HDL and LDL: Potential New Players in Breast Cancer Development
Abstract
1. Introduction
2. Association of Cholesterol in Breast Cancer Risk: Clinical and Epidemiological Studies
3. Hypercholesterolemia and Breast Cancer
4. 27-Hydroxycholesterol and Breast Cancer
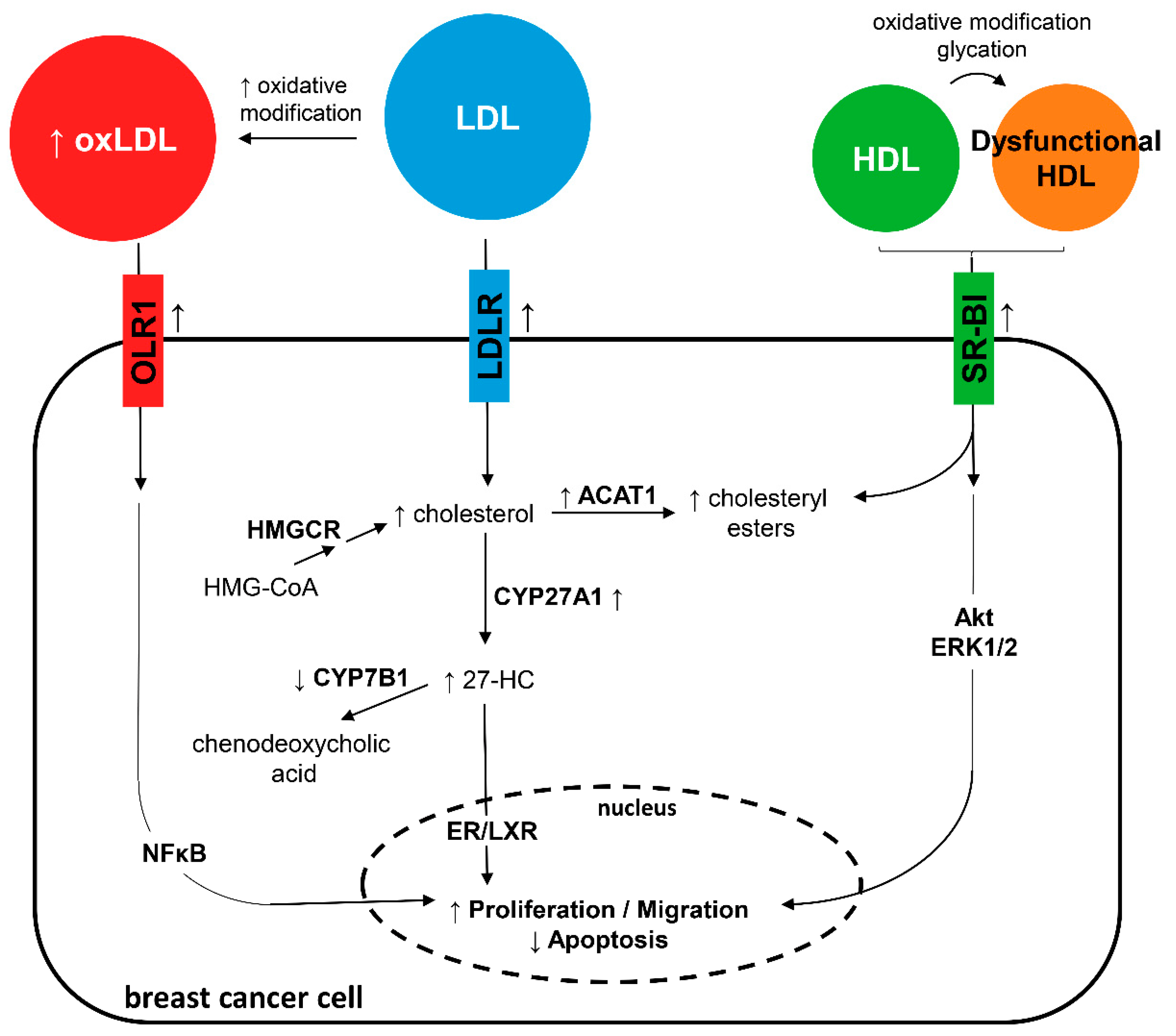
5. Low-Density Lipoprotein and Breast Cancer
Oxidized Low-Density Lipoprotein and Breast Cancer
6. High-Density Lipoprotein and Breast Cancer
Dysfunctional High-Density Lipoprotein and Breast Cancer
7. Effects of Cholesterol-Lowering Therapies on Breast Cancer
7.1. Statins
7.2. Ezetimibe
7.3. Phytosterols
7.4. Other Therapies
8. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Akinyemiju, T.F.; Al Lami, F.H.; Alam, T.; Alizadeh-Navaei, R.; Allen, C.; Alsharif, U.; Alvis-Guzman, N.; Amini, E.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2018, 4, 1553–1568. [Google Scholar] [PubMed]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Metabolic complications of obesity. Endocrine 2000, 13, 155–165. [Google Scholar] [CrossRef]
- Yung, R.L.; Ligibel, J.A. Obesity and breast cancer: Risk, outcomes, and future considerations. Clin. Adv. Hematol. Oncol. 2016, 14, 790–797. [Google Scholar] [PubMed]
- Park, J.; Morley, T.S.; Kim, M.; Clegg, D.J.; Scherer, P.E. Obesity and cancer--mechanisms underlying tumour progression and recurrence. Nat. Rev. Endocrinol. 2014, 10, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.R.; Schulze, A. Lipid metabolism in cancer. FEBS J. 2012, 279, 2610–2623. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.M.; Boatman, K.K.; McConathy, W.J. Serum lipids and apolipoproteins in women with breast masses. Breast Cancer Res. Treat. 1995, 34, 161–169. [Google Scholar] [CrossRef]
- Touvier, M.; Fassier, P.; His, M.; Norat, T.; Chan, D.S.M.; Blacher, J.; Hercberg, S.; Galan, P.; Druesne-Pecollo, N.; Latino-Martel, P. Cholesterol and breast cancer risk: A systematic review and meta-analysis of prospective studies. Br. J. Nutr. 2015, 114, 347–357. [Google Scholar] [CrossRef]
- Chandler, P.D.; Song, Y.; Lin, J.; Zhang, S.; Sesso, H.D.; Mora, S.; Giovannucci, E.L.; Rexrode, K.E.; Moorthy, M.V.; Li, C.; et al. Lipid biomarkers and long-term risk of cancer in the Women’s Health Study. Am. J. Clin. Nutr. 2016, 103, 1397–1407. [Google Scholar] [CrossRef]
- Nowak, C.; Ärnlöv, J. A Mendelian randomization study of the effects of blood lipids on breast cancer risk. Nat. Commun. 2018, 9, 3957. [Google Scholar] [CrossRef]
- Jafri, H.; Alsheikh-Ali, A.A.; Karas, R.H. Baseline and on-treatment high-density lipoprotein cholesterol and the risk of cancer in randomized controlled trials of lipid-altering therapy. J. Am. Coll. Cardiol. 2010, 55, 2846–2854. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, C.M.; Berrington de González, A.; Freedman, N.D.; Huxley, R.; Mok, Y.; Jee, S.H.; Samet, J.M. Total cholesterol and cancer risk in a large prospective study in Korea. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 1592–1598. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J.; Melnichouk, O.; Huszti, E.; Connelly, P.W.; Greenberg, C.V.; Minkin, S.; Boyd, N.F. Serum Lipids, Lipoproteins, and Risk of Breast Cancer: A Nested Case-Control Study Using Multiple Time Points. J. Natl. Cancer Inst. 2015, 107, djv032. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Sung, J.; Song, Y.-M. Serum total cholesterol and the risk of breast cancer in postmenopausal Korean women. Cancer Causes Control 2009, 20, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Liu, H.; Gao, R. Serum Lipids and Breast Cancer Risk: A Meta-Analysis of Prospective Cohort Studies. PLoS ONE 2015, 10, e0142669. [Google Scholar] [CrossRef] [PubMed]
- Bosco, J.L.F.; Palmer, J.R.; Boggs, D.A.; Hatch, E.E.; Rosenberg, L. Cardiometabolic factors and breast cancer risk in U.S. black women. Breast Cancer Res. Treat. 2012, 134, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Eliassen, A.H.; Colditz, G.A.; Rosner, B.; Willett, W.C.; Hankinson, S.E. Serum lipids, lipid-lowering drugs, and the risk of breast cancer. Arch. Intern. Med. 2005, 165, 2264–2271. [Google Scholar] [CrossRef] [PubMed]
- His, M.; Zelek, L.; Deschasaux, M.; Pouchieu, C.; Kesse-Guyot, E.; Hercberg, S.; Galan, P.; Latino-Martel, P.; Blacher, J.; Touvier, M. Prospective associations between serum biomarkers of lipid metabolism and overall, breast and prostate cancer risk. Eur. J. Epidemiol. 2014, 29, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Dos Santos, C.; Fonseca, I.; Dias, S.; Mendes de Almeida, J.C. Plasma level of LDL-cholesterol at diagnosis is a predictor factor of breast tumor progression. BMC Cancer 2014, 14, 132. [Google Scholar] [CrossRef] [PubMed]
- Borgquist, S.; Butt, T.; Almgren, P.; Shiffman, D.; Stocks, T.; Orho-Melander, M.; Manjer, J.; Melander, O. Apolipoproteins, lipids and risk of cancer. Int. J. Cancer J. Int. Cancer 2016, 138, 2648–2656. [Google Scholar] [CrossRef] [PubMed]
- Llanos, A.A.; Makambi, K.H.; Tucker, C.A.; Wallington, S.F.; Shields, P.G.; Adams-Campbell, L.L. Cholesterol, lipoproteins, and breast cancer risk in African American women. Ethn. Dis. 2012, 22, 281–287. [Google Scholar] [PubMed]
- Li, X.; Tang, H.; Wang, J.; Xie, X.; Liu, P.; Kong, Y.; Ye, F.; Shuang, Z.; Xie, Z.; Xie, X. The effect of preoperative serum triglycerides and high-density lipoprotein-cholesterol levels on the prognosis of breast cancer. Breast Edinb. Scotl. 2017, 32, 1–6. [Google Scholar] [CrossRef] [PubMed]
