The Predictive Value of Hyperuricemia on Renal Outcome after Contrast-Enhanced Computerized Tomography
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design and Clinical Data Retrieval
2.2. Outcome Variables
2.3. Statistical Analysis
3. Results
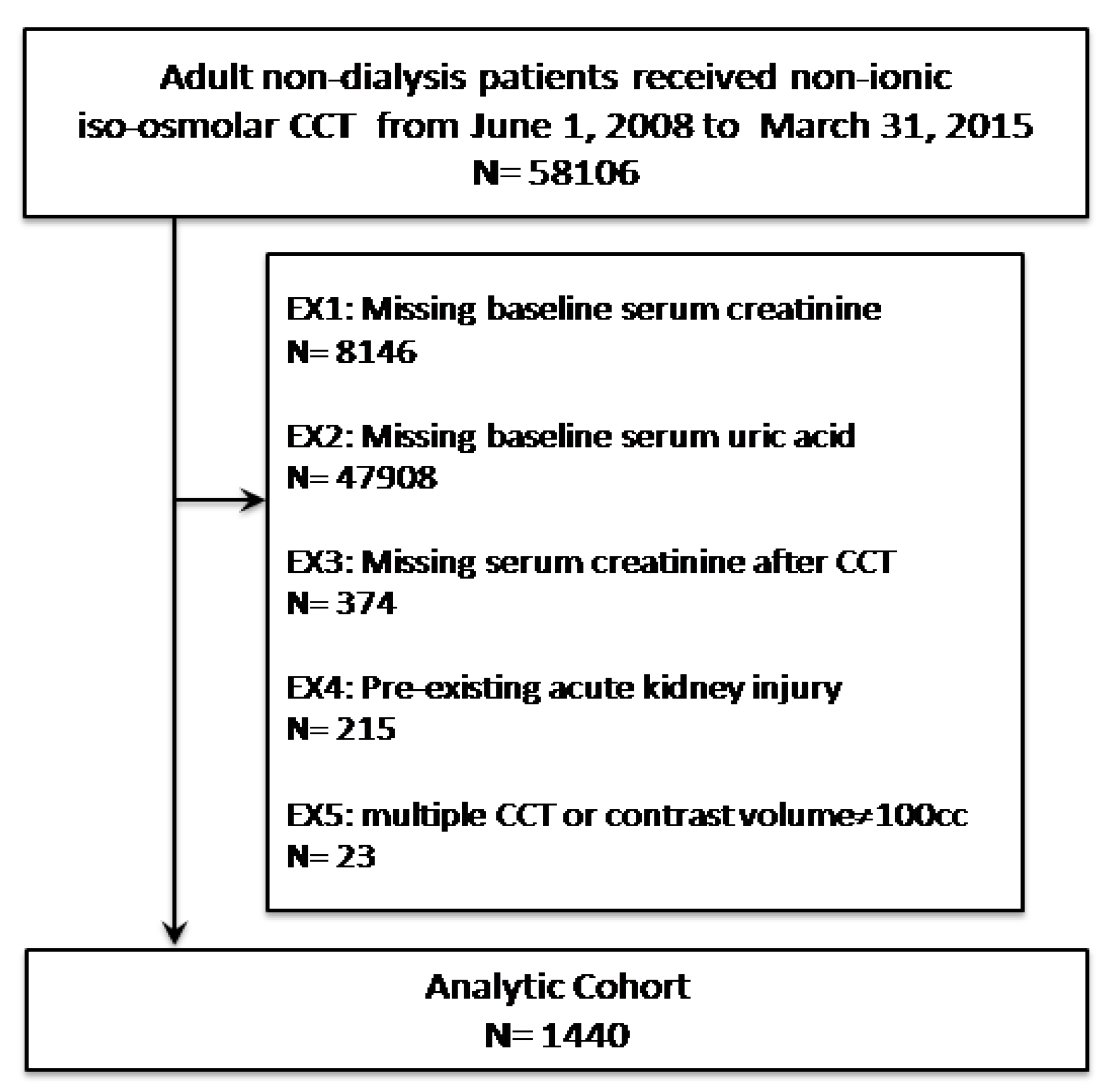
3.1. Study Population
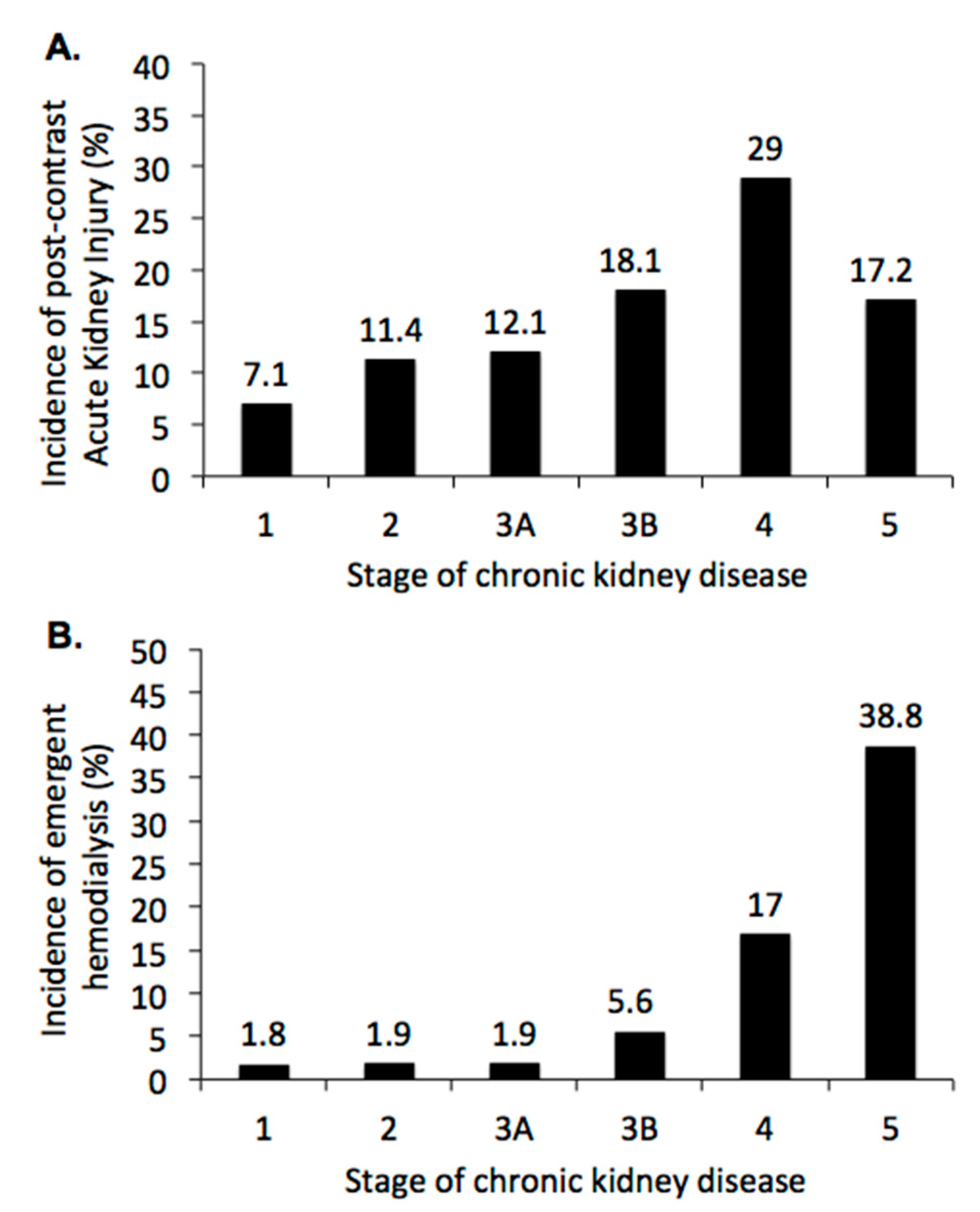
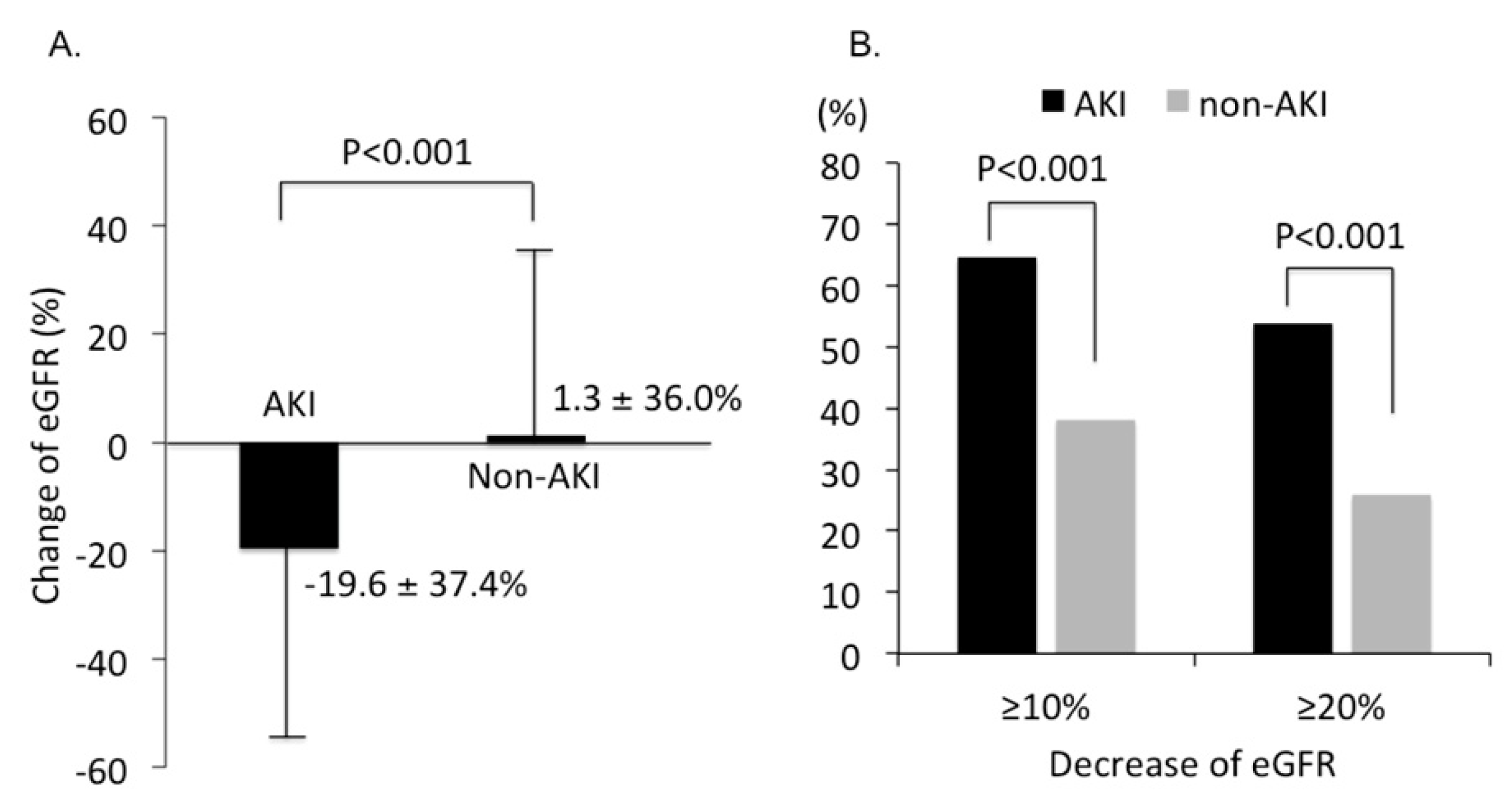
3.2. Renal Outcome Rates after CCT
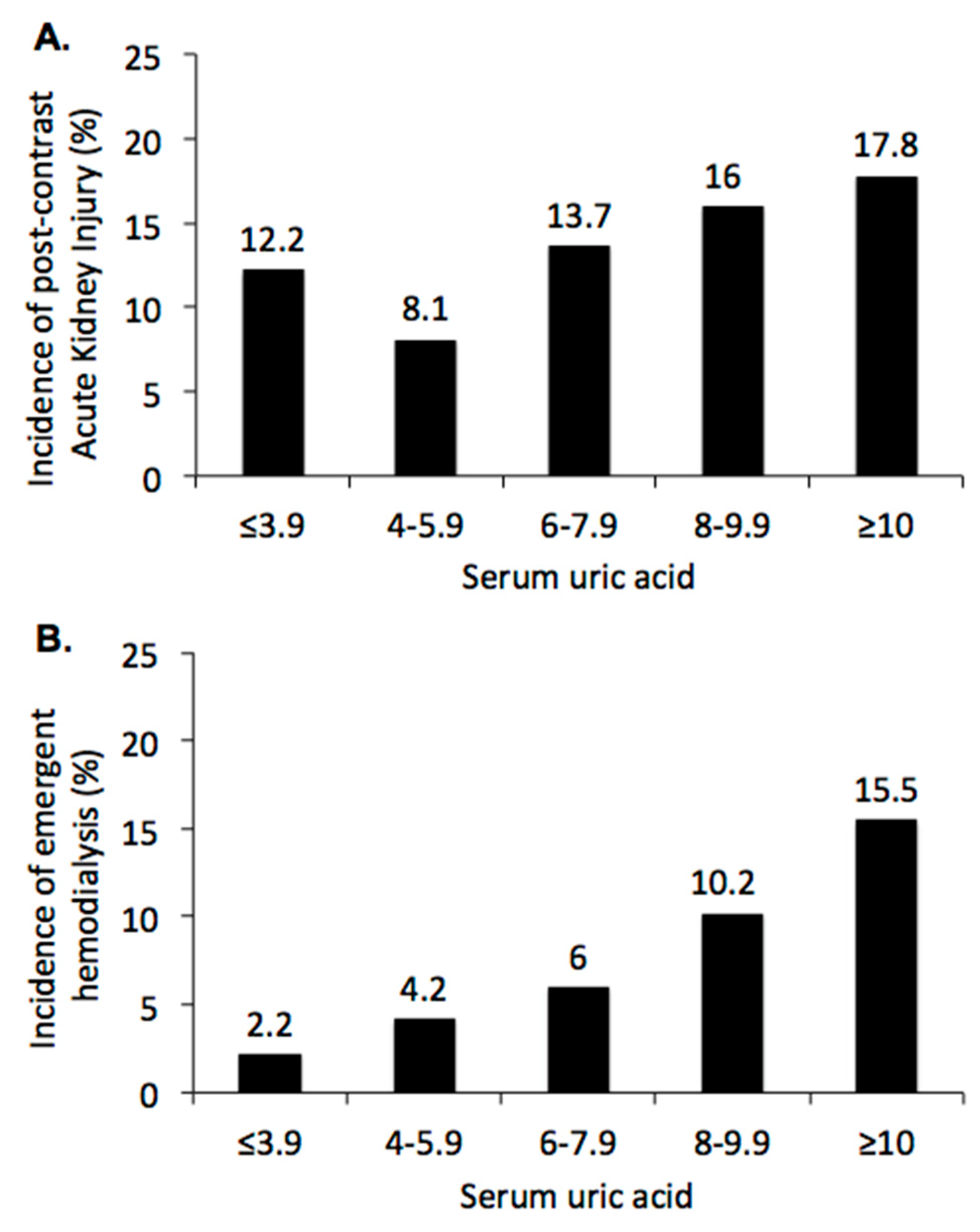
3.3. Sensitivity Analysis: Impact of Serum Acid on Renal Outcome after CCT
3.4. Analysis of Renal Outcome in three Months after CCT
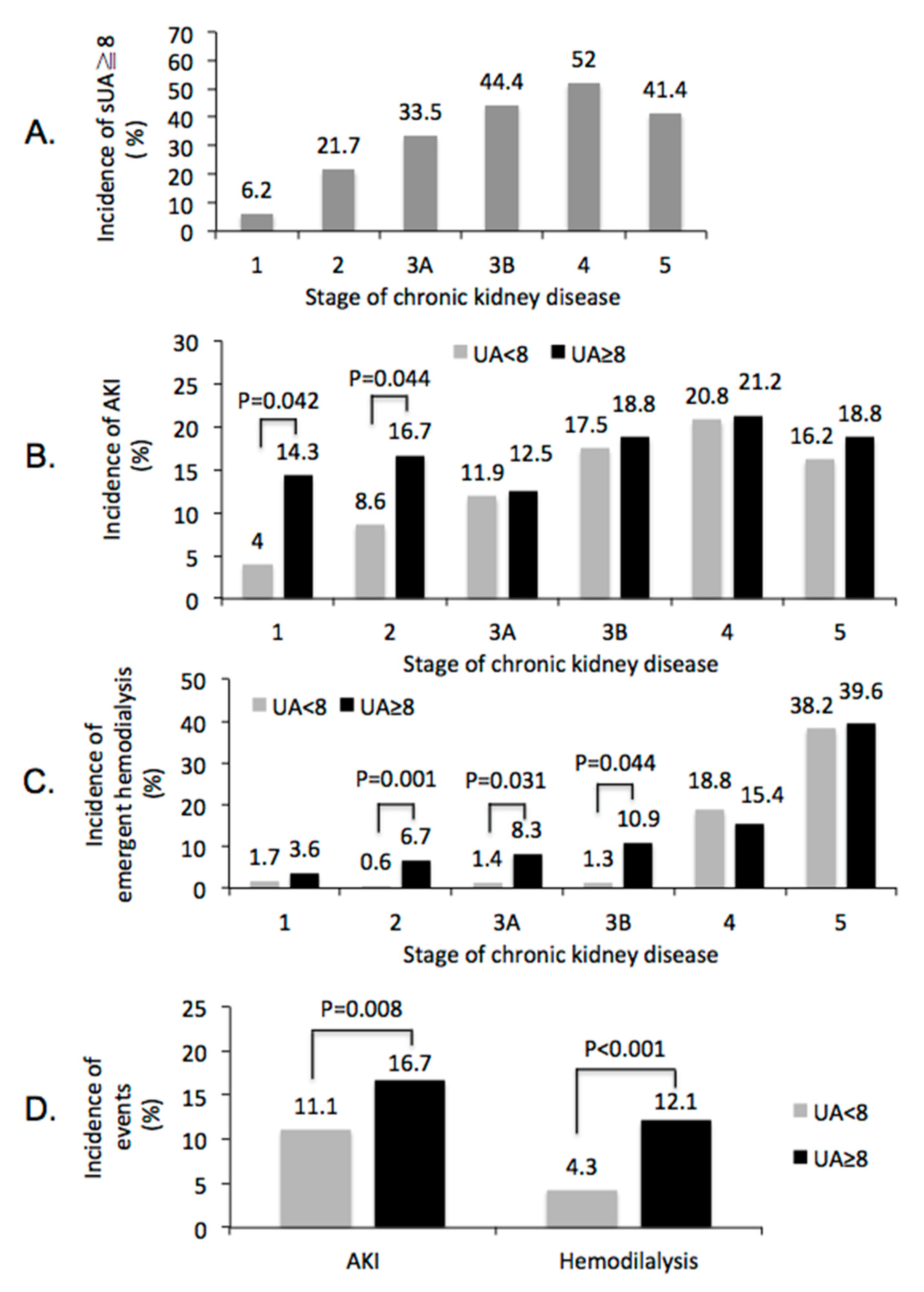
3.5. Subgroups Analysis of Renal Outcome after CCT
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Okusa, M.D.; Davenport, A. Reading between the (guide)lines—The KDIGO practice guideline on acute kidney injury in the individual patient. Kidney Int. 2014, 85, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef] [PubMed]
- Hsu, R.K.; McCulloch, C.E.; Heung, M. Exploring potential reasons for the temporal trend in dialysis-requiring AKI in the United States. Clin. J. Am. Soc. Nephrol. 2016, 11, 14–20. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.A. Contrast-induced acute kidney injury. J. Am. Coll. Cardiol. 2008, 51, 1419–1428. [Google Scholar] [CrossRef]
- Barrett, B.J. Contrast nephrotoxicity. J. Am. Soc. Nephrol. 1994, 5, 125–137. [Google Scholar] [PubMed]
