Saliva and Serum Immune Responses in Apical Periodontitis
Abstract
1. Introduction
2. Experimental Section
2.1. Population
2.2. Oral Diagnosis
2.3. Bacterial Analyses
2.4. Antibody Determinations
2.5. Calculations of Cross-Reactive Antibodies and Antibodies Binding to Bacterial Antigens
2.6. Statistical Methods
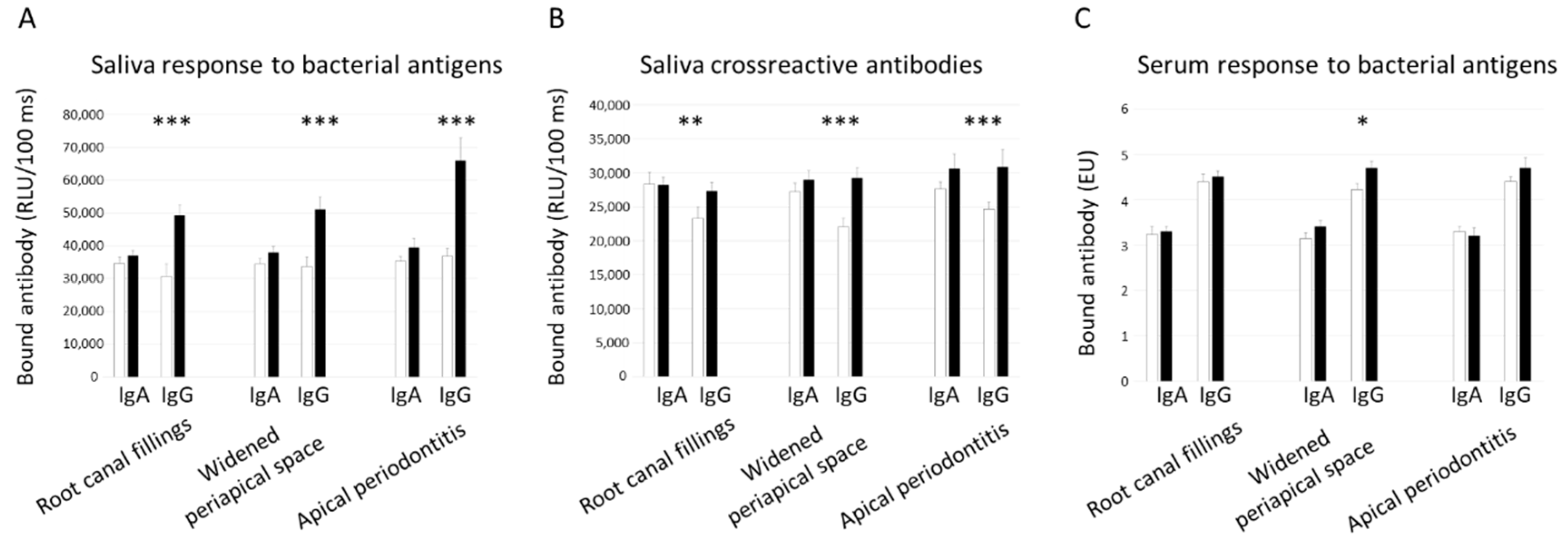
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Carrotte, P. Endodontics: Part 3. Treatment of endodontic emergencies. Br. Dent. J. 2004, 197, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Pak, J.G.; Fayazi, S.; White, S.N. Prevalence of periapical radiolucency and root canal treatment: A systematic review of cross-sectional studies. J. Endod. 2012, 38, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Persoon, I.F.; Özok, A.R. Definitions and Epidemiology of Endodontic Infections. Curr. Oral. Health Rep. 2017, 4, 278–285. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Rôças, I.N. Distinctive features of the microbiota associated with different forms of apical periodontitis. J. Oral. Microbiol. 2009, 1. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.Y.; Lee, T.K.; Lim, S.M.; Chang, S.W.; Park, J.; Han, S.H.; Zhu, Q.; Safavi, K.E.; Fouad, A.F.; Kum, K.Y. Microbial analysis in primary and persistent endodontic infections by using pyrosequencing. J. Endod. 2013, 39, 1136–1140. [Google Scholar] [CrossRef] [PubMed]
- Tzanetakis, G.N.; Azcarate-Peril, M.A.; Zachaki, S.; Panopoulos, P.; Kontakiotis, E.G.; Madianos, P.N.; Divaris, K. Comparison of Bacterial Community Composition of Primary and Persistent Endodontic Infections Using Pyrosequencing. J. Endod. 2015, 41, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Keskin, C.; Demiryürek, E.Ö.; Onuk, E.E. Pyrosequencing analysis of cryogenically ground samples from primary and secondary/persistent endodontic infections. J. Endod. 2017, 43, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Bouillaguet, S.; Manoil, D.; Girard, M.; Louis, J.; Gaïa, N.; Leo, S.; Schrenzel, J.; Lazarevic, V. Root Microbiota in Primary and Secondary Apical Periodontitis. Front. Microbiol. 2018, 9, 2374. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.M.; Luo, T.; Lee, K.H.; Guerreiro, D.; Botero, T.M.; McDonald, N.J.; Rickard, A.H. Deciphering Endodontic Microbial Communities by Next-generation Sequencing. J. Endod. 2018, 44, 1080–1087. [Google Scholar] [CrossRef]
- Gomes, B.P.; Berber, V.B.; Kokaras, A.S.; Chen, T.; Paster, B.J. Microbiomes of Endodontic-Periodontal Lesions before and after Chemomechanical Preparation. J. Endod. 2015, 4, 1975–1984. [Google Scholar] [CrossRef] [PubMed]