- His, M.; Dartois, L.; Fagherazzi, G.; Boutten, A.; Dupré, T.; Mesrine, S.; Boutron-Ruault, M.-C.; Clavel-Chapelon, F.; Dossus, L. Associations between serum lipids and breast cancer incidence and survival in the E3N prospective cohort study. Cancer Causes Control CCC 2017, 28, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Kucharska-Newton, A.M.; Rosamond, W.D.; Mink, P.J.; Alberg, A.J.; Shahar, E.; Folsom, A.R. HDL-cholesterol and incidence of breast cancer in the ARIC cohort study. Ann. Epidemiol. 2008, 18, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, S.K.; Han, W.; Kim, D.-H.; Hong, Y.-C.; Ha, E.H.; Ahn, S.-H.; Noh, D.-Y.; Kang, D.; Yoo, K.-Y. Serum high-density lipoprotein cholesterol and breast cancer risk by menopausal status, body mass index, and hormonal receptor in Korea. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2009, 18, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Furberg, A.-S.; Veierød, M.B.; Wilsgaard, T.; Bernstein, L.; Thune, I. Serum high-density lipoprotein cholesterol, metabolic profile, and breast cancer risk. J. Natl. Cancer Inst. 2004, 96, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Z.-L.; Wu, Y.-T.; Wu, H.; Dai, W.; Arshad, B.; Xu, Z.; Li, H.; Wu, K.-N.; Kong, L.-Q. Status of lipid and lipoprotein in female breast cancer patients at initial diagnosis and during chemotherapy. Lipids Health Dis. 2018, 17, 91. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.K.; Poudel, B.; Thanpari, C.; Chandra Koner, B. Assessment of biochemical profiles in premenopausal and postmenopausal women with breast cancer. Asian Pac. J. Cancer Prev. APJCP 2012, 13, 3385–3388. [Google Scholar] [CrossRef]
- Owiredu, W.K.B.A.; Donkor, S.; Addai, B.W.; Amidu, N. Serum lipid profile of breast cancer patients. Pak. J. Biol. Sci. 2009, 12, 332–338. [Google Scholar] [CrossRef]
- Michalaki, V.; Koutroulis, G.; Syrigos, K.; Piperi, C.; Kalofoutis, A. Evaluation of serum lipids and high-density lipoprotein subfractions (HDL2, HDL3) in postmenopausal patients with breast cancer. Mol. Cell. Biochem. 2005, 268, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Kotepui, M. Diet and risk of breast cancer. Contemp. Oncol. Pozn. Pol. 2016, 20, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; La Vecchia, C.; de Groh, M.; Negri, E.; Morrison, H.; Mery, L.; Canadian Cancer Registries Epidemiology Research Group. Dietary cholesterol intake and cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2012, 23, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, L.; Zhang, D.; Jiang, W. Systematic review and meta-analysis suggest that dietary cholesterol intake increases risk of breast cancer. Nutr. Res. 2016, 36, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Cleary, M.P.; Grande, J.P.; Maihle, N.J. Effect of high fat diet on body weight and mammary tumor latency in MMTV-TGF-alpha mice. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2004, 28, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Guy, C.T.; Cardiff, R.D.; Muller, W.J. Induction of mammary tumors by expression of polyomavirus middle T oncogene: A transgenic mouse model for metastatic disease. Mol. Cell. Biol. 1992, 12, 954–961. [Google Scholar] [CrossRef]
- Lin, E.Y.; Jones, J.G.; Li, P.; Zhu, L.; Whitney, K.D.; Muller, W.J.; Pollard, J.W. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am. J. Pathol. 2003, 163, 2113–2126. [Google Scholar] [CrossRef]
- Dogan, S.; Hu, X.; Zhang, Y.; Maihle, N.J.; Grande, J.P.; Cleary, M.P. Effects of high-fat diet and/or body weight on mammary tumor leptin and apoptosis signaling pathways in MMTV-TGF-α mice. Breast Cancer Res. 2007, 9, R91. [Google Scholar] [CrossRef]
- Llaverias, G.; Danilo, C.; Mercier, I.; Daumer, K.; Capozza, F.; Williams, T.M.; Sotgia, F.; Lisanti, M.P.; Frank, P.G. Role of cholesterol in the development and progression of breast cancer. Am. J. Pathol. 2011, 178, 402–412. [Google Scholar] [CrossRef]
- Pelton, K.; Coticchia, C.M.; Curatolo, A.S.; Schaffner, C.P.; Zurakowski, D.; Solomon, K.R.; Moses, M.A. Hypercholesterolemia induces angiogenesis and accelerates growth of breast tumors in vivo. Am. J. Pathol. 2014, 184, 2099–2110. [Google Scholar] [CrossRef]
- Alikhani, N.; Ferguson, R.D.; Novosyadlyy, R.; Gallagher, E.J.; Scheinman, E.J.; Yakar, S.; LeRoith, D. Mammary tumor growth and pulmonary metastasis are enhanced in a hyperlipidemic mouse model. Oncogene 2013, 32, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.H.; Reddick, R.L.; Piedrahita, J.A.; Maeda, N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science 1992, 258, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Constantinou, C.; Mpatsoulis, D.; Natsos, A.; Petropoulou, P.-I.; Zvintzou, E.; Traish, A.M.; Voshol, P.J.; Karagiannides, I.; Kypreos, K.E. The low density lipoprotein receptor modulates the effects of hypogonadism on diet-induced obesity and related metabolic perturbations. J. Lipid Res. 2014, 55, 1434–1447. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, D.P.; Park, S.; Goulet, M.T.; Jasper, J.; Wardell, S.E.; Chang, C.-Y.; Norris, J.D.; Guyton, J.R.; Nelson, E.R. Obesity, cholesterol metabolism, and breast cancer pathogenesis. Cancer Res. 2014, 74, 4976–4982. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.R.; Wardell, S.E.; Jasper, J.S.; Park, S.; Suchindran, S.; Howe, M.K.; Carver, N.J.; Pillai, R.V.; Sullivan, P.M.; Sondhi, V.; et al. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science 2013, 342, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, C.R.; Domingues, G.; Matias, I.; Matos, J.; Fonseca, I.; de Almeida, J.M.; Dias, S. LDL-cholesterol signaling induces breast cancer proliferation and invasion. Lipids Health Dis. 2014, 13, 16. [Google Scholar] [CrossRef]