- Weisbord, S.D.; Mor, M.K.; Resnick, A.L.; Hartwig, K.C.; Palevsky, P.M.; Fine, M.J. Incidence and outcomes of contrast-induced AKI following computed tomography. Clin. J. Am. Soc. Nephrol. 2008, 3, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Horton, R.; Berman, P. Eliminating acute kidney injury by 2025: An achievable goal. Lancet 2015, 385, 2551–2552. [Google Scholar] [CrossRef]
- Shimada, M.; Johnson, R.J.; May, W.S.J.; Lingegowda, V.; Sood, P.; Nakagawa, T.; Van, Q.C.; Dass, B.; Ejaz, A.A. A novel role for uric acid in acute kidney injury associated with tumour lysis syndrome. Nephrol. Dial. Transplant. 2009, 24, 2960–2964. [Google Scholar] [CrossRef]
- Umekawa, T.; Chegini, N.; Khan, S.R. Increased expression of monocyte chemoattractant protein-1 (MCP-1) by renal epithelial cells in culture on exposure to calcium oxalate, phosphate and uric acid crystals. Nephrol. Dial. Transplant. 2003, 18, 664–669. [Google Scholar] [CrossRef]
- Devarajan, P. Update on mechanisms of ischemic acute kidney injury. J. Am. Soc. Nephrol. 2006, 17, 1503–1520. [Google Scholar] [CrossRef]
- Sánchez-Lozada, L.G.; Tapia, E.; Santamaría, J.; Avila-Casado, C.; Soto, V.; Nepomuceno, T.; Rodríguez-Iturbe, B.; Johnson, R.J.; Herrera-Acosta, J. Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int. 2005, 67, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Lapsia, V.; Johnson, R.J.; Dass, B.; Shimada, M.; Kambhampati, G.; Ejaz, N.I.; Arif, A.A.; Ejaz, A.A. Elevated