- Graunaite, I.; Lodiene, G.; Maciulskiene, V. Pathogenesis of apical periodontitis: A literature review. J. Oral. Maxillofac. Res. 2011, 2, e1. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ríos, P.; Pussinen, P.J.; Vernal, R.; Hernández, M. Oxidative Stress in the Local and Systemic Events of Apical Periodontitis. Front. Physiol. 2017, 1, 869. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.S.; Blattner, T.C.; Sant’Ana Filho, M.; Grecca, F.S.; Hugo, F.N.; Fouad, A.F.; Reynolds, M.A. Can apical periodontitis modify systemic levels of inflammatory markers? A systematic review and meta-analysis. J. Endod. 2013, 39, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Vidal, F.; Fontes, T.V.; Marques, T.V.; Gonçalves, L.S. Association between apical periodontitis lesions and plasmatic levels of C-reactive protein, interleukin 6 and fibrinogen in hypertensive patients. Int. Endod. J. 2016, 49, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Garrido, M.; Cárdenas, A.M.; Astorga, J.; Quinlan, F.; Valdés, M.; Chaparro, A.; Carvajal, P.; Pussinen, P.; Huamán-Chipana, P.; Jalil, J.E.; et al. Elevated Systemic Inflammatory Burden and Cardiovascular Risk in Young Adults with Endodontic Apical Lesions. J. Endod. 2019, 45, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Greening, A.B.; Schonfeld, S.E. Apical lesions contain elevated immunoglobulin G levels. J. Endod. 1980, 12, 867–869. [Google Scholar] [CrossRef]
- Johannessen, A.C.; Nilsen, R.; Skaug, N. Deposits of immunoglobulins and complement factor C3 in human dental periapical inflammatory lesions. Scand. J. Dent. Res. 1983, 91, 191–199. [Google Scholar] [CrossRef]
- Keudell, K.; Powel, G.; Berry, H. A review of microbial and immunologic aspects of endodontics. J. Oral. Pathol. Med. 1981, 36, 39–43. [Google Scholar]
- Torres, J.O.C.; Torabinejad, M.; Matiz, R.A.R.; Mantilla, E.G. Presence of secretory IgA in human periapical lesions. J. Endod. 1994, 20, 87–89. [Google Scholar] [CrossRef]
- Liljestrand, J.M.; Mäntylä, P.; Paju, S.; Buhlin, K.; Kopra, K.A.; Persson, G.R.; Hernandez, M.; Nieminen, M.S.; Sinisalo, J.; Tjäderhane, L.; et al. Association of Endodontic Lesions with Coronary Artery Disease. J. Dent. Res. 2016, 95, 1358–1365. [Google Scholar] [CrossRef]
- Pietiäinen, M.; Liljestrand, J.M.; Kopra, E.; Pussinen, P.J. Mediators between oral dysbiosis and cardiovascular diseases. Eur. J. Oral. Sci. 2018, 126 (Suppl. 1), 26–36. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, P.B.; Bolger, A.F.; Papapanou, P.N.; Osinbowale, O.; Trevisan, M.; Levison, M.E.; Taubert, K.A.; Newburger, J.W.; Gornik, H.L.; Gewitz, M.H.; et al. Periodontal disease and atherosclerotic vascular disease: Does the evidence support an independent association? A scientific statement from the American Heart Association. Circulation 2012, 125, 2520–2544. [Google Scholar] [CrossRef] [PubMed]