- Jensen, E.V.; Jordan, V.C. The estrogen receptor: A model for molecular medicine. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2003, 9, 1980–1989. [Google Scholar]
- Umetani, M.; Shaul, P.W. 27-Hydroxycholesterol: The first identified endogenous SERM. Trends Endocrinol. Metab. 2011, 22, 130–135. [Google Scholar] [CrossRef]
- Burkard, I.; von Eckardstein, A.; Waeber, G.; Vollenweider, P.; Rentsch, K.M. Lipoprotein distribution and biological variation of 24S- and 27-hydroxycholesterol in healthy volunteers. Atherosclerosis 2007, 194, 71–78. [Google Scholar] [CrossRef]
- Russell, D.W. Oxysterol biosynthetic enzymes. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2000, 1529, 126–135. [Google Scholar] [CrossRef]
- Cruz, P.; Torres, C.; Ramírez, M.E.; Epuñán, M.J.; Valladares, L.E.; Sierralta, W.D. Proliferation of human mammary cancer cells exposed to 27-hydroxycholesterol. Exp. Ther. Med. 2010, 1, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Ishikawa, T.; Sirianni, R.; Tang, H.; McDonald, J.G.; Yuhanna, I.S.; Thompson, B.; Girard, L.; Mineo, C.; Brekken, R.A.; et al. 27-Hydroxycholesterol promotes cell-autonomous, ER-positive breast cancer growth. Cell Rep. 2013, 5, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Baek, A.E.; Yu, Y.-R.A.; He, S.; Wardell, S.E.; Chang, C.-Y.; Kwon, S.; Pillai, R.V.; McDowell, H.B.; Thompson, J.W.; Dubois, L.G.; et al. The cholesterol metabolite 27 hydroxycholesterol facilitates breast cancer metastasis through its actions on immune cells. Nat. Commun. 2017, 8, 864. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.-L.; Le Cornet, C.; Sookthai, D.; Johnson, T.S.; Kaaks, R.; Fortner, R.T. Circulating 27-Hydroxycholesterol and Breast Cancer Risk: Results From the EPIC-Heidelberg Cohort. J. Natl. Cancer Inst. 2019, 111, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.; Ohm, J.E.; Dhasarathy, A.; Schommer, J.; Roche, C.; Hammer, K.D.P.; Ghribi, O. The cholesterol metabolite 27-hydroxycholesterol regulates p53 activity and increases cell proliferation via MDM2 in breast cancer cells. Mol. Cell. Biochem. 2015, 410, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.-M.; Liang, Z.-R.; Zhou, K.-R.; Zhou, H.; Qu, L.-H. 27-Hydroxycholesterol increases Myc protein stability via suppressing PP2A, SCP1 and FBW7 transcription in MCF-7 breast cancer cells. Biochem. Biophys. Res. Commun. 2016, 480, 328–333. [Google Scholar] [CrossRef]
- Hu, B.; Zhang, K.; Li, S.; Li, H.; Yan, Z.; Huang, L.; Wu, J.; Han, X.; Jiang, W.; Mulatibieke, T.; et al. HIC1 attenuates invasion and metastasis by inhibiting the IL-6/STAT3 signalling pathway in human pancreatic cancer. Cancer Lett. 2016, 376, 387–398. [Google Scholar] [CrossRef]
- Zhu, D.; Shen, Z.; Liu, J.; Chen, J.; Liu, Y.; Hu, C.; Li, Z.; Li, Y. The ROS-mediated activation of STAT-3/VEGF signaling is involved in the 27-hydroxycholesterol-induced angiogenesis in human breast cancer cells. Toxicol. Lett. 2016, 264, 79–86. [Google Scholar] [CrossRef]
- Torres, C.G.; Ramírez, M.E.; Cruz, P.; Epuñan, M.J.; Valladares, L.E.; Sierralta, W.D. 27-hydroxycholesterol induces the transition of MCF7 cells into a mesenchymal phenotype. Oncol. Rep. 2011, 26, 389–397. [Google Scholar]
- Shen, Z.; Zhu, D.; Liu, J.; Chen, J.; Liu, Y.; Hu, C.; Li, Z.; Li, Y. 27-Hydroxycholesterol induces invasion and migration of breast cancer cells by increasing MMP9 and generating EMT through activation of STAT-3. Environ. Toxicol. Pharmacol. 2017, 51, 1–8. [Google Scholar] [CrossRef]
- Umetani, M.; Ghosh, P.; Ishikawa, T.; Umetani, J.; Ahmed, M.; Mineo, C.; Shaul, P.W. The cholesterol metabolite 27-hydroxycholesterol promotes atherosclerosis via proinflammatory processes mediated by estrogen receptor alpha. Cell Metab. 2014, 20, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, J. Cholesterol forges link between obesity and breast cancer. Science 2013, 342, 1028. [Google Scholar] [CrossRef] [PubMed]
- DuSell, C.D.; Umetani, M.; Shaul, P.W.; Mangelsdorf, D.J.; McDonnell, D.P. 27-hydroxycholesterol is an endogenous selective estrogen receptor modulator. Mol. Endocrinol. 2008, 22, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-R.; Ishikawa, T.; Umetani, M. The interaction between metabolism, cancer and cardiovascular disease, connected by 27-hydroxycholesterol. Clin. Lipidol. 2014, 9, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Pires, L.A.; Hegg, R.; Freitas, F.R.; Tavares, E.R.; Almeida, C.P.; Baracat, E.C.; Maranhão, R.C. Effect of neoadjuvant chemotherapy on low-density lipoprotein (LDL) receptor and LDL receptor-related protein 1 (LRP-1) receptor in locally advanced breast cancer. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Medicas E Biol. 2012, 45, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Stranzl, A.; Schmidt, H.; Winkler, R.; Kostner, G.M. Low-density lipoprotein receptor mRNA in human breast cancer cells: Influence by PKC modulators. Breast Cancer Res. Treat. 1997, 42, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, E.J.; Zelenko, Z.; Neel, B.A.; Antoniou, I.M.; Rajan, L.; Kase, N.; LeRoith, D. Elevated tumor LDLR expression accelerates LDL cholesterol-mediated breast cancer growth in mouse models of hyperlipidemia. Oncogene 2017, 36, 6462–6471. [Google Scholar] [CrossRef]
- Antalis, C.J.; Arnold, T.; Rasool, T.; Lee, B.; Buhman, K.K.; Siddiqui, R.A. High ACAT1 expression in estrogen receptor negative basal-like breast cancer cells is associated with LDL-induced proliferation. Breast Cancer Res. Treat. 2010, 122, 661–670. [Google Scholar] [CrossRef]
