Sex Moderates the Effect of Aerobic Exercise on Some Aspects of Cognition in Cognitively Intact Younger and Middle-Age Adults
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Study Outcomes
2.2.1. Cardiorespiratory Exercise Test
2.2.2. Cognitive Assessment
2.2.3. Statistical Analysis
2.2.4. Data Availability
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Northey, J.M.; Cherbuin, N.; Pumpa, K.L.; Smee, D.J.; Rattray, B. Exercise interventions for cognitive function in adults older than 50: A systematic review with meta-analysis. Br. J. Sports Med. 2018, 52, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Angevaren, M.; Aufdemkampe, G.; Verhaar, H.; Aleman, A.; Vanhees, L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst. Rev. 2008, CD005381. [Google Scholar] [CrossRef]
- Colcombe, S.; Kramer, A.F. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol. Sci. 2003, 14, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Etnier, J.L.; Nowell, P.M.; Landers, D.M.; Sibley, B.A. A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain Res. Rev. 2006, 52, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Young, J.; Angevaren, M.; Rusted, J.; Tabet, N. Aerobic exercise to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst. Rev. 2015, 4, CD005381. [Google Scholar] [CrossRef] [PubMed]
- Stern, Y.; MacKay-Brandt, A.; Lee, S.; McKinley, P.; McIntyre, K.; Razlighi, Q.; Agarunov, E.; Bartels, M.; Sloan, R.P. Effect of aerobic exercise on cognition in younger adults: A randomized clinical trial. Neurology 2019, 92, e905–e916. [Google Scholar] [CrossRef] [PubMed]
- Lindwall, M.; Rennemark, M.; Berggren, T. Movement in mind: The relationship of exercise with cognitive status for older adults in the Swedish National Study on Aging and Care (SNAC). Aging Ment. Health 2008, 12, 212–220. [Google Scholar] [CrossRef]
- Coleman, M.; Offen, K.; Markant, J. Exercise Similarly Facilitates Men and Women’s Selective Attention Task Response Times but Differentially Affects Memory Task Performance. Front. Psychol. 2018, 9, 1405. [Google Scholar] [CrossRef]
- Fallah, N.; Mitnitski, A.; Middleton, L.; Rockwood, K. Modeling the impact of sex on how exercise is associated with cognitive changes and death in older Canadians. Neuroepidemiology 2009, 33, 47–54. [Google Scholar] [CrossRef]
- Dregan, A.; Gulliford, M.C. Leisure-time physical activity over the life course and cognitive functioning in late mid-adult years: A cohort-based investigation. Psychol. Med. 2013, 43, 2447–2458. [Google Scholar] [CrossRef]