- Khalighinejad, N.; Aminoshariae, M.R.; Aminoshariae, A.; Kulild, J.C.; Mickel, A.; Fouad, A.F. Association between systemic diseases and apical periodontitis. J. Endod. 2016, 42, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Berlin-Broner, Y.; Febbraio, M.; Levin, L. Association between apical periodontitis and cardiovascular diseases: A systematic review of the literature. Int. Endod. J. 2017, 50, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Vaara, S.; Nieminen, M.S.; Lokki, M.L.; Perola, M.; Pussinen, P.J.; Allonen, J.; Parkkonen, O.; Sinisalo, J. Cohort Profile: The Corogene study. Int. J. Epidemiol. 2012, 41, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Buhlin, K.; Mäntylä, P.; Paju, S.; Peltola, J.S.; Nieminen, M.S.; Sinisalo, J.; Pussinen, P.J. Periodontitis is associated with angiographically verified coronary artery disease. J. Clin. Periodontol. 2011, 38, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Liljestrand, J.M.; Paju, S.; Buhlin, K.; Persson, G.R.; Sarna, S.; Nieminen, M.S.; Sinisalo, J.; Mäntylä, P.; Pussinen, P.J. Lipopolysaccharide, a possible molecular mediator between periodontitis and coronary artery disease. J. Clin. Periodontol. 2017, 44, 784–792. [Google Scholar] [CrossRef]
- Mäntylä, P.; Buhlin, K.; Paju, S.; Persson, G.R.; Nieminen, M.S.; Sinisalo, J.; Pussinen, P.J. Subgingival Aggregatibacter actinomycetemcomitans associates with the risk of coronary artery disease. J. Clin. Periodontol. 2013, 40, 583–590. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Smith, C.; Martin, L.; Haffajee, J.A.; Uzel, N.G.; Goodson, J.M. Use of checkerboard DNA-DNA hybridization to study complex microbial ecosystems. Oral. Microbiol. Immunol. 2004, 19, 352–362. [Google Scholar] [CrossRef]
- Hyvärinen, K.; Mäntylä, P.; Buhlin, K.; Paju, S.; Nieminen, M.S.; Sinisalo, J.; Pussinen, P.J. A common periodontal pathogen has an adverse association with both acute and stable coronary artery disease. Atherosclerosis 2012, 223, 478–484. [Google Scholar] [CrossRef]
- Liljestrand, J.M.; Paju, S.; Pietiäinen, M.; Buhlin, K.; Persson, G.R.; Nieminen, M.S.; Sinisalo, J.; Mäntylä, P.; Pussinen, P.J. Immunologic burden links periodontitis to acute coronary syndrome. Atherosclerosis 2018, 268, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Pussinen, P.J.; Könönen, E.; Paju, S.; Hyvärinen, K.; Gursoy, U.K.; Huumonen, S.; Knuuttila, M.; Suominen, A.L. Periodontal pathogen carriage, rather than periodontitis, determines the serum antibody levels. J. Clin. Periodontol. 2011, 38, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Hörkkö, S.; Bird, D.A.; Miller, E.; Itabe, H.; Leitinger, N.; Subbanagounder, G.; Berliner, J.A.; Friedman, P.; Dennis, E.A.; Curtiss, L.K.; et al. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J. Clin. Investig. 1999, 103, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Turunen, S.P.; Kummu, O.; Harila, K.; Veneskoski, M.; Soliymani, R.; Baumann, M.; Pussinen, P.J.; Hörkkö, S. Recognition of Porphyromonas gingivalis gingipain epitopes by natural IgM binding to malondialdehyde modified low-density lipoprotein. PLoS ONE 2012, 7, e34910. [Google Scholar] [CrossRef][Green Version]