- Rotheneder, M.; Kostner, G.M. Effects of low- and high-density lipoproteins on the proliferation of human breast cancer cells in vitro: Differences between hormone-dependent and hormone-independent cell lines. Int. J. Cancer 1989, 43, 875–879. [Google Scholar] [CrossRef]
- Lu, C.-W.; Lo, Y.-H.; Chen, C.-H.; Lin, C.-Y.; Tsai, C.-H.; Chen, P.-J.; Yang, Y.-F.; Wang, C.-H.; Tan, C.-H.; Hou, M.-F.; et al. VLDL and LDL, but not HDL, promote breast cancer cell proliferation, metastasis and angiogenesis. Cancer Lett. 2017, 388, 130–138. [Google Scholar] [CrossRef]
- Antalis, C.J.; Uchida, A.; Buhman, K.K.; Siddiqui, R.A. Migration of MDA-MB-231 breast cancer cells depends on the availability of exogenous lipids and cholesterol esterification. Clin. Exp. Metastasis 2011, 28, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, G.L.; Wang, K.A. Dietary fat reduction and breast cancer outcome: Results from the Women’s Intervention Nutrition Study (WINS). Am. J. Clin. Nutr. 2007, 86, s878–s881. [Google Scholar] [CrossRef] [PubMed]
- Neve, R.M.; Chin, K.; Fridlyand, J.; Yeh, J.; Baehner, F.L.; Fevr, T.; Clark, L.; Bayani, N.; Coppe, J.-P.; Tong, F.; et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 2006, 10, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, K.M.; Kandil, D.; Khan, A.; Cosar, E.F. Theranostic and molecular classification of breast cancer. Arch. Pathol. Lab. Med. 2014, 138, 44–56. [Google Scholar] [CrossRef] [PubMed]
- De Gonzalo-Calvo, D.; López-Vilaró, L.; Nasarre, L.; Perez-Olabarria, M.; Vázquez, T.; Escuin, D.; Badimon, L.; Barnadas, A.; Lerma, E.; Llorente-Cortés, V. Intratumor cholesteryl ester accumulation is associated with human breast cancer proliferation and aggressive potential: A molecular and clinicopathological study. BMC Cancer 2015, 15, 460. [Google Scholar] [CrossRef]
- Sánchez-Pérez, Y.; Carrasco-Legleu, C.; García-Cuellar, C.; Pérez-Carreón, J.; Hernández-García, S.; Salcido-Neyoy, M.; Alemán-Lazarini, L.; Villa-Treviño, S. Oxidative stress in carcinogenesis. Correlation between lipid peroxidation and induction of preneoplastic lesions in rat hepatocarcinogenesis. Cancer Lett. 2005, 217, 25–32. [Google Scholar] [CrossRef]
- Wiseman, H.; Halliwell, B. Damage to DNA by reactive oxygen and nitrogen species: Role in inflammatory disease and progression to cancer. Biochem. J. 1996, 313 Pt 1, 17–29. [Google Scholar] [CrossRef]
- Delimaris, I.; Faviou, E.; Antonakos, G.; Stathopoulou, E.; Zachari, A.; Dionyssiou-Asteriou, A. Oxidized LDL, serum oxidizability and serum lipid levels in patients with breast or ovarian cancer. Clin. Biochem. 2007, 40, 1129–1134. [Google Scholar] [CrossRef]
- Khaidakov, M.; Mehta, J.L. Oxidized LDL triggers pro-oncogenic signaling in human breast mammary epithelial cells partly via stimulation of MiR-21. PLoS ONE 2012, 7, e46973. [Google Scholar] [CrossRef]
- Pucci, S.; Polidoro, C.; Greggi, C.; Amati, F.; Morini, E.; Murdocca, M.; Biancolella, M.; Orlandi, A.; Sangiuolo, F.; Novelli, G. Pro-oncogenic action of LOX-1 and its splice variant LOX-1Δ4 in breast cancer phenotypes. Cell Death Dis. 2019, 10, 53. [Google Scholar] [CrossRef]
- Khaidakov, M.; Mitra, S.; Kang, B.-Y.; Wang, X.; Kadlubar, S.; Novelli, G.; Raj, V.; Winters, M.; Carter, W.C.; Mehta, J.L. Oxidized LDL receptor 1 (OLR1) as a possible link between obesity, dyslipidemia and cancer. PLoS ONE 2011, 6, e20277. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Zhang, P.; Fu, J. Up-regulation of LOX-1 expression by TNF-α promotes trans-endothelial migration of MDA-MB-231 breast cancer cells. Cancer Lett. 2007, 258, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, H.A.; Iliopoulos, D.; Joshi, A.; Zhang, Y.; Jaeger, S.A.; Bulyk, M.; Tsichlis, P.N.; Shirley Liu, X.; Struhl, K. A transcriptional signature and common gene networks link cancer with lipid metabolism and diverse human diseases. Cancer Cell 2010, 17, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhao, H.; Zhao, L.; Zhang, Y.; Wan, Q.; Shen, Y.; Bu, X.; Wan, M.; Shen, C. Up-regulation of OLR1 expression by TBC1D3 through activation of TNFα/NF-κB pathway promotes the migration of human breast cancer cells. Cancer Lett. 2017, 408, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Gospodarowicz, D.; Lui, G.M.; Gonzalez, R. High-density lipoproteins and the proliferation of human tumor cells maintained on extracellular matrix-coated dishes and exposed to defined medium. Cancer Res. 1982, 42, 3704–3713. [Google Scholar] [CrossRef]
- Danilo, C.; Gutierrez-Pajares, J.L.; Mainieri, M.A.; Mercier, I.; Lisanti, M.P.; Frank, P.G. Scavenger receptor class B type I regulates cellular cholesterol metabolism and cell signaling associated with breast cancer development. Breast Cancer Res. 2013, 15, R87. [Google Scholar] [CrossRef] [PubMed]
- Pussinen, P.J.; Karten, B.; Wintersperger, A.; Reicher, H.; McLean, M.; Malle, E.; Sattler, W. The human breast carcinoma cell line HBL-100 acquires exogenous cholesterol from high-density lipoprotein via CLA-1 (CD-36 and LIMPII analogous 1)-mediated selective cholesteryl ester uptake. Biochem. J. 2000, 349, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.M.; Murao, K.; Imachi, H.; Yu, X.; Abe, H.; Yamauchi, A.; Niimi, M.; Miyauchi, A.; Wong, N.C.W.; Ishida, T. A mutant high-density lipoprotein receptor inhibits proliferation of human breast cancer cells. Cancer Res. 2004, 64, 1515–1521. [Google Scholar] [CrossRef]
