Analgesic and Antidepressant Effects of Oltipraz on Neuropathic Pain in Mice by Modulating Microglial Activation
Abstract
1. Introduction
2. Experimental Section
2.1. Animals
2.2. Induction of Neuropathic Pain
2.3. Nociceptive Testing
2.4. Measurement of Depressive-Like Behavior
2.5. Western Blot Analysis
2.6. Experimental Procedures
2.7. Drugs
2.8. Statistical Analyses
3. Results
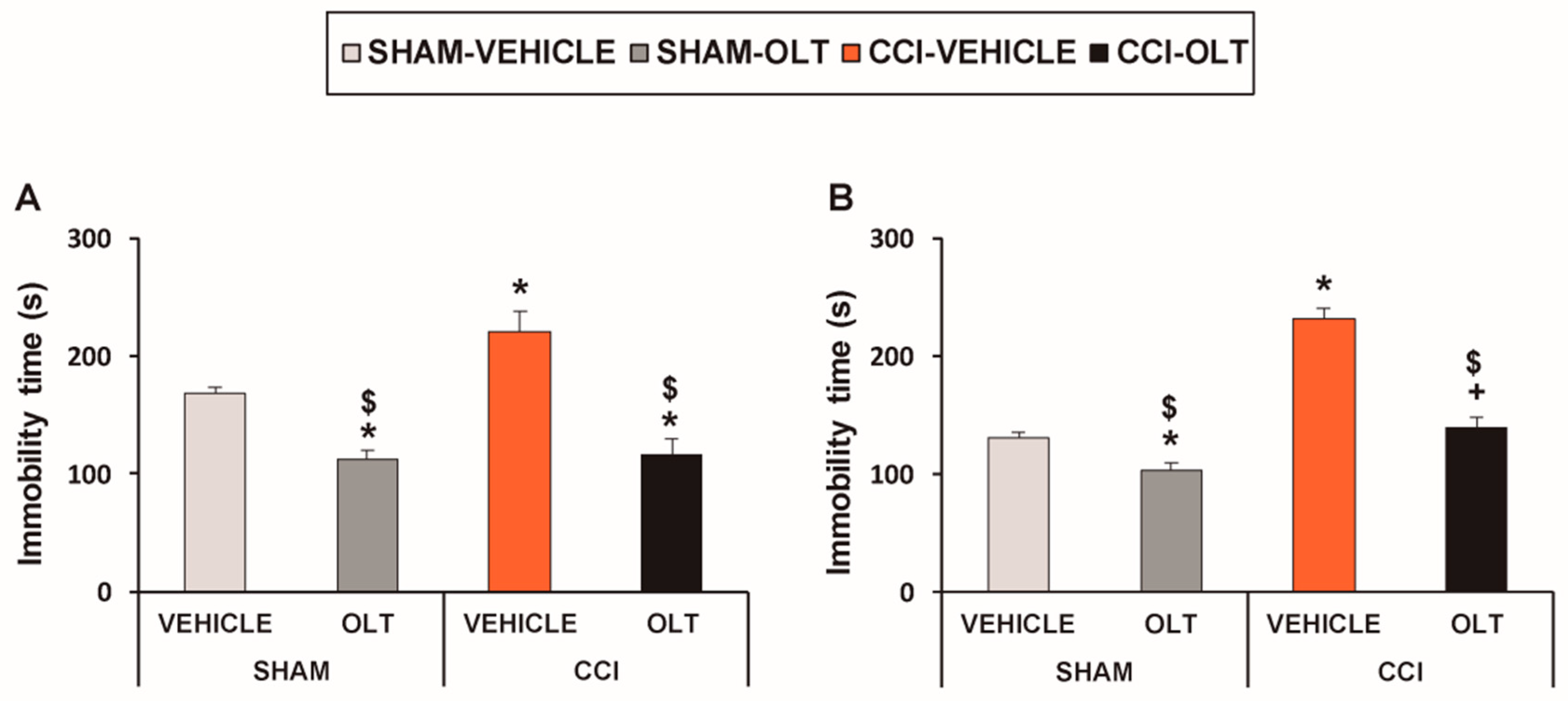
3.1. Treatment with Oltipraz Produces Antinociceptive and Antidepressant Effects in CCI-Injured Mice
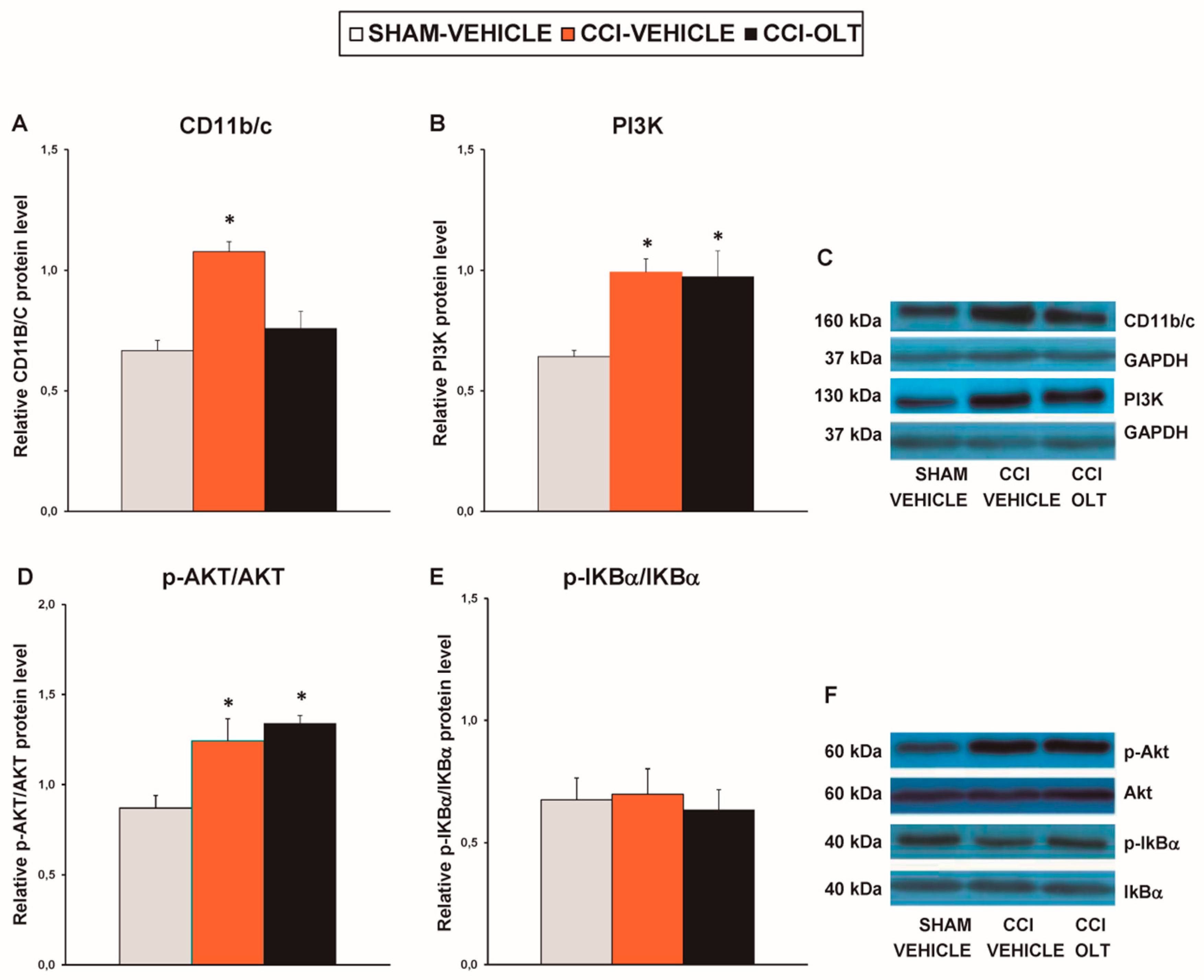
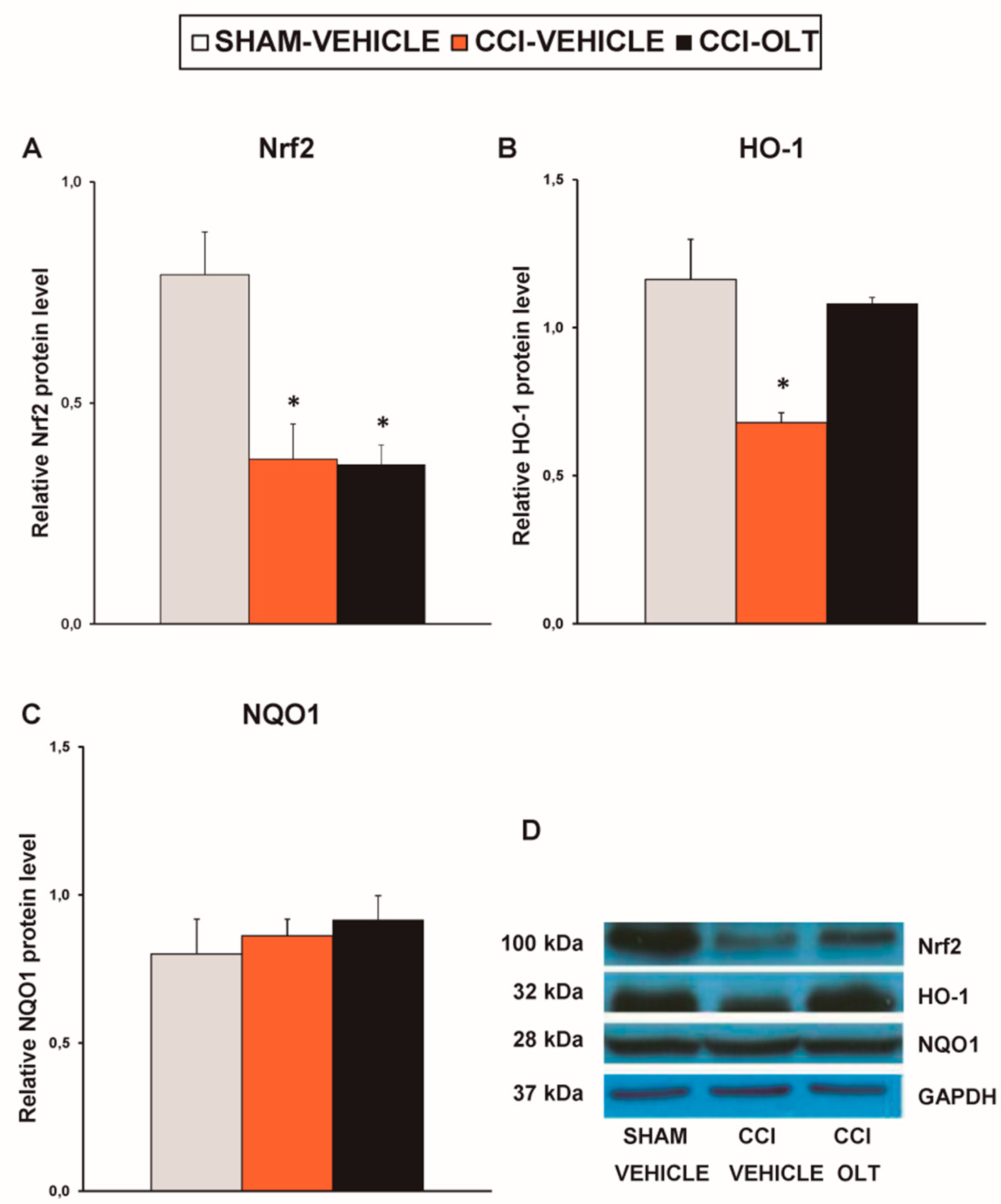
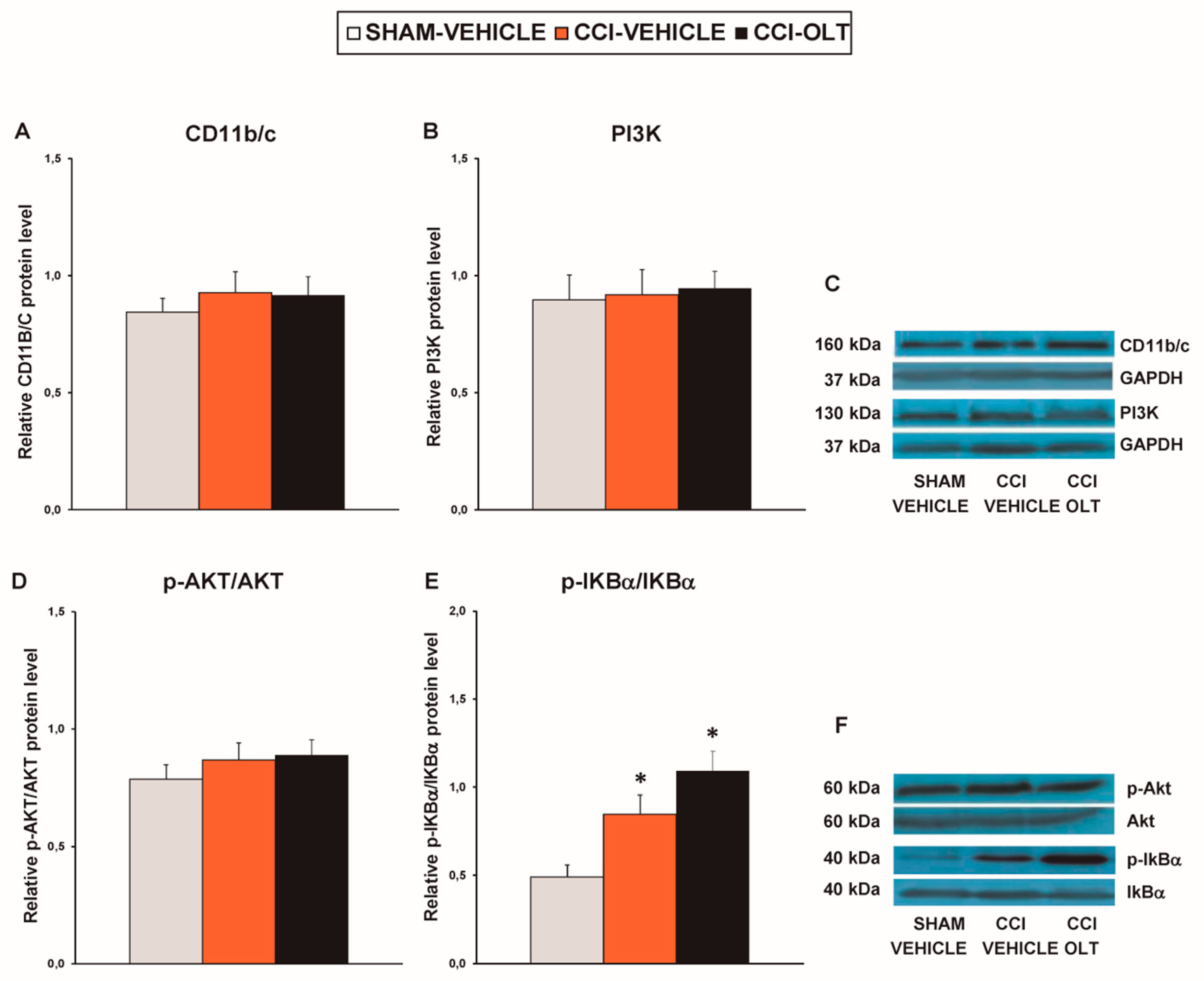
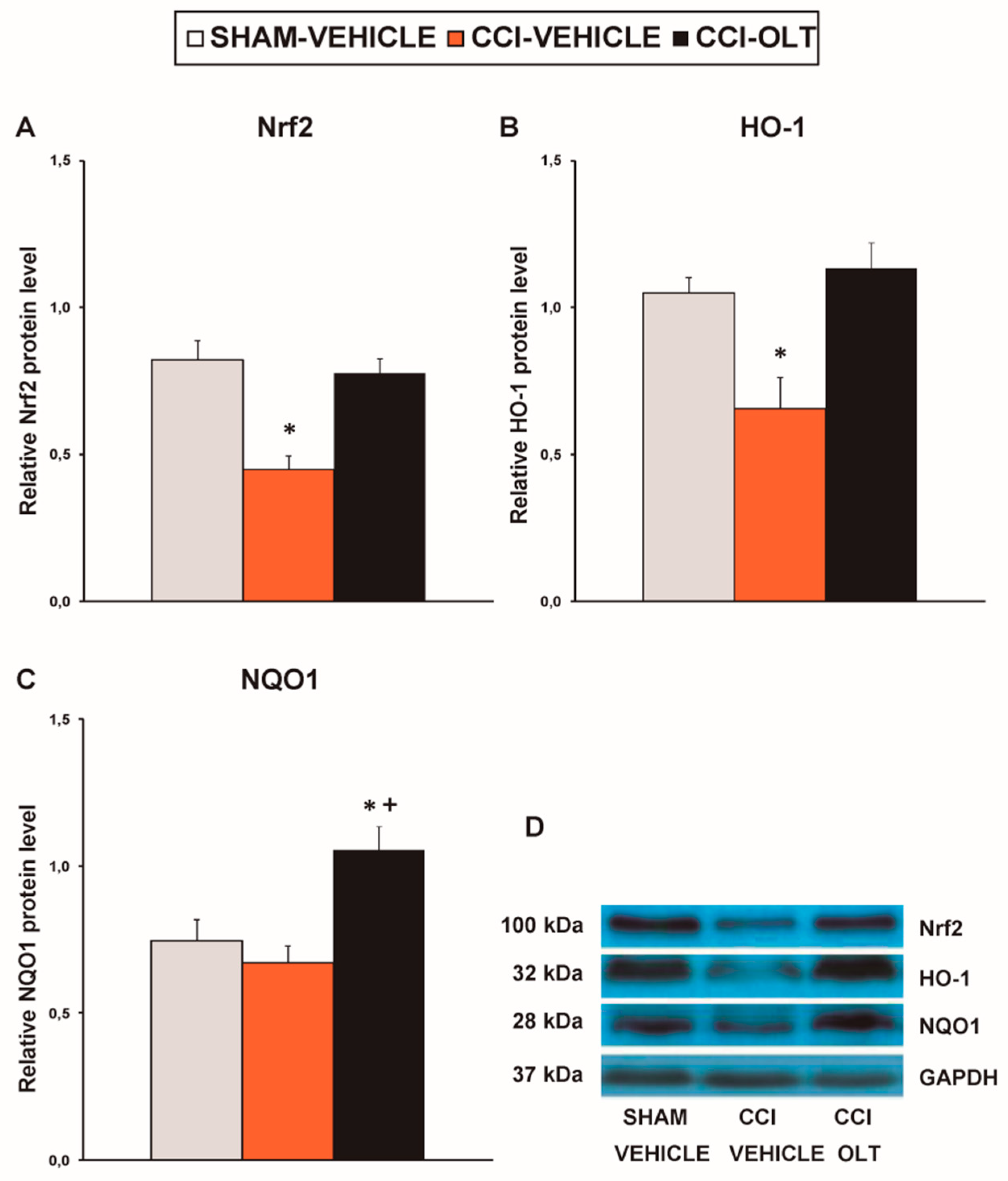
3.2. Effect of Oltipraz on Expression of CD11b/c, PI3K/p-Akt, p-IkBα, Nrf2, HO-1, and NQO1 in Spinal Cord of CCI-Injured Mice
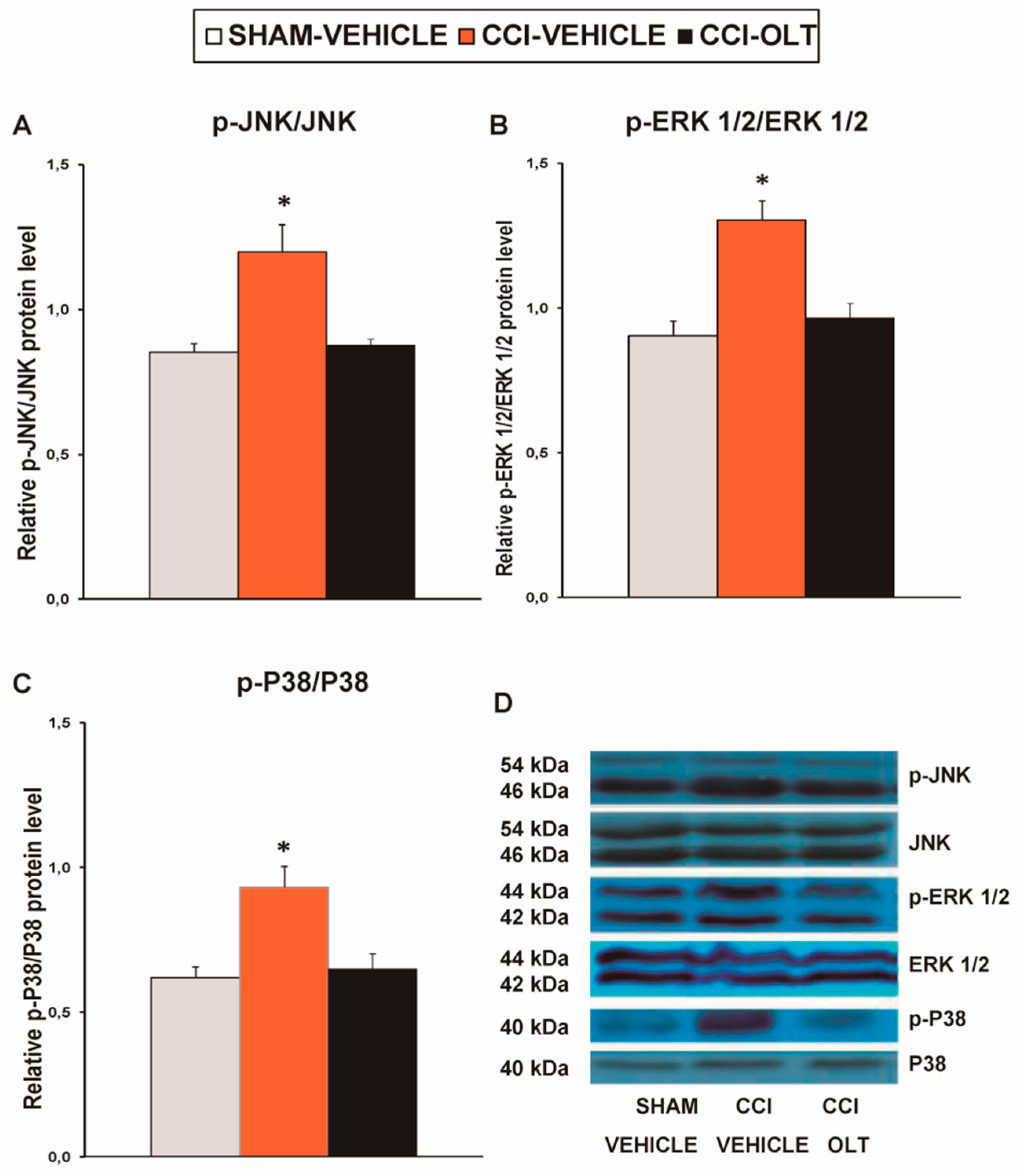
3.3. Effect of Oltipraz on Expression of p-JNK, p-ERK ½, and p-P38 in Spinal Cord of CCI-Injured Mice
3.4. Effect of oltipraz on Expression of CD11b/c, PI3K/p-Akt, p-IkBα, Nrf2, HO-1, and NQO1 in Hippocampus of CCI-Injured Mice
3.5. Effect of Oltipraz on Expression of CD11b/c, PI3K/p-Akt, p-IkBα, Nrf2, HO-,1 and NQO1 in Prefrontal Cortex of the CCI-Injured Mice
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Gonçalves, L.; Silva, R.; Pinto-Ribeiro, F.; Pêgo, J.M.; Bessa, J.M.; Pertovaara, A.; Sousa, N.; Almeida, A. Neuropathic pain is associated with depressive behaviour and induces neuroplasticity in the amygdala of the rat. Exp. Neurol. 2008, 213, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Kawai, A.T.; Wollan, P.; Yawn, B.P. Adverse impacts of chronic pain on health-related quality of life, work productivity, depression and anxiety in a community-based study. Fam. Pract. 2017, 34, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Popiolek-Barczyk, K.; Mika, J. Targeting the microglial signaling pathways: New insights in the modulation of neuropathic pain. Curr. Med. Chem. 2016, 23, 2908–2928. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.J.; Jia, L.; Zhang, X.; Wei, H.; Yue, S.W. MAPK pathways are involved in neuropathic pain in rats with chronic compression of the dorsal root ganglion. Evid. Based Complement. Alternat. Med. 2016, 2016, 6153215. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, Y.Q.; Qadri, Y.J.; Serhan, C.N.; Ji, R.R. Microglia in pain: Detrimental and protective roles in pathogenesis and resolution of pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Lv, Y.; Ren, F. PI3K/Akt pathway is required for spinal central sensitization in neuropathic pain. Cell. Mol. Neurobiol. 2018, 38, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.X.; Zhuang, Z.Y.; Woolf, C.J.; Ji, R.R. P38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J. Neurosci. 2003, 23, 4017–4022. [Google Scholar] [CrossRef] [PubMed]
- Hervera, A.; Leánez, S.; Negrete, R.; Motterlini, R.; Pol, O. Carbon monoxide reduces neuropathic pain and spinal microglial activation by inhibiting nitric oxide synthesis in mice. PLoS ONE 2012, 7, e43693. [Google Scholar] [CrossRef] [PubMed]
- Galan-Arriero, I.; Avila-Martin, G.; Ferrer-Donato, A.; Gomez-Soriano, J.; Bravo-Esteban, E.; Taylor, J. Oral administration of the p38α MAPK inhibitor, UR13870, inhibits affective pain behavior after spinal cord injury. Pain 2014, 155, 2188–2198. [Google Scholar] [CrossRef] [PubMed]
- Sawada, A.; Niiyama, Y.; Ataka, K.; Nagaishi, K.; Yamakage, M.; Fujimiya, M. Suppression of bone marrow-derived microglia in the amygdala improves anxiety-like behavior induced by chronic partial sciatic nerve ligation in mice. Pain 2014, 155, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, L.J.; Wang, J.; Li, D.; Ren, W.J.; Peng, J.; Wei, X.; Xu, T.; Xin, W.J.; Pang, R.P.; et al. TNF-α differentially regulates synaptic plasticity in the hippocampus and spinal cord by microglia-dependent mechanisms after peripheral nerve injury. J. Neurosci. 2017, 37, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Riego, G.; Redondo, A.; Leánez, S.; Pol, O. Mechanism implicated in the anti-allodynic and anti-hyperalgesic effects induced by the activation of heme oxygenase 1/carbon monoxide signaling pathway in the central nervous system of mice with neuropathic pain. Biochem. Pharmacol. 2018, 148, 52–63. [Google Scholar] [CrossRef]
- Zhang, F.; Vadakkan, K.; Kim, S.S.; Wu, L.J.; Shang, Y.; Zhuo, M. Selective activation of microglia in spinal cord but not higher cortical regions following nerve injury in adult mouse. Mol. Pain 2008, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Q.; Liu, D.Q.; Chen, S.; Sun, J.; Wang, X.M.; Tian, Y.K.; Wu, W.; Ye, D.W. Minocycline as a promising therapeutic strategy for chronic pain. Pharmacol. Res. 2018, 134, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Mizokoshi, A.; Shigemoto-Mogami, Y.; Koizumi, S.; Inoue, K. Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia 2004, 45, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Obata, K.; Katsura, H.; Mizushima, T.; Sakurai, J.; Kobayashi, K.; Yamanaka, H.; Dai, Y.; Fukuoka, T.; Noguchi, K. Roles of extracellular signal-regulated protein kinases 5 in spinal microglia and primary sensory neurons for neuropathic pain. J. Neurochem. 2007, 102, 1569–1584. [Google Scholar] [CrossRef]
- Ji, R.R.; Suter, M.R. P38 MAPK, microglial signaling, and neuropathic pain. Mol. Pain 2007, 3, 33. [Google Scholar] [CrossRef]
- Fu, E.S.; Zhang, Y.P.; Sagen, J.; Candiotti, K.A.; Morton, P.D.; Liebl, D.J.; Bethea, J.R.; Brambilla, R. Transgenic inhibition of glial NF-kappa B reduces pain behavior and inflammation after peripheral nerve injury. Pain 2010, 148, 509–518. [Google Scholar] [CrossRef]
- Guo, J.R.; Wang, H.; Jin, X.J.; Jia, D.L.; Zhou, X.; Tao, Q. Effect and mechanism of inhibition of PI3K/Akt/mTOR signal pathway on chronic neuropathic pain and spinal microglia in a rat model of chronic constriction injury. Oncotarget 2017, 8, 52923–52934. [Google Scholar] [CrossRef]
- Staurengo-Ferrari, L.; Badaro-Garcia, S.; Hohmann, M.S.N.; Manchope, M.F.; Zaninelli, T.H.; Casagrande, R.; Verri, W.A., Jr. Contribution of Nrf2 modulation to the mechanism of action of analgesic and anti-inflammatory drugs in pre-clinical and clinical stages. Front. Pharmacol. 2019, 9, 1536. [Google Scholar] [CrossRef]
- Li, S.; Yang, C.; Fang, X.; Zhan, G.; Huang, N.; Gao, J.; Xu, H.; Hashimoto, K.; Luo, A. Role of Keap1-Nrf2 signaling in anhedonia symptoms in a rat model of chronic neuropathic pain: Improvement with sulforaphane. Front. Pharmacol. 2018, 9, 887. [Google Scholar] [CrossRef] [PubMed]
- Negi, G.; Kumar, A.; Sharma, S.S. Nrf2 and NF-κB modulation by sulforaphane counteracts multiple manifestations of diabetic neuropathy in rats and high glucose-induced changes. Curr. Neurovasc. Res. 2011, 8, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Castany, S.; Carcolé, M.; Leánez, S.; Pol, O. The induction of heme oxygenase 1 decreases painful diabetic neuropathy and enhances the antinociceptive effects of morphine in diabetic mice. PLoS ONE 2016, 11, e0146427. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, C.; Leánez, S.; Pol, O. The induction of the transcription factor Nrf2 enhances the antinociceptive effects of delta-opioid receptors in diabetic mice. PLoS ONE 2017, 12, e0180998. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, C.; Leánez, S.; Pol, O. The Inhibitory Effects of cobalt protoporphyrin ix and cannabinoid 2 receptor agonists in type 2 diabetic mice. Int. J. Mol. Sci. 2017, 18, 2268. [Google Scholar] [CrossRef] [PubMed]
- Hervera, A.; Gou, G.; Leánez, S.; Pol, O. Effects of treatment with a carbon monoxide-releasing molecule and a heme oxygenase 1 inducer in the antinociceptive effects of morphine in different models of acute and chronic pain in mice. Psychopharmacology 2013, 228, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Carcolé, M.; Castany, S.; Leánez, S.; Pol, O. Treatment with a heme oxygenase 1 inducer enhances the antinociceptive effects of µ-opioid, δ-opioid, and cannabinoid 2 receptors during inflammatory pain. J. Pharmacol. Exp. Ther. 2014, 351, 224–232. [Google Scholar] [CrossRef]
- Redondo, A.; Chamorro, P.A.F.; Riego, G.; Leánez, S.; Pol, O. Treatment with sulforaphane produces antinociception and improves morphine effects during inflammatory pain in mice. J. Pharmacol. Exp. Ther. 2017, 363, 293–302. [Google Scholar] [CrossRef]
- Calixto-Campos, C.; Carvalho, T.T.; Hohmann, M.S.; Pinho-Ribeiro, F.A.; Fattori, V.; Manchope, M.F.; Zarpelon, A.C.; Baracat, M.M.; Georgetti, S.R.; Casagrande, R.; et al. Vanillic acid inhibits inflammatory pain by inhibiting neutrophil recruitment, oxidative stress, cytokine production, and NFκB activation in mice. J. Nat. Prod. 2015, 78, 1799–1808. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C. Anti-nociceptive and anti-inflammatory actions of sulforaphane in chronic constriction injury-induced neuropathic pain mice. Inflammopharmacology 2017, 25, 99–106. [Google Scholar] [CrossRef]
- Ferreira-Chamorro, P.; Redondo, A.; Riego, G.; Leánez, S.; Pol, O. Sulforaphane inhibited the nociceptive responses, anxiety- and depressive-like behaviors associated with neuropathic pain and improved the anti-allodynic effects of morphine in mice. Front. Pharmacol. 2018, 9, 1332. [Google Scholar] [CrossRef] [PubMed]
- Boccella, S.; Guida, F.; De Logu, F.; De Gregorio, D.; Mazzitelli, M.; Belardo, C.; Iannotta, M.; Serra, N.; Nassini, R.; De Novellis, V.; et al. Ketones and pain: Unexplored role of hydroxyl carboxylic acid receptor type 2 in the pathophysiology of neuropathic pain. FASEB J. 2019, 33, 1062–1073. [Google Scholar] [CrossRef] [PubMed]