- Wang, C.; Kankaanpää, J.; Kummu, O.; Turunen, S.P.; Akhi, R.; Bergmann, U.; Pussinen, P.; Remes, A.M.; Hörkkö, S. Characterization of a natural mouse monoclonal antibody recognizing epitopes shared by oxidized lowdensity lipoprotein and chaperonin 60 of Aggregatibacter actinomycetemcomitans. Immunol. Res. 2016, 64, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Karvonen, J.; Päivänsalo, M.; Kesäniemi, Y.A.; Hörkkö, S. Immunoglobulin M type of autoantibodies to oxidized low-density lipoprotein has an inverse relation to carotid artery atherosclerosis. Circulation 2003, 108, 2107–2112. [Google Scholar] [CrossRef]
- Akhi, R.; Wang, C.; Nissinen, A.E.; Kankaanpää, J.; Bloigu, R.; Paju, S.; Mäntylä, P.; Buhlin, K.; Sinisalo, J.; Pussinen, P.J.; et al. Salivary IgA to MAA-LDL and Oral Pathogens Are Linked to Coronary Disease. J. Dent. Res. 2019, 98, 296–303. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, S.; Koh, D.; Hsu, C.Y. Salivary biomarkers for dental caries. Periodontology 2000, 70, 128–141. [Google Scholar] [CrossRef]
- Brandtzaeg, P. Secretory immunity with special reference to the oral cavity. J. Oral. Microbiol. 2013, 5, 20401. [Google Scholar] [CrossRef]
- Marton, I.J.; Kiss, C. Protective and destructive immune reactions in apical periodontitis. Oral. Microbiol. Immunol. 2000, 15, 139–150. [Google Scholar] [CrossRef]
- Matsuo, T.; Nakanishi, T.; Ebisu, S. Immunoglobulins in periapical exudates of infected root canals: Correlation with the clinical findings of the involved teeth. Endod. Dent. Traumatol. 1995, 11, 95–99. [Google Scholar] [CrossRef]
- Gomes, B.P.; Jacinto, R.C.; Pinheiro, E.T.; Sousa, E.L.; Zaia, A.A.; Ferraz, C.C.; Souza-Filho, F.J. Porphyromonas gingivalis, Porphyromonas endodontalis, Prevotella intermedia and Prevotella nigrescens in endodontic lesions detected by culture and by PCR. Oral. Microbiol. Immunol. 2005, 20, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Tomazinho, L.F.; Avila-Campos, M.J. Detection of Porphyromonas gingivalis, Porphyromonas endodontalis, Prevotella intermedia, and Prevotella nigrescens in chronic endodontic infection. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 103, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Martinho, F.C.; Chiesa, W.M.; Leite, F.R.; Cirelli, J.A.; Gomes, B.P. Antigenic activity of bacterial endodontic contents from primary root canal infection with periapical lesions against macrophage in the release of interleukin-1beta and tumor necrosis factor alpha. J. Endod. 2010, 36, 1467–1474. [Google Scholar] [CrossRef]