uric acid increases the risk for acute kidney injury. Am. J. Med. 2012, 125, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Choi, J.H.; Joung, K.W.; Kim, J.Y.; Baek, S.H.; Ji, S.M.; Chin, J.H.; Choi, I.C. Relationship between serum uric acid concentration and acute kidney injury after coronary artery bypass surgery. J. Korean Med. Sci. 2015, 30, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Durante, P.; Romero, F.; Pérez, M.; Chávez, M.; Parra, G. Effect of uric acid on nephrotoxicity induced by mercuric chloride in rats. Toxicol. Ind. Health 2010, 26, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Hillis, G.S.; Cuthbertson, B.H.; Gibson, P.H.; McNeilly, J.D.; Maclennan, G.S.; Jeffrey, R.R.; Buchan, K.G.; El-Shafei, H.; Gibson, G.; Croal, B.L. Uric acid levels and outcome from coronary artery bypass grafting. J. Thorac. Cardiovasc. Surg. 2009, 138, 200–205. [Google Scholar] [CrossRef]
- Joung, K.W.; Jo, J.Y.; Kim, W.J.; Choi, D.K.; Chin, J.H.; Lee, E.H.; Choi, I.C. Association of preoperative uric acid and acute kidney injury following cardiovascular surgery. J. Cardiothorac. Vasc. Anesth. 2014, 28, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tan, N.; Chen, J.; Zhou, Y.L.; Chen, L.L.; Chen, S.Q.; Chen, Z.J.; Li, L.W. The relationship between hyperuricemia and the risk of contrast-induced acute kidney injury after percutaneous coronary intervention in patients with relatively normal serum creatinine. Clinics 2013, 68, 19–25. [Google Scholar] [CrossRef]