- Kensler, T.W.; Egner, P.A.; Dolan, P.M.; Groopman, J.D.; Roebuck, B.D. Mechanism of protection against aflatoxin tumorigenicity in rats fed 5-(2-pyrazinyl)-4-methyl-1,2-dithiol-3-thione (oltipraz) and related 1,2-dithiol-3-thiones and 1,2-dithiol-3-ones. Cancer Res. 1987, 47, 4271–4277. [Google Scholar] [PubMed]
- Kensler, T.W.; Groopman, J.D.; Sutter, T.R.; Curphey, T.J.; Roebuck, B.D. Development of cancer chemopreventive agents: oltipraz as a paradigm. Chem. Res. Toxicol. 1999, 12, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Killeen, M.E.; Englert, J.A.; Stolz, D.B.; Song, M.; Han, Y.; Delude, R.L.; Kellum, J.A.; Fink, M.P. The phase 2 enzyme inducers ethacrynic acid, DL-sulforaphane, and oltipraz inhibit lipopolysaccharide-induced high-mobility group box 1 secretion by RAW 264.7 cells. J. Pharmacol. Exp. Ther. 2006, 316, 1070–1079. [Google Scholar] [CrossRef] [PubMed]
- Noorafshan, A.; Kardeh, S.; Ashkani-Esfahani, S.; Namazi, M.R.; Saleh, E. The effects of oltipraz on tissue regeneration in the process of wound healing: a stereological study. Bull. Emerg. Trauma 2014, 2, 161–165. [Google Scholar]
- Yu, Z.; Shao, W.; Chiang, Y.; Foltz, W.; Zhang, Z.; Ling, W.; Fantus, I.G.; Jin, T. Oltipraz upregulates the nuclear respiratory factor 2 alpha subunit (NRF2) antioxidant system and prevents insulin resistance and obesity induced by a high-fat diet in C57BL/6J mice. Diabetologia 2011, 54, 922–934. [Google Scholar] [CrossRef]
- Tang, Y.; Guo, M.; Ma, X.Y.; Sun, W.P.; Hao, M.H.; Zhu, H.Y. Oltipraz attenuates the progression of heart failure in rats through inhibiting oxidative stress and inflammatory response. Eur. Rev. Med. Pharmacol. Sci. 2018, 24, 8918–8923. [Google Scholar]
- Kim, S.G.; Kim, Y.M.; Choi, Y.H.; Lee, M.G.; Choi, J.Y.; Han, J.Y.; Cho, S.H.; Jang, J.W.; Um, S.H.; Chon, C.Y.; et al. Pharmacokinetics of oltipraz and its major metabolite (RM) in patients with liver fibrosis or cirrhosis: relationship with suppression of circulating TGF-beta1. Clin. Pharmacol. Ther. 2010, 88, 360–368. [Google Scholar] [CrossRef]