- Solomon, C.; Chalfin, H.; Kellert, M.; Weseley, P. The endodontic-periodontal lesion: A rational approach to treatment. J. Am. Dent. Assoc. 1995, 126, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Potempa, J.; Pike, R.; Travis, J. Titration and mapping of the active site of cysteine proteinases from Porphyromonas gingivalis (gingipains) using peptidyl chloromethanes. Biol. Chem. 1997, 378, 223–230. [Google Scholar] [CrossRef]
- Ford, P.J.; Gemmell, E.; Hamlet, S.M.; Hasan, A.; Walker, P.J.; West, M.J.; Cullinan, M.P.; Seymour, G.J. Cross-reactivity of GroEL antibodies with human heat shock protein 60 and quantification of pathogens in atherosclerosis. Oral. Microbiol. Immunol. 2005, 20, 296–302. [Google Scholar] [CrossRef]
- Montebugnoli, L.; Servidio, D.; Miaton, R.A.; Prati, C.; Tricoci, P.; Melloni, C.; Melandri, G. Periodontal health improves systemic inflammatory and haemostatic status in subjects with coronary heart disease. J. Clin. Periodontol. 2005, 32, 188–192. [Google Scholar] [CrossRef]
- Monteiro, A.M.; Jardini, M.A.; Alves, S.; Giampaoli, V.; Aubin, E.C.; Figueiredo Neto, A.M.; Gidlund, M. Cardiovascular disease parameters in periodontitis. J. Periodontol. 2009, 80, 378–388. [Google Scholar] [CrossRef]
- Buhlin, K.; Holmer, J.; Gustafsson, A.; Hörkkö, S.; Pockley, A.G.; Johansson, A.; Paju, S.; Klinge, B.; Pussinen, P.J. Association of periodontitis with persistent, pro-atherogenic antibody responses. J. Clin. Periodontol. 2015, 42, 1006–1014. [Google Scholar] [CrossRef]
- Russell, M.W.; Hajishengallis, G.; Childers, N.K.; Michalek, S.M. Secretory Immunity in Defense against Cariogenic Mutans Streptococci. Caries Res. 1999, 33, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Mattila, K.J.; Nieminen, M.S.; Valtonen, V.V.; Rasi, V.P.; Kesäniemi, Y.A.; Syrjälä, S.L.; Jungell, P.S.; Isoluoma, M.; Hietaniemi, K.; Jokinen, M.J. Association between dental health and acute myocardial infarction. BMJ 1989, 298, 779–781. [Google Scholar] [CrossRef] [PubMed]
- Cotti, E.; Dessi, C.; Piras, A.; Flore, G.; Deidda, M.; Madeddu, C.; Zedda, A.; Longu, G.; Mercuro, G. Association of endodontic infection with detection of an initial lesion to the cardiovascular system. J. Endod. 2011, 37, 1624–1629. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.; Glaßl, E.M.; Nasseri, P.; Crismani, A.; Luger, A.K.; Schoenherr, E.; Bertl, K.; Glodny, B. The association of chronic apical periodontitis and endodontic therapy with atherosclerosis. Clin. Oral Investig. 2014, 18, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Pasqualini, D.; Bergandi, L.; Palumbo, L.; Borraccino, A.; Dambra, V.; Alovisi, M.; Migliaretti, G.; Ferraro, G.; Ghigo, D.; Bergerone, S.; et al. Association among oral health, apical periodontitis, CD14 polymorphisms, and coronary heart disease in middle-aged adults. J. Endod. 2012, 38, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Aminoshariae, A.; Kulild, J.C.; Fouad, A.F. The Impact of Endodontic Infections on the Pathogenesis of Cardiovascular Disease(s): A Systematic Review with Meta-analysis Using GRADE. J. Endod. 2018, 44, 1361–1366.e3. [Google Scholar] [CrossRef] [PubMed]