- Park, S.H.; Shin, W.Y.; Lee, E.Y.; Gil, H.W.; Lee, S.W.; Lee, S.J.; Jin, D.K.; Hong, S.Y. The impact of hyperuricemia on in-hospital mortality and incidence of acute kidney injury in patients undergoing percutaneous coronary intervention. Circ. J. 2011, 75, 692–697. [Google Scholar] [CrossRef]
- Cheungpasitporn, W.; Thongprayoon, C.; Harrison, A.M.; Erickson, S.B. Admission hyperuricemia increases the risk of acute kidney injury in hospitalized patients. Clin. Kidney J. 2016, 9, 51–56. [Google Scholar] [CrossRef]
- Mehran, R.; Dangas, G.D.; Weisbord, S.D. Contrast-Associated Acute Kidney Injury. N. Engl. J. Med. 2019, 380, 2146–2155. [Google Scholar] [CrossRef]
- Silver, S.A.; Shah, P.M.; Chertow, G.M.; Harel, S.; Wald, R.; Harel, Z. Risk prediction models for contrast induced nephropathy: Systematic review. BMJ 2015, 351, h4395. [Google Scholar] [CrossRef] [PubMed]
- Toprak, O. Conflicting and new risk factors for contrast induced nephropathy. J. Urol. 2007, 178, 2277–2283. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Coresh, J.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Int. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, A.A.; Dass, B.; Kambhampati, G.; Ejaz, N.I.; Maroz, N.; Dhatt, G.S.; Arif, A.A.; Faldu, C.; Lanaspa, M.A.; Shah, G.; et al. Lowering serum uric acid to prevent acute kidney injury. Med. Hypotheses 2012, 78, 796–799. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, A.A.; Beaver, T.M.; Shimada, M.; Sood, P.; Lingegowda, V.; Schold, J.D.; Kim, T.; Johnson, R.J. Uric acid: A novel risk factor for acute kidney injury in high-risk cardiac surgery patients? Am. J. Nephrol. 2009, 30, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, M.; Bjounstad, P.; Hisatome, I.; Niwa, K.; Roncal-Jimenez, C.A.; Andres-Hernando, A.; Jensen, T.; Milagres, T.; Sato, Y.; Garcia, G.; et al. Elevated Serum Uric Acid Level Predicts Rapid Decline in Kidney Function. Am. J. Nephrol. 2017, 45, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, A.A.; Dass, B.; Lingegowda, V.; Shimada, M.; Beaver, T.M.; Ejaz, N.I.; Abouhamze, A.S.; Johnson, R.J. Effect of uric acid lowering therapy on the prevention of acute kidney injury in cardiovascular surgery. Int. Urol. Nephrol. 2013, 45, 449–458. [Google Scholar] [CrossRef]
- Steinhäuslin, F.; Burnier, M.; Magnin, J.L.; Munafo, A.; Buclin, T.; Diezi, J.; Biollaz, J. Fractional excretion of trace lithium and uric acid in acute renal failure. J. Am. Soc. Nephrol. 1994, 4, 1429–1437. [Google Scholar]
- Kaneko, K.; Taniguchi, N.; Tanabe, Y.; Nakano, T.; Hasui, M.; Nozu, K. Oxidative imbalance in idiopathic renal hypouricemia. Pediatr. Nephrol. 2009, 24, 869–871. [Google Scholar] [CrossRef]
- Sánchez-Lozada, L.G.; Soto, V.; Tapia, E.; Avila-Casado, C.; Sautin, Y.Y.; Nakagawa, T.; Franco, M.; Rodríguez-Iturbe, B.; Johnson, R.J. Role of oxidative stress in the renal abnormalities induced by experimental hyperuricemia. Am. J. Physiol. Renal Physiol. 2008, 295, F1134–F1141. [Google Scholar] [CrossRef]