- Dik, M.; Deeg, D.J.; Visser, M.; Jonker, C. Early life physical activity and cognition at old age. J. Clin. Exp. Neuropsychol. 2003, 25, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Dimech, C.J.; Anderson, J.A.E.; Lockrow, A.W.; Spreng, R.N.; Turner, G.R. Sex differences in the relationship between cardiorespiratory fitness and brain function in older adulthood. J. Appl. Physiol. 2019, 126, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Dao, E.; Barha, C.K.; Best, J.R.; Hsiung, G.-Y.; Tam, R.; Liu-Ambrose, T. The Effect of Aerobic Exercise on White Matter Hyperintensity Progression May Vary by Sex. Can. J. Aging 2019, 38, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Barha, C.K.; Davis, J.C.; Falck, R.S.; Nagamatsu, L.S.; Liu-Ambrose, T. Sex differences in exercise efficacy to improve cognition: A systematic review and meta-analysis of randomized controlled trials in older humans. Front. Neuroendocrinol. 2017, 46, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.D.; Ainsworth, B.E.; Hartman, T.J.; Leon, A.S. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med. Sci. Sports Exerc. 1993, 25, 81–91. [Google Scholar] [CrossRef]
- Mattis, S. Mental Status ecamination for organic mental syndrome in the elderly patient. In Geriatric Psychiatry; Bellak, L., Karasu, T.B., Eds.; Grune & Stratton: New York, NY, USA, 1976; pp. 77–121. [Google Scholar]
- Slade, S.C.; Dionne, C.E.; Underwood, M.; Buchbinder, R. Consensus on Exercise Reporting Template (CERT): Explanation and Elaboration Statement. Br. J. Sports Med. 2016, 50, 1428–1437. [Google Scholar] [CrossRef] [PubMed]
- Mcgee, V.E.; Carleton, W.T. Piecewise Regression. J. Am. Stat. Assoc. 1970, 65, 1109–1124. [Google Scholar] [CrossRef]
- Fox, S.M., 3rd; Naughton, J.P.; Haskell, W.L. Physical activity and the prevention of coronary heart disease. Ann. Clin. Res. 1971, 3, 404–432. [Google Scholar] [CrossRef]
- Robergs, R.A.; Dwyer, D.; Astorino, T. Recommendations for improved data processing from expired gas analysis indirect calorimetry. Sports Med. 2010, 40, 95–111. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Test of Adult Reading; The Psychological Corporation: San Antonio, TX, USA, 2001. [Google Scholar]
- Lim, Y.Y.; Jaeger, J.; Harrington, K.; Ashwood, T.; Ellis, K.A.; Stoffler, A.; Szoeke, C.; Lachovitzki, R.; Martins, R.N.; Villemagne, V.L.; et al. Three-month stability of the CogState brief battery in healthy older adults, mild cognitive impairment, and Alzheimer’s disease: Results from the Australian Imaging, Biomarkers, and Lifestyle-rate of change substudy (AIBL-ROCS). Arch. Clin. Neuropsychol. 2013, 28, 320–330. [Google Scholar] [CrossRef]
- Schmidt, M. Rey Auditory and Verbal Learing Test: A Handbook; Western Psychological Services: Los Angeles, CA, USA, 1996. [Google Scholar]