- Pussinen, P.J.; Jousilahti, P.; Alfthan, G.; Palosuo, T.; Asikainen, S.; Salomaa, V. Antibodies to periodontal pathogens are associated with coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1250–1254. [Google Scholar] [CrossRef]
- Pussinen, P.J.; Alfthan, G.; Rissanen, H.; Reunanen, A.; Asikainen, S.; Knekt, P. Antibodies to periodontal pathogens and stroke risk. Stroke 2004, 35, 2020–2023. [Google Scholar] [CrossRef]
- Pussinen, P.J.; Nyyssönen, K.; Alfthan, G.; Salonen, R.; Laukkanen, J.A.; Salonen, J.T. Serum antibody levels to Actinobacillus actinomycetemcomitans predict the risk for coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 833–838. [Google Scholar] [CrossRef]
- Beck, J.D.; Eke, P.; Heiss, G.; Madianos, P.; Couper, D.; Lin, D.; Moss, K.; Elter, J.; Offenbacher, S. Periodontal disease and coronary heart disease: A reappraisal of the exposure. Circulation 2005, 112, 19–24. [Google Scholar] [CrossRef]


| Character | Mean (SD) | |
|---|---|---|
| Age (years) | 62.9 (9.1) | |
| BMI (kg/m2) | 27.8 (5.1) | |
| Number of teeth | 21.4 (7.5) | |
| Mean (95% CI) | ||
| Number of implants | 0.12 (0.05–0.18) | |
| Carious teeth | 0.99 (0.84–1.14) | |
| Root canal fillings | 2.17 (1.96–2.39) | |
| Inadequate root fillings | 1.08 (0.95–1.20) | |
| Widened periapical space | 0.80 (0.71–0.89) | |
| Apical periodontitis | 0.36 (0.27–0.45) | |
| With root canal fillings Without root canal fillings | 0.16 (0.12–0.21) | |
| 0.19 (0.11–0.27) | ||
| N (%) | ||
| Gender (males) | 284 (67.0) | |
| Smoking (ever) | 220 (51.9) | |
| Hypertension | 266 (62.9) | |
| Diabetes (type I or II) | 92 (21.9) | |
| Dyslipidemia | 340 (80.6) | |
| Carious teeth | 198 (47.4) | |
| Root canal fillings | 304 (71.7) | |
| Widened periapical spaces | 224 (53.8) | |
| Apical periodontitis | 101 (23.8) | |
| Endodontic lesion score | No endodontic lesions | 162 (38.2) |
| ≥1 tooth with widened periapical space or one tooth with apical periodontitis | 194 (45.8) | |
| ≥2 teeth with apical periodontitis | 68 (16.0) | |
| Endodontic treatment score | No endodontic lesions | 323 (76.2) |
| Apical periodontitis in teeth with root canal fillings | 51 (12.0) | |
| Apical periodontitis in teeth without root canal fillings | 50 (11.8) | |
| Marginal periodontitis | Healthy | 42 (9.9) |
| Gingivitis | 61 (14.4) | |
| Periodontitis | 320 (75.5) | |
| Root Canal Fillings | Widened Periapical Space | Apical Periodontitis | ||||
|---|---|---|---|---|---|---|
| Beta, p-Value | OR (95% CI), p | Beta, p-Value | OR (95% CI), p | Beta, p-Value | OR (95% CI), p | |
| Saliva IgA against bacteria * | 0.096, 0.041 | 2.162 (0.934–5.004), 0.072 | 0.062, 0.204 | 2.090 (1.007–4.337), 0.048 | 0.059, 0.235 | 1.995 (0.870–4.573), 0.103 |