- Zuo, T.; Jiang, L.; Mao, S.; Liu, X.; Guo, L. Hyperuricemia and contrast-induced acute kidney injury: A systematic review and meta-analysis. Int. J. Cardiol. 2016, 224, 286–294. [Google Scholar] [CrossRef] [PubMed]
| Serum Uric Acid (Number) | ≤3.9 (N = 270) | 4.0–5.9 (N = 430) | 6.0–7.9 (N = 386) | 8–9.9 (N = 225) | ≥10 (N = 129) | Total (N = 1440) | p Value |
|---|---|---|---|---|---|---|---|
| Clinical characteristics | |||||||
| Age (years) | 63.2 ± 17.0 | 65.2 ± 15.5 | 67.5 ± 14.5 | 68.8 ± 14.5 | 67.5 ± 16.9 | 66.2 ± 15.7 | <0.0001 |
| Age ≥ 65 years * | 131 (48.5%) | 229 (53.3%) | 244 (63.2%) | 141 (62.7%) | 76 (59%) | 821 (57%) | <0.0001 |
| Male sex * | 176 (65.2%) | 279 (64.9%) | 254 (65.8%) | 162 (72%) | 93 (72.1%) | 964 (66.9%) | 0.233 |
| Status of renal function | |||||||
| Stage 1 CKD | 175 (64.8%) | 177 (41.2%) | 71 (18.4%) | 20 (8.9%) | 8 (6.2%) | 451 (31.3%) | <0.0001 |
| Stage 2 CKD | 56 (20.7%) | 135 (31.4%) | 133 (34.5%) | 60 (26.7%) | 30 (23.3%) | 414 (28.8%) | |
| Stage 3A CKD | 14 (5.2%) | 57 (13.3%) | 72 (18.7%) | 53 (23.6%) | 19 (14.7%) | 215 (14.9%) | |
| Stage 3B CKD | 10 (3.7%) | 27 (6.3%) | 43 (11.1%) | 31 (13.8%) | 33 (25.6%) | 144 (10%) | |
| Stage 4 CKD | 4 (1.5%) | 14 (3.3%) | 30 (7.8%) | 29 (12.9%) | 23 (17.8%) | 100 (6.9%) | |