- Brickman, A.M.; Khan, U.A.; Provenzano, F.A.; Yeung, L.K.; Suzuki, W.; Schroeter, H.; Wall, M.; Sloan, R.P.; Small, S.A. Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nat. Neurosci. 2014, 17, 1798–1803. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D. Wechsler Memory Scale-III; Psychological Coorporation: San Antonio, TX, USA, 1997. [Google Scholar]
- Benton, A.L.; Hamsher, K.; Sivan, A.B. Multiligual Aphasia Examination; AJA Associates, Inc.: Iowa City, IA, USA, 1994. [Google Scholar]
- Goodglass, H.; Kaplan, E. The Assessment of Aphasia and Related Disorders; Lea & Febiger: Philadelphia, PA, USA, 1972. [Google Scholar]
- Ruff, R.M.; Allen, C.C.; Farrow, C.E.; Niemann, H.; Wylie, T. Figural Fluency-Differential Impairment in Patients with Left Versus Right Frontal-Lobe Lesions. Arch. Clin. Neuropsychol. 1994, 9, 41–55. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wechsler, D. Wechsler Adult Intelligence Scale-III; Psychological Coorporation: San Antonio, TX, USA, 1997. [Google Scholar]
- Gross, R.A.; Johnston, K.C. Levels of evidence: Taking Neurology to the next level. Neurology 2009, 72, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Tingley, D.; Yamamoto, T.; Hirose, K.; Keele, L.; Imai, K. Mediation: R Package for Causal Mediation Analysis. J. Stat. Softw. 2014, 59, 38. [Google Scholar] [CrossRef]
- Johnson, L.; Loprinzi, P.D. The effects of acute exercise on episodic memory function among young university students: Moderation considerations by biological sex. Health Promot. Perspect. 2019, 9, 99–104. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Frith, E. The Role of Sex in Memory Function: Considerations and Recommendations in the Context of Exercise. J. Clin. Med. 2018, 7, 132. [Google Scholar] [CrossRef] [PubMed]
- Venezia, A.C.; Guth, L.M.; Sapp, R.M.; Spangenburg, E.E.; Roth, S.M. Sex-dependent and independent effects of long-term voluntary wheel running on Bdnf mRNA and protein expression. Physiol. Behav. 2016, 156, 8–15. [Google Scholar] [CrossRef]
- Watts, A.; Andrews, S.J.; Anstey, K.J. Sex Differences in the Impact of BDNF Genotype on the Longitudinal Relationship between Physical Activity and Cognitive Performance. Gerontology 2018, 64, 361–372. [Google Scholar] [CrossRef]
- Barha, C.K.; Hsiung, G.-Y.R.; Best, J.R.; Davis, J.C.; Eng, J.J.; Jacova, C.; Lee, P.E.; Munkacsy, M.; Cheung, W.; Liu-Ambrose, T. Sex Difference in Aerobic Exercise Efficacy to Improve Cognition in Older Adults with Vascular Cognitive Impairment: Secondary Analysis of a Randomized Controlled Trial. J. Alzheimer’s Dis. JAD 2017, 60, 1397–1410. [Google Scholar] [CrossRef]
- Barha, C.K.; Liu-Ambrose, T. Exercise and the Aging Brain: Considerations for Sex Differences. Brain Plast. 2018, 4, 53–63. [Google Scholar] [CrossRef]