- Yuan, B.; Wu, C.; Wang, X.; Wang, D.; Liu, H.; Guo, L.; Li, X.-A.; Han, J.; Feng, H. High scavenger receptor class B type I expression is related to tumor aggressiveness and poor prognosis in breast cancer. Tumor Biol. 2016, 37, 3581–3588. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Li, M.; Yin, L.; Li, X.-A.; Zhang, T.-G. Up-regulated expression of scavenger receptor class B type 1 (SR-B1) is associated with malignant behaviors and poor prognosis of breast cancer. Pathol. Res. Pract. 2016, 212, 555–559. [Google Scholar] [CrossRef]
- Lee-Rueckert, M.; Escola-Gil, J.C.; Kovanen, P.T. HDL functionality in reverse cholesterol transport—Challenges in translating data emerging from mouse models to human disease. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2016, 1861, 566–583. [Google Scholar] [CrossRef] [PubMed]
- Mineo, C.; Shaul, P.W. Novel Biological Functions of High-Density Lipoprotein Cholesterol. Circ. Res. 2012, 111, 1079–1090. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-L.; Stasyk, T.; Morandell, S.; Dieplinger, H.; Falkensammer, G.; Griesmacher, A.; Mogg, M.; Schreiber, M.; Feuerstein, I.; Huck, C.W.; et al. Biomarker discovery in breast cancer serum using 2-D differential gel electrophoresis/ MALDI-TOF/TOF and data validation by routine clinical assays. Electrophoresis 2006, 27, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-J.; Hou, M.-F.; Tsai, S.-M.; Wu, S.-H.; Hou, L.A.; Ma, H.; Shann, T.-Y.; Wu, S.-H.; Tsai, L.-Y. The association between lipid profiles and breast cancer among Taiwanese women. Clin. Chem. Lab. Med. 2007, 45, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Cedó, L.; García-León, A.; Baila-Rueda, L.; Santos, D.; Grijalva, V.; Martínez-Cignoni, M.R.; Carbó, J.M.; Metso, J.; López-Vilaró, L.; Zorzano, A.; et al. ApoA-I mimetic administration, but not increased apoA-I-containing HDL, inhibits tumour growth in a mouse model of inherited breast cancer. Sci. Rep. 2016, 6, 36387. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Vaca, F.; Escolà-Gil, J.C.; Martín-Campos, J.M.; Julve, J. Role of apoA-II in lipid metabolism and atherosclerosis: Advances in the study of an enigmatic protein. J. Lipid Res. 2001, 42, 1727–1739. [Google Scholar] [PubMed]
- Julve, J.; Escolà-Gil, J.C.; Rotllan, N.; Fiévet, C.; Vallez, E.; de la Torre, C.; Ribas, V.; Sloan, J.H.; Blanco-Vaca, F. Human apolipoprotein A-II determines plasma triglycerides by regulating lipoprotein lipase activity and high-density lipoprotein proteome. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 232–238. [Google Scholar] [CrossRef]
- Ribas, V.; Sánchez-Quesada, J.L.; Antón, R.; Camacho, M.; Julve, J.; Escolà-Gil, J.C.; Vila, L.; Ordóñez-Llanos, J.; Blanco-Vaca, F. Human apolipoprotein A-II enrichment displaces paraoxonase from HDL and impairs its antioxidant properties: A new mechanism linking HDL protein composition and antiatherogenic potential. Circ. Res. 2004, 95, 789–797. [Google Scholar] [CrossRef]
- Pan, B.; Ren, H.; Lv, X.; Zhao, Y.; Yu, B.; He, Y.; Ma, Y.; Niu, C.; Kong, J.; Yu, F.; et al. Hypochlorite-induced oxidative stress elevates the capability of HDL in promoting breast cancer metastasis. J. Transl. Med. 2012, 10, 65. [Google Scholar] [CrossRef]
- Kontush, A.; Chapman, M.J. Why is HDL functionally deficient in type 2 diabetes? Curr. Diab. Rep. 2008, 8, 51–59. [Google Scholar] [CrossRef]
- Larsson, S.C.; Mantzoros, C.S.; Wolk, A. Diabetes mellitus and risk of breast cancer: A meta-analysis. Int. J. Cancer 2007, 121, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Ren, H.; Ma, Y.; Liu, D.; Yu, B.; Ji, L.; Pan, L.; Li, J.; Yang, L.; Lv, X.; et al. High-density lipoprotein of patients with type 2 diabetes mellitus elevates the capability of promoting migration and invasion of breast cancer cells. Int. J. Cancer 2012, 131, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Ren, H.; He, Y.; Lv, X.; Ma, Y.; Li, J.; Huang, L.; Yu, B.; Kong, J.; Niu, C.; et al. HDL of patients with type 2 diabetes mellitus elevates the capability of promoting breast cancer metastasis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; He, D.; Ming, J.; He, Y.; Zhou, C.; Ren, H.; He, X.; Wang, C.; Jin, J.; Ji, L.; et al. High-density lipoprotein of patients with breast cancer complicated with type 2 diabetes mellitus promotes cancer cells adhesion to vascular endothelium via ICAM-1 and VCAM-1 upregulation. Breast Cancer Res. Treat. 2016, 155, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.L.; Brown, M.S. Regulation of the mevalonate pathway. Nature 1990, 343, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Cauley, J.A.; Zmuda, J.M.; Lui, L.-Y.; Hillier, T.A.; Ness, R.B.; Stone, K.L.; Cummings, S.R.; Bauer, D.C. Lipid-lowering drug use and breast cancer in older women: A prospective study. J. Womens Health 2003, 12, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Anothaisintawee, T.; Udomsubpayakul, U.; McEvoy, M.; Lerdsitthichai, P.; Attia, J.; Thakkinstian, A. Effect of Lipophilic and Hydrophilic Statins on Breast Cancer Risk in Thai Women: A Cross-sectional Study. J. Cancer 2016, 7, 1163–1168. [Google Scholar] [CrossRef]
- Bonovas, S.; Filioussi, K.; Tsavaris, N.; Sitaras, N.M. Use of statins and breast cancer: A meta-analysis of seven randomized clinical trials and nine observational studies. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 8606–8612. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Yang, H.-C.; Nguyen, P.-A.; Poly, T.N.; Huang, C.-W.; Kekade, S.; Khalfan, A.M.; Debnath, T.; Li, Y.-C.J.; Abdul, S.S. Exploring association between statin use and breast cancer risk: An updated meta-analysis. Arch. Gynecol. Obstet. 2017, 296, 1043–1053. [Google Scholar] [CrossRef]