- Polat, E.C.; Besiroglu, H.; Ozcan, L.; Otunctemur, A.; Eruyar, A.T.; Somay, A.; Ozbay, N.; Cekmen, M.; Eraldemir, C.; Ozbek, E. Beneficial effects of Oltipraz, nuclear factor-erythroid-2-related factor 2 (Nrf2), on renal damage in unilateral ureteral obstruction rat model. Int. Braz. J. Urol. 2018, 44, 1243–1251. [Google Scholar] [CrossRef]
- Atilano-Roque, A.; Wen, X.; Aleksunes, L.M.; Joy, M.S. Nrf2 activators as potential modulators of injury in human kidney cells. Toxicol. Rep. 2016, 3, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Hargreaves, K.; Dubner, R.; Brown, F.; Flores, C.; Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988, 32, 77–88. [Google Scholar] [CrossRef]
- Bennett, G.J.; Xie, Y.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988, 33, 87–107. [Google Scholar] [CrossRef]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Le Pichon, M.; Jalfre, M. Depression: A new animal model sensitive to antidepressant treatments. Nature 1977, 266, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Sindrup, S.H.; Jensen, T.S. Efficacy of pharmacological treatments of neuropathic pain: An update and effect related to mechanism of drug action. Pain 1999, 83, 389–400. [Google Scholar] [CrossRef]
- Dworkin, R.H.; Backonja, M.; Rowbotham, M.C.; Allen, R.R.; Argoff, C.R.; Bennett, G.J.; Bushnell, M.C.; Farrar, J.T.; Galer, B.S.; Haythornthwaite, J.A.; et al. Advances in neuropathic pain: Diagnosis, mechanisms, and treatment recommendations. Arch. Neurol. 2003, 60, 1524–1534. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S.S. NF-kappaB inhibitory action of resveratrol: A probable mechanism of neuroprotection in experimental diabetic neuropathy. Biochem. Biophys. Res. Commun. 2010, 394, 360–365. [Google Scholar] [CrossRef]
- Isacchi, B.; Iacopi, R.; Bergonzi, M.C.; Ghelardini, C.; Galeotti, N.; Norcini, M.; Vivoli, E.; Vincieri, F.F.; Bilia, A.R. Antihyperalgesic activity of verbascoside in two models of neuropathic pain. J. Pharm. Pharmacol. 2011, 63, 594–601. [Google Scholar] [CrossRef]
- Arruri, V.; Komirishetty, P.; Areti, A.; Dungavath, S.K.N.; Kumar, A. Nrf2 and NF-κB modulation by Plumbagin attenuates functional, behavioural and biochemical deficits in rat model of neuropathic pain. Pharmacol. Rep. 2017, 69, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Luo, L.; Cai, X.; Fang, Y.; Wang, J.; Chen, G.; Yang, J.; Zhou, Q.; Sun, X.; Cheng, X.; et al. Nrf2 inhibits oxaliplatin-induced peripheral neuropathy via protection of mitochondrial function. Free Radic. Biol. Med. 2018, 120, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Johnson, J.A. An important role of Nrf2-ARE pathway in the cellular defense mechanism. J. Biochem. Mol. Biol. 2004, 37, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Saykally, J.N.; Rachmany, L.; Hatic, H.; Shaer, A.; Rubovitch, V.; Pick, C.G.; Citron, B.A. The nuclear factor erythroid 2-like 2 activator, tert-butylhydroquinone, improves cognitive performance in mice after mild traumatic brain injury. Neuroscience 2012, 223, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, I.; Bohren, Y.; Waltisperger, E.; Sage-Ciocca, D.; Yin, J.C.; Freund-Mercier, M.J.; Barrot, M. A time-dependent history of mood disorders in a murine model of neuropathic pain. Biol. Psychiatry 2011, 70, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Liu, S.; Wang, Y.; Cui, R.; Zhang, X. The link between depression and chronic pain: Neural mechanisms in the brain. Neural Plast. 2017, 2017, 9724371. [Google Scholar] [CrossRef]
- Wu, S.; Gao, Q.; Zhao, P.; Gao, Y.; Xi, Y.; Wang, X.; Liang, Y.; Shi, H.; Ma, Y. Sulforaphane produces antidepressant- and anxiolytic-like effects in adult mice. Behav. Brain Res. 2016, 301, 55–62. [Google Scholar] [CrossRef]
- Yao, W.; Zhang, J.C.; Ishima, T.; Dong, C.; Yang, C.; Ren, Q.; Ma, M.; Han, M.; Wu, J.; Suganuma, H.; et al. Role of Keap1-Nrf2 signaling in depression and dietary intake of glucoraphanin confers stress resilience in mice. Sci. Rep. 2016, 6, 30659. [Google Scholar] [CrossRef]
- Yirmiya, R.; Rimmerman, N.; Reshef, R. Depression as a microglial disease. Trends Neurosci. 2015, 38, 637–658. [Google Scholar] [CrossRef]
- Loggia, M.L.; Chonde, D.B.; Akeju, O.; Arabasz, G.; Catana, C.; Edwards, R.R.; Hill, E.; Hsu, S.; Izquierdo-Garcia, D.; Ji, R.R.; et al. Evidence for brain glial activation in chronic pain patients. Brain 2015, 138, 604–615. [Google Scholar] [CrossRef]
- Xu, N.; Tang, X.H.; Pan, W.; Xie, Z.M.; Zhang, G.F.; Ji, M.H.; Yang, J.J.; Zhou, M.T.; Zhou, Z.Q. Spared nerve injury increases the expression of microglia M1 markers in the prefrontal cortex of rats and provokes depression-like behaviors. Front. Neurosci. 2017, 11, 209. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Ding, Z.; Zhang, J.; Xu, W.; Guo, Q.; Zou, W.; Xiong, Y.; Weng, Y.; Yang, Y.; Chen, S.; et al. Minocycline relieves depressive-like behaviors in rats with bone cancer pain by inhibiting microglia activation in hippocampus. Anesth. Analg. 2019. [Google Scholar] [CrossRef]