- Baker, L.D.; Frank, L.L.; Foster-Schubert, K.; Green, P.S.; Wilkinson, C.W.; McTiernan, A.; Plymate, S.R.; Fishel, M.A.; Watson, G.S.; Cholerton, B.A.; et al. Effects of aerobic exercise on mild cognitive impairment: A controlled trial. Arch. Neurol. 2010, 67, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Pierce, G.L.; Eskurza, I.; Walker, A.E.; Fay, T.N.; Seals, D.R. Sex-specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clin. Sci. 2011, 120, 13–23. [Google Scholar] [CrossRef]
- Parker, B.A.; Kalasky, M.J.; Proctor, D.N. Evidence for sex differences in cardiovascular aging and adaptive responses to physical activity. Eur. J. Appl. Physiol. 2010, 110, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.F.; Hahn, S.; Cohen, N.J.; Banich, M.T.; McAuley, E.; Harrison, C.R.; Chason, J.; Vakil, E.; Bardell, L.; Boileau, R.A.; et al. Ageing, fitness and neurocognitive function. Nature 1999, 400, 418–419. [Google Scholar] [CrossRef]
- Kramer, A.F.; Colcombe, S.J.; McAuley, E.; Eriksen, K.I.; Scalf, P.; Jerome, G.J.; Marquez, D.X.; Elavsky, S.; Webb, A.G. Enhancing brain and cognitive function of older adults through fitness training. J. Mol. Neurosci. 2003, 20, 213–221. [Google Scholar] [CrossRef]
- Voss, M.W.; Soto, C.; Yoo, S.; Sodoma, M.; Vivar, C.; Van Praag, H. Exercise and Hippocampal Memory Systems. Trends Cogn. Sci. 2019, 23, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Bale, T.L. Sex matters. Neuropsychopharmacology 2019, 44, 1–3. [Google Scholar] [CrossRef]
- Yagi, S.; Galea, L.A.M. Sex differences in hippocampal cognition and neurogenesis. Neuropsychopharmacology 2019, 44, 200–213. [Google Scholar] [CrossRef]

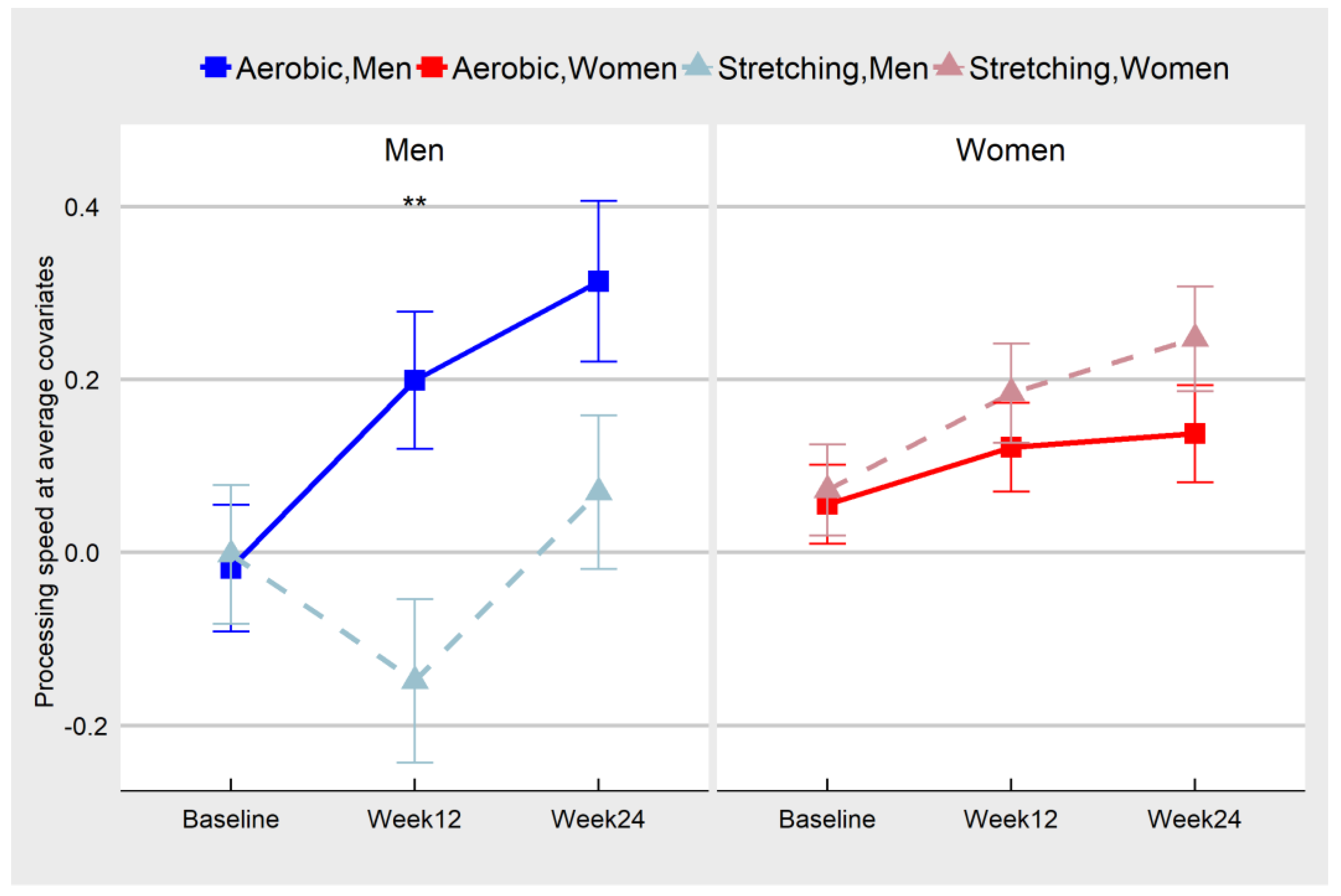
| Aerobic Condition | Stretching/Toning Condition | Sex Difference 1 | |||
|---|---|---|---|---|---|
| Women (n = 47) | Men (n = 19) | Women (n = 46) | Men (n = 20) | ||
| Age, y | 43 (12.9) | 38.1 (10.2) | 41.9 (15.0) | 33.5 (11.3) | 0.0039 ** |
| Education, y | 16.2 (2.6) | 14.8 (2.5) | 16.6 (1.9) | 15.8 (2.7) | 0.0306 * |
| Estimated IQ | 111.8 (12.8) | 113.9 (12.9) | 111 (13.8) | 112.1 (17.1) | 0.566 |
| Weight, lb | 156.7 (36.3) | 194.2 (31) | 152.7 (36.5) | 187 (26.3) | <0.0001 *** |
| Height, in | 64 (3.2) | 69.6 (2.2) | 64.3 (3.1) | 69.3 (3.9) | <0.0001 *** |
| Body mass index | 26.6 (5.3) | 28.1 (4.1) | 25.9 (5) | 27.4 (3.9) | 0.072 |
| VO2 max, (mL/kg/min) | 25.44 (6.3) | 33.06 (7.48) | 26.6 (5.87) | 33.77 (5.63) | <0.0001 *** |
| Processing Speed | –0.02 (0.79) | −0.14 (0.83) | 0.11 (0.71) | 0.11 (0.83) | 0.7210 |
| Episodic memory | 0.14 (0.73) | −0.29 (0.92) | 0.10 (0.80) | −0.01 (1.04) | 0.1404 |
| Working memory | –0.01 (0.78) | 0.18 (0.63) | 0 (0.75) | 0.05 (0.75) | 0.3671 |
| Language | 0.06 (0.95) | −0.12 (0.58) | 0.09 (0.93) | 0.05 (0.71) | 0.4365 |
| Attention | 0.14 (0.63) | −0.15 (1.18) | 0.03 (0.77) | −0.27 (1.35) | 0.1750 |