- Schairer, C.; Freedman, D.M.; Gadalla, S.M.; Pfeiffer, R.M. Lipid-lowering drugs, dyslipidemia, and breast cancer risk in a Medicare population. Breast Cancer Res. Treat. 2018, 169, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Undela, K.; Srikanth, V.; Bansal, D. Statin use and risk of breast cancer: A meta-analysis of observational studies. Breast Cancer Res. Treat. 2012, 135, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Dale, K.M.; Coleman, C.I.; Henyan, N.N.; Kluger, J.; White, C.M. Statins and cancer risk: A meta-analysis. JAMA 2006, 295, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Ray, K.K.; Catapano, A.L.; Ference, T.B.; Burgess, S.; Neff, D.R.; Oliver-Williams, C.; Wood, A.M.; Butterworth, A.S.; Di Angelantonio, E.; et al. Mendelian Randomization Study of ACLY and Cardiovascular Disease. N. Engl. J. Med. 2019, 380, 1033–1042. [Google Scholar] [CrossRef]
- McDougall, J.A.; Malone, K.E.; Daling, J.R.; Cushing-Haugen, K.L.; Porter, P.L.; Li, C.I. Long-Term Statin Use and Risk of Ductal and Lobular Breast Cancer among Women 55 to 74 Years of Age. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Borgquist, S.; Giobbie-Hurder, A.; Ahern, T.P.; Garber, J.E.; Colleoni, M.; Láng, I.; Debled, M.; Ejlertsen, B.; von Moos, R.; Smith, I.; et al. Cholesterol, Cholesterol-Lowering Medication Use, and Breast Cancer Outcome in the BIG 1-98 Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Mansourian, M.; Haghjooy-Javanmard, S.; Eshraghi, A.; Vaseghi, G.; Hayatshahi, A.; Thomas, J. Statins Use and Risk of Breast Cancer Recurrence and Death: A Systematic Review and Meta-Analysis of Observational Studies. J. Pharm. Pharm. Sci. Publ. Can. Soc. Pharm. Sci. Soc. Can. Sci. Pharm. 2016, 19, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Sakellakis, M.; Akinosoglou, K.; Kostaki, A.; Spyropoulou, D.; Koutras, A. Statins and risk of breast cancer recurrence. Breast Cancer Dove Med. Press 2016, 8, 199–205. [Google Scholar] [PubMed]
- Chae, Y.K.; Valsecchi, M.E.; Kim, J.; Bianchi, A.L.; Khemasuwan, D.; Desai, A.; Tester, W. Reduced risk of breast cancer recurrence in patients using ACE inhibitors, ARBs, and/or statins. Cancer Investig. 2011, 29, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.-J.; Tu, C.; Li, Y.-Y.; Zhu, J.; Qian, K.-Q.; Li, W.-J.; Wu, L. Statin use and breast cancer survival and risk: A systematic review and meta-analysis. Oncotarget 2015, 6, 42988–43004. [Google Scholar] [CrossRef] [PubMed]
- Shaitelman, S.F.; Stauder, M.C.; Allen, P.; Reddy, S.; Lakoski, S.; Atkinson, B.; Reddy, J.; Amaya, D.; Guerra, W.; Ueno, N.; et al. Impact of Statin Use on Outcomes in Triple Negative Breast Cancer. J. Cancer 2017, 8, 2026–2032. [Google Scholar] [CrossRef]
- Smith, A.; Murphy, L.; Zgaga, L.; Barron, T.I.; Bennett, K. Pre-diagnostic statin use, lymph node status and mortality in women with stages I-III breast cancer. Br. J. Cancer 2017, 117, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Murtola, T.J.; Visvanathan, K.; Artama, M.; Vainio, H.; Pukkala, E. Statin use and breast cancer survival: A nationwide cohort study from Finland. PLoS ONE 2014, 9, e110231. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yi, Z.; Guan, X.; Zeng, Y.-X.; Ma, F. The relationship between statins and breast cancer prognosis varies by statin type and exposure time: A meta-analysis. Breast Cancer Res. Treat. 2017, 164, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Manthravadi, S.; Shrestha, A.; Madhusudhana, S. Impact of statin use on cancer recurrence and mortality in breast cancer: A systematic review and meta-analysis: Breast cancer: A systematic review and meta-analysis. Int. J. Cancer 2016, 139, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Ahern, T.P.; Pedersen, L.; Tarp, M.; Cronin-Fenton, D.P.; Garne, J.P.; Silliman, R.A.; Sørensen, H.T.; Lash, T.L. Statin prescriptions and breast cancer recurrence risk: A Danish nationwide prospective cohort study. J. Natl. Cancer Inst. 2011, 103, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Brewer, T.M.; Masuda, H.; Liu, D.D.; Shen, Y.; Liu, P.; Iwamoto, T.; Kai, K.; Barnett, C.M.; Woodward, W.A.; Reuben, J.M.; et al. Statin use in primary inflammatory breast cancer: A cohort study. Br. J. Cancer 2013, 109, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Afzali, M.; Vatankhah, M.; Ostad, S.N. Investigation of simvastatin-induced apoptosis and cell cycle arrest in cancer stem cells of MCF-7. J. Cancer Res. Ther. 2016, 12, 725–730. [Google Scholar]
- Alarcon Martinez, T.; Zeybek, N.D.; Müftüoğlu, S. Evaluation of the Cytotoxic and Autophagic Effects of Atorvastatin on MCF-7 Breast Cancer Cells. Balk. Med. J. 2018, 35, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.J.; Esserman, L.J.; Zhou, Y.; Shoemaker, M.; Lobo, M.; Borman, E.; Baehner, F.; Kumar, A.S.; Adduci, K.; Marx, C.; et al. Breast cancer growth prevention by statins. Cancer Res. 2006, 66, 8707–8714. [Google Scholar] [CrossRef]
- Göbel, A.; Breining, D.; Rauner, M.; Hofbauer, L.C.; Rachner, T.D. Induction of 3-hydroxy-3-methylglutaryl-CoA reductase mediates statin resistance in breast cancer cells. Cell Death Dis. 2019, 10, 91. [Google Scholar] [CrossRef]
- Kimbung, S.; Lettiero, B.; Feldt, M.; Bosch, A.; Borgquist, S. High expression of cholesterol biosynthesis genes is associated with resistance to statin treatment and inferior survival in breast cancer. Oncotarget 2016, 7, 59640–59651. [Google Scholar] [CrossRef] [PubMed]