| Saliva IgG against bacteria * | 0.169, <0.001 | 2.519 (1.431–4.434), 0.001 | 0.138, 0.005 | 2.246 (1.397–3.611), 0.001 | 0.182, <0.001 | 2.904 (1.714–4.922), <0.001 |
| Serum IgA against bacteria * | 0.031, 0.527 | 1.144 (0.516–2.539), 0.741 | 0.017, 0.738 | 1.513 (0.753–3.040), 0.245 | 0.037, 0.476 | 0.922 (0.421–2.018), 0.838 |
| Serum IgG against bacteria * | 0.010, 0.838 | 1.272 (0.443–3.656), 0.655 | 0.002, 0.963 | 2.048 (0.840–4.995), 0.115 | 0.083, 0.102 | 1.710 (0.615–4.754), 0.304 |
| Saliva pathogen sum | 0.012, 0.802 | 0.996 (0.932–1.064), 0.900 | 0.068, 0.163 | 1.046 (0.989–1.107), 0.117 | 0.079, 0.111 | 0.998 (0.936–1.064), 0.951 |
| Subgingival pathogen sum | 0.053, 0.273 | 0.991 (0.934–1.051), 0.760 | 0.086, 0.085 | 1.051 (0.999–1.105), 0.056 | 0.098, 0.049 | 1.047 (0.987–1.112), 0.127 |
| 45 gram-positive taxa | 0.025, 0.603 | 0.754 (0.515–1.104), 0.147 | 0.121, 0.014 | 1.395 (1.016–1.917), 0.040 | 0.045, 0.375 | 1.096 (0.761–1.578), 0.622 |
| 33 gram-negative taxa | 0.056, 0.237 | 0.945 (0.660–1.353), 0.757 | 0.121, 0.014 | 1.446 (1.046–1.999), 0.026 | 0.059, 0.238 | 1.249 (0.850–1.836), 0.257 |
| Saliva cross-reactive IgA ** | 0.086, 0.067 | 1.248 (0.926–1.680), 0.146 | 0.096, 0.049 | 1.295 (0.995–1.685), 0.055 | 0.064, 0.196 | 1.327 (0.986–1.788), 0.062 |
| Saliva cross-reactive IgG ** | 0.128, 0.006 | 1.240 (0.942–1.632), 0.125 | 0.160, 0.001 | 1.555 (1.217–1.987), <0.001 | 0.159, 0.001 | 1.615 (1.237–2.108), <0.001 |
| Character | Endodontic Lesion Score | Endodontic Treatment Score | ||||||
|---|---|---|---|---|---|---|---|---|
| Score I | Score II | Score III | Score I | Score II | Score III | |||
| Mean (SD) | P 1 | Mean (SD) | P 1 | |||||
| Age (years) | 63.3 (9.2) | 63.1 (8.7) | 63.7 (9.8 | 0.924 | 63.3 (9.0) | 64.3 (9.0) | 62.5 (9.5) | 0.578 |
| BMI (kg/m2) | 27.8 (5.0) | 27.8 (5.1) | 27.8 (4.4) | 0.990 | 27.7 (5.0) | 27.9 (4.8) | 28.2 (4.8) | 0.852 |
| Number of teeth | 18.1 (10.5) | 21.5 (7.3) | 22.0 (6.7) | <0.001 | 19.7 (9.3) | 23.2 (6.0) | 20.5 (8.0) | 0.031 |
| Mean (95% CI) | Mean (95% CI) | |||||||
| Number of implants | 0.17 (0.04–0.29) | 0.10 (0.01–0.20) | 0.12 (0.04–0.28) | 0.696 | 0.15 (0.06–0.23) | 0.14 (0.07–0.35) | 0.02 (0.02–0.06) | 0.546 |
| Carious teeth | 0.68 (0.52–0.84) | 0.97 (0.75–1.18) | 1.74 (1.20–2.27) | <0.001 | 0.83 (0.69–0.98) | 1.24 (0.76–1.71) | 1.82 (1.10–2.53) | <0.001 |
| Root canal fillings | 0.95 (0.73–1.17) | 2.54 (2.26–2.82) | 3.91 (3.21–4.61) | <0.001 | 1.82 (1.61–2.02) | 4.65 (3.84–5.45) | 1.91 (1.28–2.54) | <0.001 |
| Inadequate root fillings | 0.41 (0.29–0.52) | 1.26 (1.10–1.42) | 2.07 (1.64–2.51) | <0.001 | 0.88 (0.76–1.00) | 2.33 (1.83–2.84) | 1.05 (0.65–1.44) | <0.001 |
| N (%) | P 2 | N (%) | P 2 | |||||
| N (%) | 189 (41.7) | 196 (43.3) | 68 (15.0) | 352 (77.7) | 51 (11.3) | 50 (11.0) | ||