| Executive function | −0.02 (0.74) | −0.18 (0.94) | −0.04 (0.54) | 0.36 (0.67) | 0.3952 |
| TIADL total error | 0.62 (0.68) | 0.74 (1.24) | 0.28 (0.54) | 0.50 (0.76) | 0.3551 |
| Race n (%) | |||||
| White | 19 (40.43) | 11 (57.89) | 16 (34.78) | 12 (60) | 0.0886 2 |
| Black | 16 (34.04) | 5 (26.32) | 9 (19.57) | 3 (15) | |
| Others | 12 (25.53) | 3 (15.79) | 21 (45.65) | 5 (25) | |
| Aerobic | Stretching | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Women | Men | Women | Men | ||||||
| Week | n | n | n | n | |||||
| VO2 Max (mL/kg/min) | 12 | 38 | 3.3 (4.78) | 15 | 4.48 (6.02) | 33 | 0.33 (3.54) | 14 | 0.82 (5.56) |
| 24 | 30 | 3.35 (4.96) | 11 | 3.25 (4.42) | 28 | 0.25 (4.41) | 14 | 0.84 (6.22) | |
| Executive Function | 12 | 38 | 0.29 (0.58) | 16 | 0.42 (0.52) | 38 | 0.19 (0.85) | 14 | 0.02 (0.37) |
| 24 | 32 | 0.5 (0.59) | 12 | 0.52 (0.3) | 34 | 0.35 (0.74) | 16 | 0.05 (0.73) | |
| Attention | 12 | 38 | −0.45 (1.15) | 16 | −0.08 (1.05) | 38 | −0.19 (0.65) | 13 | −0.36 (1.23) |
| 24 | 32 | −0.65 (2.23) | 12 | −0.41 (1.44) | 34 | −0.51 (1.19) | 16 | −0.06 (0.93) | |
| Language | 12 | 38 | 0.09 (0.59) | 16 | 0.08 (0.51) | 38 | 0.01 (0.51) | 14 | 0.15 (0.49) |
| 24 | 32 | 0.13 (0.58) | 12 | 0.12 (0.57) | 34 | 0.2 (0.69) | 16 | 0.23 (0.54) | |
| Processing Speed | 12 | 38 | 0.06 (0.38) | 16 | 0.21 (0.46) | 38 | 0.12 (0.52) | 14 | −0.14 (0.51) |
| 24 | 32 | 0.06 (0.47) | 12 | 0.33 (0.42) | 34 | 0.19 (0.43) | 16 | 0.06 (0.51) | |
| Episodic Memory | 12 | 38 | 0.27 (0.59) | 16 | 0.36 (0.51) | 38 | 0.32 (0.52) | 14 | 0.1 (0.87) |
| 24 | 32 | 0.36 (0.59) | 12 | 0.59 (0.52) | 34 | 0.58 (0.54) | 16 | 0.26 (0.96) | |
| Working Memory | 12 | 38 | 0.15 (0.88) | 16 | 0.06 (0.46) | 38 | 0.38 (1.09) | 14 | 0.2 (0.73) |
| 24 | 32 | 0.43 (0.67) | 12 | 0.22 (0.53) | 34 | 0.36 (0.6) | 16 | 0.35 (0.85) | |
| BMI | 12 | 36 | −0.5 (1.4) | 16 | −0.51 (1.19) | 36 | −0.12 (0.97) | 14 | −0.17 (1.54) |
| 24 | 31 | −0.85 (1.2) | 12 | −1.05 (1.77) | 31 | −0.07 (0.97) | 15 | −0.67 (1.49) | |
| Three-Way Interaction Models | Four-Way Interaction Model | |||||
|---|---|---|---|---|---|---|
| Age × Session × Group | Sex × Session × Group | Age × Sex × Session × Group | ||||
| F | p-Value | F | p-Value | F | p-Value | |
| VO2 max | 0.06 | 0.9426 | 0.09 | 0.9097 | 1.59 | 0.2065 |
| Executive Function | 10.84 | <0.0001 ** | 3.07 | 0.0486 * | 0.77 | 0.4639 |
| Attention | 0.47 | 0.6242 | 0.68 | 0.5099 | 1.03 | 0.3595 |
| Episodic Memory | 0.81 | 0.4445 | 2.22 | 0.1112 | 1.34 | 0.2656 |
| Language | 0.43 | 0.6531 | 0.12 | 0.8833 | 0.42 | 0.6554 |
| Speed | 0.05 | 0.9536 | 3.09 | 0.0478 * | 0.83 | 0.4368 |
| Working Memory | 0.07 | 0.9305 | 0.66 | 0.5173 | 0.48 | 0.6203 |
| BMI | 2.55 | 0.0808 | 0.23 | 0.7987 | 0.42 | 0.6571 |