- Ghosh-Choudhury, N.; Mandal, C.C.; Ghosh-Choudhury, N.; Ghosh Choudhury, G. Simvastatin induces derepression of PTEN expression via NFkappaB to inhibit breast cancer cell growth. Cell. Signal. 2010, 22, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Vintonenko, N.; Jais, J.-P.; Kassis, N.; Abdelkarim, M.; Perret, G.-Y.; Lecouvey, M.; Crepin, M.; Di Benedetto, M. Transcriptome analysis and in vivo activity of fluvastatin versus zoledronic acid in a murine breast cancer metastasis model. Mol. Pharmacol. 2012, 82, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Lubet, R.A.; Boring, D.; Steele, V.E.; Ruppert, J.M.; Juliana, M.M.; Grubbs, C.J. Lack of efficacy of the statins atorvastatin and lovastatin in rodent mammary carcinogenesis. Cancer Prev. Res. Phila. 2009, 2, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Krause, B.R.; Princen, H.M. Lack of predictability of classical animal models for hypolipidemic activity: A good time for mice? Atherosclerosis 1998, 140, 15–24. [Google Scholar] [CrossRef]
- Garwood, E.R.; Kumar, A.S.; Baehner, F.L.; Moore, D.H.; Au, A.; Hylton, N.; Flowers, C.I.; Garber, J.; Lesnikoski, B.-A.; Hwang, E.S.; et al. Fluvastatin reduces proliferation and increases apoptosis in women with high grade breast cancer. Breast Cancer Res. Treat. 2010, 119, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Bjarnadottir, O.; Romero, Q.; Bendahl, P.-O.; Jirström, K.; Rydén, L.; Loman, N.; Uhlén, M.; Johannesson, H.; Rose, C.; Grabau, D.; et al. Targeting HMG-CoA reductase with statins in a window-of-opportunity breast cancer trial. Breast Cancer Res. Treat. 2013, 138, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Kimbung, S.; Chang, C.-Y.; Bendahl, P.-O.; Dubois, L.; Thompson, J.W.; McDonnell, D.P.; Borgquist, S. Impact of 27-hydroxylase (CYP27A1) and 27-hydroxycholesterol in breast cancer. Endocr. Relat. Cancer 2017, 24, 339–349. [Google Scholar] [CrossRef]
- Mc Menamin, Ú.C.; Murray, L.J.; Hughes, C.M.; Cardwell, C.R. Statin use and breast cancer survival: A nationwide cohort study in Scotland. BMC Cancer 2016, 16, 600. [Google Scholar] [CrossRef]
- Cedó, L.; Blanco-Vaca, F.; Escolà-Gil, J.C. Antiatherogenic potential of ezetimibe in sitosterolemia: Beyond plant sterols lowering. Atherosclerosis 2017, 260, 94–96. [Google Scholar] [CrossRef]
- Kobberø Lauridsen, B.; Stender, S.; Frikke-Schmidt, R.; Nordestgaard, B.G.; Tybjærg-Hansen, A. Using genetics to explore whether the cholesterol-lowering drug ezetimibe may cause an increased risk of cancer. Int. J. Epidemiol. 2017, 46, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, T.A.; Puska, P.; Gylling, H.; Vanhanen, H.; Vartiainen, E. Reduction of serum cholesterol with sitostanol-ester margarine in a mildly hypercholesterolemic population. N. Engl. J. Med. 1995, 333, 1308–1312. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.B.; Downie, A.; Fink, C.S.; Kim, U. Dietary phytosterol inhibits the growth and metastasis of MDA-MB-231 human breast cancer cells grown in SCID mice. Anticancer Res. 2000, 20, 821–824. [Google Scholar] [PubMed]
- Ju, Y.H.; Clausen, L.M.; Allred, K.F.; Almada, A.L.; Helferich, W.G. beta-Sitosterol, beta-Sitosterol Glucoside, and a Mixture of beta-Sitosterol and beta-Sitosterol Glucoside Modulate the Growth of Estrogen-Responsive Breast Cancer Cells In Vitro and in Ovariectomized Athymic Mice. J. Nutr. 2004, 134, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Llaverias, G.; Escolà-Gil, J.C.; Lerma, E.; Julve, J.; Pons, C.; Cabré, A.; Cofán, M.; Ros, E.; Sánchez-Quesada, J.L.; Blanco-Vaca, F. Phytosterols inhibit the tumor growth and lipoprotein oxidizability induced by a high-fat diet in mice with inherited breast cancer. J. Nutr. Biochem. 2013, 24, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Bradford, P.G.; Awad, A.B. Phytosterols as anticancer compounds. Mol. Nutr. Food Res. 2007, 51, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Vaca, F.; Cedo, L.; Julve, J. Phytosterols in cancer: From molecular mechanisms to preventive and therapeutic potentials. Curr. Med. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Després, J.-P.; Lemieux, I.; Robins, S.J. Role of fibric acid derivatives in the management of risk factors for coronary heart disease. Drugs 2004, 64, 2177–2198. [Google Scholar] [CrossRef] [PubMed]
- Bonovas, S.; Nikolopoulos, G.K.; Bagos, P.G. Use of Fibrates and Cancer Risk: A Systematic Review and Meta-Analysis of 17 Long-Term Randomized Placebo-Controlled Trials. PLoS ONE 2012, 7, e45259. [Google Scholar] [CrossRef]
- Kwiterovich, P.O. The antiatherogenic role of high-density lipoprotein cholesterol. Am. J. Cardiol. 1998, 82, 13Q–21Q. [Google Scholar] [CrossRef]
- Navab, M.; Anantharamaiah, G.M.; Reddy, S.T.; Hama, S.; Hough, G.; Grijalva, V.R.; Yu, N.; Ansell, B.J.; Datta, G.; Garber, D.W.; et al. Apolipoprotein A-I mimetic peptides. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
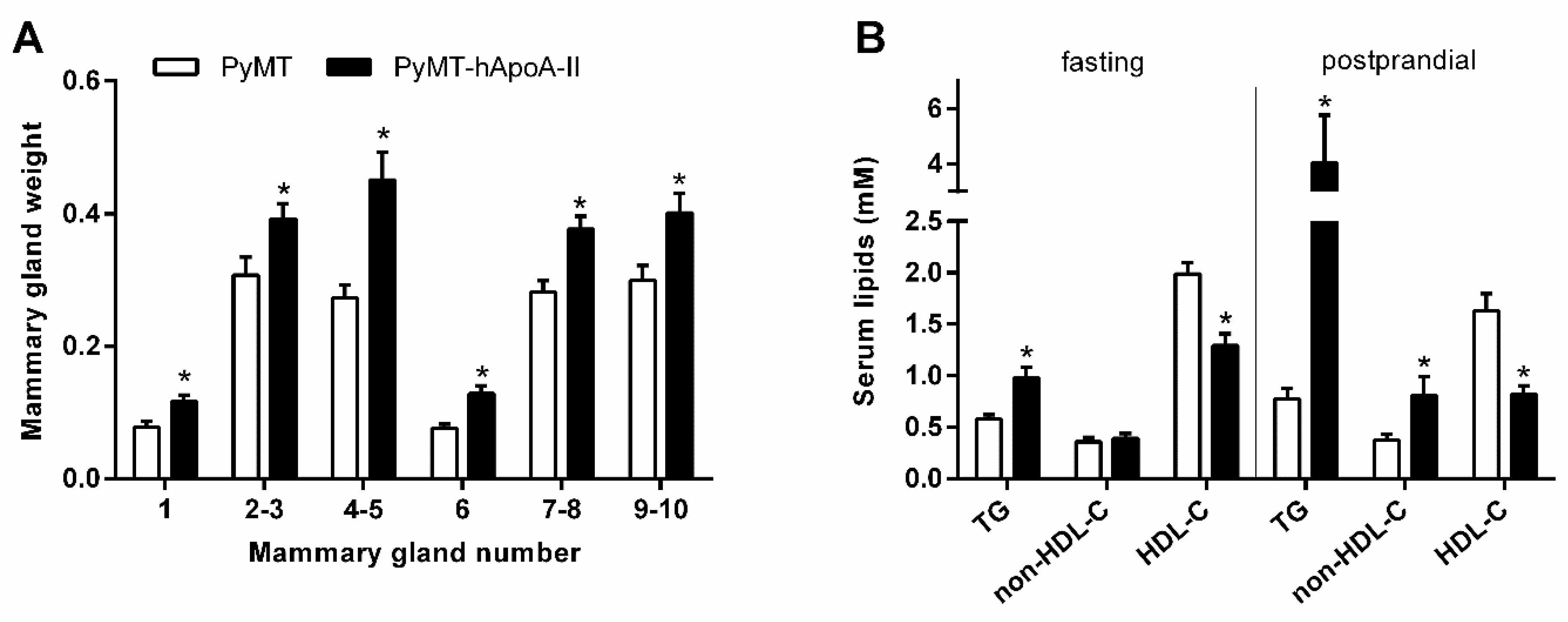
| Reference | Year | Study Design | Participants | Main Findings |
|---|---|---|---|---|
| Nowak et al. [11] | 2018 | Mendelian randomization | >400,000 | Raised LDL-C increased the risk of breast cancer (OR = 1.09 (1.02–1.18)) and ER-positive breast cancer (OR = 1.14 (1.05–1.24)). Raised HDL-C increased the risk of ER-positive breast cancer (OR = 1.13 (1.01–1.26)). |
| Ni et al. [16] | 2015 | Meta-analysis | 1,189,635 | Inverse association between HDL-C and breast cancer risk among postmenopausal women (RR = 0.45 (0.64–0.93)). No association in premenopausal women. No association between LDL-C and breast cancer risk. |
| Touvier et al. [9] | 2015 | Meta-analysis | 1,489,484 | Inverse association between HDL-C and breast cancer risk among premenopausal women (HR = 0.77 (0.31–0.67)). No association in postmenopausal women. No association between LDL-C and breast cancer risk. |