| Stage 5 CKD | 11 (4.1%) | 20 (4.7%) | 37 (9.6%) | 32 (14.2%) | 16 (12.4%) | 116 (8.1%) | |
| Comorbidity | |||||||
| Cancer * | 82 (30.4%) | 141 (32.8%) | 115 (29.8%) | 77 (34.2%) | 37 (28.7%) | 452 (31.4%) | 0.689 |
| Diabetic mellitus * | 93 (34.4%) | 153 (35.6%) | 132 (34.2%) | 87 (38.7%) | 49 (38%) | 514 (35.7%) | 0.786 |
| Hypertension * | 125 (46.3%) | 233 (54.2%) | 271 (70.2%) | 154 (68.4%) | 87 (67.4%) | 870 (60.4%) | <0.0001 |
| CAD * | 48 (17.8%) | 107 (24.9%) | 127 (32.9%) | 60 (26.7%) | 47 (36.4%) | 389 (27%) | <0.0001 |
| Heart failure * | 15 (5.6%) | 30 (7%) | 28 (7.3%) | 14 (6.2%) | 23 (17.8%) | 110 (7.6%) | <0.0001 |
| Atrial fibrillation * | 27 (10%) | 45 (10.5%) | 59 (15.3%) | 25 (11.1%) | 35 (27.1%) | 191 (13.3%) | <0.0001 |
| CVA * | 43 (15.9%) | 100 (23.3%) | 87 (22.5%) | 47 (20.9%) | 27 (20.9%) | 304 (21.1%) | 0.197 |
| Chronic liver disease * | 21 (7.8%) | 34 (7.9%) | 32 (8.3%) | 24 (10.7%) | 21 (16.3%) | 132 (9.2%) | 0.036 |
| PAOD * | 5 (1.9%) | 19 (4.4%) | 24 (6.2%) | 14 (6.2%) | 9 (7%) | 71 (4.9%) | 0.061 |
| Shock * | 30 (11.1%) | 18 (4.2%) | 14 (3.6%) | 11 (4.9%) | 14 (10.9%) | 87 (6%) | <0.0001 |
| GI bleeding * | 12 (4.4%) | 9 (2.1%) | 15 (3.9%) | 11 (4.9%) | 9 (7%) | 56 (3.9%) | 0.098 |
| Laboratory data | |||||||
| Serum Albumin | 3.0 ± 0.6 | 3.4 ± 0.8 | 3.4 ± 0.7 | 3.4 ± 0.7 | 3.3 ± 0.7 | 3.3 ± 0.7 | <0.0001 |
| Hemoglobin | 10.3 ± 2.4 | 11.5 ± 2.6 | 11.3 ± 2.6 | 11.3 ± 2.6 | 10.8 ± 2.6 | 11.1 ± 2.6 | <0.0001 |