| Table 4a Aerobic—Stretching Executive Function | |||||||
| Age | Session | Sex | Estimate | S.E | t | p | |
| 30 | Baseline | W | 0.09 | 0.12 | 0.77 | 0.4445 | |
| M | −0.09 | 0.14 | −0.63 | 0.5278 | |||
| 12 Weeks | W | −0.24 | 0.13 | −1.90 | 0.0585 | ||
| M | 0.09 | 0.16 | 0.55 | 0.5839 | |||
| 24 Weeks | W | −0.08 | 0.14 | −0.53 | 0.5987 | ||
| M | 0.21 | 0.17 | 1.20 | 0.2335 | |||
| 40 | Baseline | W | 0.03 | 0.09 | 0.37 | 0.7122 | |
| M | −0.15 | 0.14 | −1.02 | 0.3097 | |||
| 12 Weeks | W | 0.06 | 0.10 | 0.57 | 0.5674 | ||
| M | 0.39 | 0.16 | 2.36 | 0.0192 | * | ||
| 24 Weeks | W | 0.10 | 0.11 | 0.93 | 0.3531 | ||
| M | 0.38 | 0.17 | 2.25 | 0.0253 | * | ||
| 50 | Baseline | W | −0.02 | 0.10 | −0.21 | 0.8325 | |
| M | −0.20 | 0.17 | −1.22 | 0.2255 | |||
| 12 Weeks | W | 0.36 | 0.11 | 3.18 | 0.0017 | ** | |
| M | 0.69 | 0.19 | 3.63 | 0.0004 | *** | ||
| 24 Weeks | W | 0.28 | 0.12 | 2.44 | 0.0158 | * | |
| M | 0.56 | 0.19 | 2.89 | 0.0043 | ** | ||
| 60 | Baseline | W | −0.08 | 0.14 | −0.56 | 0.5786 | |
| M | −0.26 | 0.20 | −1.26 | 0.2076 | |||
| 12 Weeks | W | 0.66 | 0.15 | 4.28 | <0.0001 | *** | |
| M | 0.99 | 0.23 | 4.29 | <0.0001 | *** | ||
| 24 Weeks | W | 0.46 | 0.15 | 2.97 | 0.0034 | ** | |
| M | 0.74 | 0.24 | 3.13 | 0.0021 | ** | ||
| Table 4b Aerobic—Stretching Processing Speed | |||||||
| Session | Sex | n1 | Estimate | S.E. | t | p | |
| Baseline | M | 39 | −0.02 | 0.11 | −0.15 | 0.8836 | |
| W | 93 | −0.02 | 0.01 | −0.24 | 0.8120 | ||
| 12 Weeks | M | 30 | 0.35 | 0.12 | 2.83 | 0.0051 | ** |
| W | 76 | −0.06 | 0.08 | –0.82 | 0.4156 | ||
| 24 Weeks | M | 28 | 0.244 | 0.13 | 1.91 | 0.0578 | |
| W | 66 | −0.11 | 0.08 | −1.33 | 0.1841 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stern, Y.; Lee, S.; Predovan, D.; P. Sloan, R. Sex Moderates the Effect of Aerobic Exercise on Some Aspects of Cognition in Cognitively Intact Younger and Middle-Age Adults. J. Clin. Med. 2019, 8, 886. https://doi.org/10.3390/jcm8060886
Stern Y, Lee S, Predovan D, P. Sloan R. Sex Moderates the Effect of Aerobic Exercise on Some Aspects of Cognition in Cognitively Intact Younger and Middle-Age Adults. Journal of Clinical Medicine. 2019; 8(6):886. https://doi.org/10.3390/jcm8060886
Chicago/Turabian StyleStern, Yaakov, Seonjoo Lee, David Predovan, and Richard P. Sloan. 2019. "Sex Moderates the Effect of Aerobic Exercise on Some Aspects of Cognition in Cognitively Intact Younger and Middle-Age Adults" Journal of Clinical Medicine 8, no. 6: 886. https://doi.org/10.3390/jcm8060886
APA StyleStern, Y., Lee, S., Predovan, D., & P. Sloan, R. (2019). Sex Moderates the Effect of Aerobic Exercise on Some Aspects of Cognition in Cognitively Intact Younger and Middle-Age Adults. Journal of Clinical Medicine, 8(6), 886. https://doi.org/10.3390/jcm8060886