- De Morais, H.; De Souza, C.P.; Da Silva, L.M.; Ferreira, D.M.; Werner, M.F.; Andreatini, R.; Da Cunha, J.M.; Zanoveli, J.M. Increased oxidative stress in prefrontal cortex and hippocampus is related to depressive-like behavior in streptozotocin-diabetic rats. Behav. Brain Res. 2014, 258, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Martín-Hernández, D.; Bris, Á.G.; MacDowell, K.S.; García-Bueno, B.; Madrigal, J.L.; Leza, J.C.; Caso, J.R. Modulation of the antioxidant nuclear factor (erythroid 2-derived)-like 2 pathway by antidepressants in rats. Neuropharmacology 2016, 103, 79–91. [Google Scholar] [CrossRef] [PubMed]



| Time of Treatment (Days) | |||||
|---|---|---|---|---|---|
| 0 | 1 | 4 | 8 | 11 | |
| Mechanical | F (3,20) = 96.8 | F (3,20) = 124.9 | F (3,20) = 124.2 | F (3,20) = 60.9 | F(3,20) = 180.6 |
| allodynia | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| Thermal | F (3,20) = 214.0 | F (3,20) = 334.7 | F (3,20) = 269.5 | F (3,20) = 413.6 | F(3,20) = 27.1 |
| hyperalgesia | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| Thermal | F (3,20) = 59.2 | F (3,20) = 58.9 | F (3,20) = 32.4 | F (3,20) = 23.1 | F(3,20) = 28.1 |
| allodynia | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz, A.F.; Polo, S.; Gallardo, N.; Leánez, S.; Pol, O. Analgesic and Antidepressant Effects of Oltipraz on Neuropathic Pain in Mice by Modulating Microglial Activation. J. Clin. Med. 2019, 8, 890. https://doi.org/10.3390/jcm8060890
Díaz AF, Polo S, Gallardo N, Leánez S, Pol O. Analgesic and Antidepressant Effects of Oltipraz on Neuropathic Pain in Mice by Modulating Microglial Activation. Journal of Clinical Medicine. 2019; 8(6):890. https://doi.org/10.3390/jcm8060890
Chicago/Turabian StyleDíaz, Andrés Felipe, Sara Polo, Núria Gallardo, Sergi Leánez, and Olga Pol. 2019. "Analgesic and Antidepressant Effects of Oltipraz on Neuropathic Pain in Mice by Modulating Microglial Activation" Journal of Clinical Medicine 8, no. 6: 890. https://doi.org/10.3390/jcm8060890
APA StyleDíaz, A. F., Polo, S., Gallardo, N., Leánez, S., & Pol, O. (2019). Analgesic and Antidepressant Effects of Oltipraz on Neuropathic Pain in Mice by Modulating Microglial Activation. Journal of Clinical Medicine, 8(6), 890. https://doi.org/10.3390/jcm8060890