| Odd Ratio | 95% Confident Interval | p Value | |
|---|---|---|---|
| Risk of acute kidney injury | |||
| Unadjusted | 1.54 | 1.10~2.18 | 0.013 |
| Adjusted, model 1 | 2.40 | 1.31~4.42 | 0.005 |
| Adjusted, model 2 | 2.62 | 1.27~5.38 | 0.009 |
| Risk of dialysis within 30 days after CCT | |||
| Unadjusted | 2.93 | 1.90~4.52 | <0.0001 |
| Adjusted, model 1 | 6.42 | 1.91~21.56 | 0.003 |
| Adjusted, model 2 | 5.40 | 1.39~21.04 | 0.015 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.-J.; Tsai, S.-F.; Lee, C.-T.; Wu, C.-Y. The Predictive Value of Hyperuricemia on Renal Outcome after Contrast-Enhanced Computerized Tomography. J. Clin. Med. 2019, 8, 1003. https://doi.org/10.3390/jcm8071003
Wu M-J, Tsai S-F, Lee C-T, Wu C-Y. The Predictive Value of Hyperuricemia on Renal Outcome after Contrast-Enhanced Computerized Tomography. Journal of Clinical Medicine. 2019; 8(7):1003. https://doi.org/10.3390/jcm8071003
Chicago/Turabian StyleWu, Ming-Ju, Shang-Feng Tsai, Cheng-Ting Lee, and Chun-Yi Wu. 2019. "The Predictive Value of Hyperuricemia on Renal Outcome after Contrast-Enhanced Computerized Tomography" Journal of Clinical Medicine 8, no. 7: 1003. https://doi.org/10.3390/jcm8071003
APA StyleWu, M.-J., Tsai, S.-F., Lee, C.-T., & Wu, C.-Y. (2019). The Predictive Value of Hyperuricemia on Renal Outcome after Contrast-Enhanced Computerized Tomography. Journal of Clinical Medicine, 8(7), 1003. https://doi.org/10.3390/jcm8071003