| Sex (males) | 123 (65.1) | 132 (67.3) | 47 (69.1) | 0.803 | 234 (66.5) | 33 (64.7) | 35 (70.0) | 0.842 |
| Smoking (ever) | 92 (48.9) | 112 (57.1) | 35 (51.5) | 0.265 | 190 (54.1) | 23 (45.1) | 26 (52.0) | 0.478 |
| Hypertension | 122 (64.6) | 123 (63.4) | 41 (61.2) | 0.885 | 226 (64.6) | 30 (58.8) | 30 (61.2) | 0.683 |
| Diabetes (type I/II) | 39 (20.9) | 47 (24.2) | 14 (21.2) | 0.711 | 78 (22.4) | 11 (22.0) | 11 (22.4) | 0.998 |
| Dyslipidemia | 148 (78.3) | 165 (85.5) | 49 (73.1) | 0.050 | 285 (81.7) | 42 (84.0) | 35 (70.0) | 0.121 |
| Marginal periodontitis | 111 (58.7) | 154 (78.6) | 56 (82.4) | <0.001 | 244 (69.3) | 37 (72.5) | 40 (80.0) | 0.287 |
| OR (95% CI), P-Value | |||||
|---|---|---|---|---|---|
| Saliva IgG Against Bacteria * | Saliva Cross-Reacting IgG ** | ||||
| Univariate | Multivariate 1 | Univariate | Multivariate 1 | ||
| Endodontic lesion score | No endodontic lesions | 1.0 | 1.0 | 1.0 | 1.0 |
| ≥1 tooth with widened periapical space or apical periodontitis | 2.92 (1.17–7.26), 0.022 | 2.67 (1.03–6.94), 0.044 | 2.06 (1.31–3.22), 0.002 | 1.97 (1.24–3.11), 0.004 | |
| ≥2 teeth with apical periodontitis | 3.88 (0.99–15.5), 0.050 | 3.45 (0.83–14.3), 0.088 | 1.32 (0.65–2.69), 0.435 | 1.30 (0.64–2.62), 0.472 | |
| Endodontic treatment score | No endodontic lesions | 1.0 | 1.0 | 1.0 | 1.0 |
| Apical periodontitis in teeth with root canal fillings | 2.52 (0.70–9.06), 0.157 | 2.44 (0.66–9.06), 0.184 | 1.11 (0.60–2.03), 0.746 | 1.12 (0.60–2.08), 0.731 | |
| Apical periodontitis in teeth without root canal fillings | 5.88 (1.32–26.2), 0.020 | 4.77 (1.05–21.7), 0.043 | 1.43 (0.73–2.80), 0.297 | 1.39 (0.70–2.78), 0.351 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietiäinen, M.; Liljestrand, J.M.; Akhi, R.; Buhlin, K.; Johansson, A.; Paju, S.; Salminen, A.; Mäntylä, P.; Sinisalo, J.; Tjäderhane, L.; et al. Saliva and Serum Immune Responses in Apical Periodontitis. J. Clin. Med. 2019, 8, 889. https://doi.org/10.3390/jcm8060889
Pietiäinen M, Liljestrand JM, Akhi R, Buhlin K, Johansson A, Paju S, Salminen A, Mäntylä P, Sinisalo J, Tjäderhane L, et al. Saliva and Serum Immune Responses in Apical Periodontitis. Journal of Clinical Medicine. 2019; 8(6):889. https://doi.org/10.3390/jcm8060889
Chicago/Turabian StylePietiäinen, Milla, John M. Liljestrand, Ramin Akhi, Kåre Buhlin, Anders Johansson, Susanna Paju, Aino Salminen, Päivi Mäntylä, Juha Sinisalo, Leo Tjäderhane, and et al. 2019. "Saliva and Serum Immune Responses in Apical Periodontitis" Journal of Clinical Medicine 8, no. 6: 889. https://doi.org/10.3390/jcm8060889
APA StylePietiäinen, M., Liljestrand, J. M., Akhi, R., Buhlin, K., Johansson, A., Paju, S., Salminen, A., Mäntylä, P., Sinisalo, J., Tjäderhane, L., Hörkkö, S., & Pussinen, P. J. (2019). Saliva and Serum Immune Responses in Apical Periodontitis. Journal of Clinical Medicine, 8(6), 889. https://doi.org/10.3390/jcm8060889






