 (–1.76,1.48) | 0.867 | |
| Educational level | 0.644 4 | ||||||
| General secondary school | 137 | 55.65 ± 8.77 | 147 | 54.54 ± 7.91 | 2.01 (1.29,2.74) | <0.001 | |
| Intermediate secondary school | 410 | 58.18 ± 7.71 | 396 | 56.75 ± 8.33 | 0.89 (–0.55,2.33) | 0.226 | |
| High school | 401 | 61.75 ± 7.81 | 384 | 60.03 ± 8.21 | 1.31 (–0.18,2.81) | 0.086 | |
| T0 | T1 | |||||
|---|---|---|---|---|---|---|
| n | Adjusted Effect Size 1 (95% CI) | Adjusted p Value 1 | n | Adjusted Effect Size 1 (95% CI) | Adjusted p Value 1 | |
| Soft drinks (200 mL/day) | 1774 | 0.06 (–0.01,0.14) | 0.097 | 1744 | 0.04 (–0.05,0.13) | 0.347 |
| Vegetables (150 g/day) | 1772 | 0.16 (–0.08,0.39) | 0.185 | 1744 | 0.05 (–0.17,0.27) | 0.671 |
| Fruit (150 g/day) | 1772 | 0.11 (–0.01,0.22) | 0.071 | 1744 | 0.10 (–0.02,0.23) | 0.105 |
| Nuts (25 g/day) | 1769 | 0.05 (–1.13,1.23) | 0.933 | 1740 | 0.69 (–0.38,1.75) | 0.205 |
| Cereal (50 g/day) | 1774 | 0.02 (–0.13,0.17) | 0.782 | 1746 | –0.03 (–0.20,0.14) | 0.741 |
| Side dishes (100 g/day) | 1774 | 0.28 (–0.00,0.55) | 0.052 | 1746 | –0.26 (–0.53,0.01) | 0.058 |
| Dairy products (200 g/day) | 1774 | 0.13 (–0.02,0.27) | 0.082 | 1746 | 0.25 (0.12,0.38) | <0.001 |
| Cheese (30 g/day) | 1768 | 0.22 (0.01,0.44) | 0.045 | 1735 | –0.03 (–0.28,0.21) | 0.792 |
| Eggs (60 g/day) | 1763 | 1.09 (0.23,1.95) | 0.013 | 1741 | 0.49 (–0.24,1.23) | 0.188 |
| Fat spread (5 g/day) | 1763 | –0.06 (–0.26,0.14) | 0.558 | 1736 | –0.03 (–0.23,0.17) | 0.759 |
| Fish (90 g/day) | 1774 | 0.56 (–0.83,1.95) | 0.428 | 1742 | –0.08 (–1.86,1.69) | 0.926 |
| Meat and meat products | 1774 | 0.62 (–0.03,1.26) | 0.061 | 1746 | 0.65 (–0.04,1.34) | 0.066 |
| Red meat (150 g/day) | 1770 | 0.67 (–0.62,1.96) | 0.307 | 1740 | 0.80 (–0.56,2.16) | 0.250 |
| Processed meat (150 g/day) | 1774 | 0.16 (–1.06,1.38) | 0.793 | 1746 | 1.48 (0.16,2.81) | 0.028 |
| Sweets and snacks (50 g/day) | 1775 | 0.13 (–0.07,0.33) | 0.195 | 1746 | 0.31 (0.14,0.49) | 0.001 |
| Fast food (250 g/day) | 1774 | 3.28 (1.64,4.92) | <0.001 | 1746 | 2.69 (0.75,4.63) | 0.007 |
| Low-fat milk/yoghurt | 1752 | 0.76 (0.23,1.28) | 0.005 | 1727 | 0.95 (0.42,1.47) | <0.001 |
| Low-fat cheese/sausage | 1697 | 0.39 (–0.10,0.89) | 0.116 | 1698 | 0.65 (0.16,1.14) | 0.009 |
| Sugar in coffee/tea | 1723 | –0.09 (–0.59,0.40) | 0.707 | 1677 | –0.02 (–0.53,0.48) | 0.930 |
| Vegetarian | 1759 | –0.04 (–0.98,0.91) | 0.941 | 1727 | –0.10 (–1.12,0.93) | 0.854 |
| Energy (100 kcal/day) | 1621 | 0.04 (–0.00,0.08) | 0.059 | 1630 | 0.07 (0.03,0.11) | <0.001 |
| Carbohydrates (10 E%) | 1621 | –0.09 (–0.40,0.23) | 0.585 | 1630 | –0.12 (–0.46,0.22) | 0.473 |
| Saccharose (10 g/day) | 1621 | –0.01 (–0.09,0.06) | 0.737 | 1630 | 0.12 (0.04,0.20) | 0.004 |
| Protein (10 E%) | 1621 | 0.44 (–0.34,1.22) | 0.270 | 1630 | 0.05 (–0.76,0.85) | 0.914 |
| Fat (10 E%) | 1621 | 0.02 (–0.37,0.42) | 0.909 | 1630 | 0.18 (–0.24,0.59) | 0.397 |
| Saturated fat (10 E%) | 1621 | –0.04 (–0.77,0.69) | 0.912 | 1630 | 0.30 (–0.45,1.06) | 0.429 |
| Fiber (10 g/day) | 1621 | 0.19 (–0.04,0.42) | 0.109 | 1630 | 0.20 (–0.05,0.44) | 0.114 |
| Healthy Eating Index | 1775 | 0.07 (–0.21,0.34) | 0.641 | 1746 | –0.05 (-0.33,0.24) | 0.757 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Günther, J.; Hoffmann, J.; Kunath, J.; Spies, M.; Meyer, D.; Stecher, L.; Rosenfeld, E.; Kick, L.; Rauh, K.; Hauner, H. Effects of a Lifestyle Intervention in Routine Care on Prenatal Dietary Behavior—Findings from the Cluster-Randomized GeliS Trial. J. Clin. Med. 2019, 8, 960. https://doi.org/10.3390/jcm8070960
Günther J, Hoffmann J, Kunath J, Spies M, Meyer D, Stecher L, Rosenfeld E, Kick L, Rauh K, Hauner H. Effects of a Lifestyle Intervention in Routine Care on Prenatal Dietary Behavior—Findings from the Cluster-Randomized GeliS Trial. Journal of Clinical Medicine. 2019; 8(7):960. https://doi.org/10.3390/jcm8070960
Chicago/Turabian StyleGünther, Julia, Julia Hoffmann, Julia Kunath, Monika Spies, Dorothy Meyer, Lynne Stecher, Eva Rosenfeld, Luzia Kick, Kathrin Rauh, and Hans Hauner. 2019. "Effects of a Lifestyle Intervention in Routine Care on Prenatal Dietary Behavior—Findings from the Cluster-Randomized GeliS Trial" Journal of Clinical Medicine 8, no. 7: 960. https://doi.org/10.3390/jcm8070960
APA StyleGünther, J., Hoffmann, J., Kunath, J., Spies, M., Meyer, D., Stecher, L., Rosenfeld, E., Kick, L., Rauh, K., & Hauner, H. (2019). Effects of a Lifestyle Intervention in Routine Care on Prenatal Dietary Behavior—Findings from the Cluster-Randomized GeliS Trial. Journal of Clinical Medicine, 8(7), 960. https://doi.org/10.3390/jcm8070960