| Borgquist et al. [21] | 2016 | Prospective | 5281 | No evident associations between LDL-C or HDL-C and breast cancer incidence. |
| Chandler et al. [10] | 2016 | Prospective | 15,602 | No association between LDL-C or HDL-C and breast cancer risk. |
| His et al. [19] | 2014 | Prospective | 7557 | HDL-C was inversely associated with breast cancer risk (HR 1 mmol L−1 increment = 0.48 (0.28–0.83)). |
| Rodrigues dos Santos et al. [20] | 2014 | Prospective | 244 | Systemic levels of LDL-C correlated positively with tumor size (Spearman’s r = 0.199, p = 0.002). |
| Kucharska-Newton et al. [25] | 2008 | Prospective | 7575 | Modest association of low HDL-C (<50 mg dL−1) with breast cancer among premenopausal women (HR = 1.67 (1.06–2.63)). No association in postmenopausal women. |
| Furberg et al. [27] | 2004 | Prospective | 30,546 | The risk of postmenopausal breast cancer was reduced in women in the highest quartile of HDL-C (>1.64 mmol L−1) compared with women in the lowest quartile (<1.20 mmol L−1; RR = 0.73 (0.55–0.95)). No association was found in premenopausal women. |
| Li et al. [23] | 2017 | Retrospective | 1044 | Decreased HDL-C levels showed significant association with worse overall survival (HR = 0.528 (0.302–0.923)). |
| Li et al. [28] | 2018 | Case–control | Total: 3537 Cases: 1054 Controls: 2483 | The levels of LDL-C and HDL-C were lower in breast cancer patients than controls (p < 0.001). |
| His et al. [24] | 2017 | Case–control | Total: 1626 Cases: 583 Controls: 1043 | No association between LDL-C or HDL-C and breast cancer risk or survival. |
| Martin et al. [14] | 2015 | Case–control | Total: 837 Cases: 279 Controls: 558 | HDL-C was positively associated (75th vs. 25th percentile: 23% higher, p = 0.05) and non-HDL-C was negatively associated (75th vs. 25th percentile: 19% lower, p = 0.03) with breast cancer risk. |
| Llanos et al. [22] | 2012 | Case–control | Total: 199 Cases: 97 Controls: 102 | Increasing levels of LDL-C were inversely associated with breast cancer risk (OR = 0.41 (0.21–0.81)). Lower levels of HDL-C were associated with a significant increase in breast cancer risk (OR = 1.99 (1.06–3.74)). |
| Yadav et al. [29] | 2012 | Case–control | Total: 139 Cases: 69 Controls: 70 | Postmenopausal breast cancer patients had higher LDL-C levels (p < 0.001) and lower HDL-C levels (p = 0.025) than controls. No significant changes in premenopausal women. |
| Kim et al. [26] | 2009 | Case–control | Total: 2070 Cases: 690 Controls: 1380 | Protective effect of HDL-C on breast cancer was only observed among premenopausal women (OR = 0.49 (0.33–0.72) for HDL-C ≥ 60 vs. <50 mg dL−1 (p < 0.01)). |
| Owiredu et al. [30] | 2009 | Case–control | Total: 200 Cases: 100 Controls: 100 | Increased LDL-C levels in postmenopausal breast cancer patients vs. controls (p < 0.05). No significant changes in premenopausal women. No changes in HDL-C levels between cases and controls. |
| Michalaki et al. [31] | 2005 | Case–control | Total: 100 Cases:56 Controls: 44 | A decrease in HDL-cholesterol was observed in patients with breast cancer vs. controls (p < 0.05). |
| Reference | Year | Study Design | Participants | Main Findings |
|---|---|---|---|---|
| Ference et al. [113] | 2019 | Mendelian randomization | 654,783 | Genetic inhibition of HMGCR did not affect breast cancer risk. |
| Islam et al. [109] | 2017 | Meta-analysis | 121,399 | There was no association between statin use and breast cancer risk. |
| Liu et al. [123] | 2017 | Meta-analysis | 197,048 | Significant protective effects of lipophilic statin use, but not hydrophilic statins, against cancer-specific mortality (HR = 0.57 (0.46–0.70)). |
| Mansourian et al. [116] | 2016 | Meta-analysis | 124,669 | Significant reduction in breast cancer recurrence (OR = 0.792 (0.735–0.853)) and death (OR = 0.849 (0.827–0.870)) among statin users. |
| Manthravadi et al. [124] | 2016 | Meta-analysis | 75,684 | Lipophilic statin use was associated with improved recurrence-free survival (HR = 0.72 (0.59–0.89)). |
| Wu et al. [119] | 2015 | Meta-analysis | 144,830 | There was a significantly negative association between prediagnosis statin use and breast cancer mortality (for overall survival: HR = 0.68 (0.54–0.84), and for disease-specific survival (HR = 0.72 (0.53–0.99)). There was also a significant inverse association between postdiagnosis statin use and breast cancer disease-specific survival (HR = 0.65 (0.43–0.98)). No significant association was detected between statin use and breast cancer risk. |
| Undela et al. [111] | 2012 | Meta-analysis | >2.4 million | Statin use and long-term statin use did not significantly affect breast cancer risk. |
| Bonovas et al. [108] | 2005 | Meta-analysis | 327,238 | Statin use did not significantly affect breast cancer risk. |
| Dale et al. [112] | 2005 | Meta-analysis | 86,936 | Statins did not reduce the incidence of breast cancer. |
| Borgquist et al. [115] | 2017 | Prospective | 8010 | Initiation of cholesterol-lowering medication in postmenopausal women with early stage, hormone receptor-positive invasive breast cancer during endocrine therapy was related to improved disease-free survival (HR = 0.79 (0.66–0.95)), breast cancer-free interval (HR = 0.76 (0.60–0.97)), and distant recurrence-free interval (HR = 0.74 (0.56–0.97)). |
| Murtola et al. [122] | 2014 | Prospective | 31,236 | Both postdiagnostic and prediagnostic statin uses were associatedwith a lowered risk of breast cancer death (HR = 0.46 (0.38–0.55) and HR = 0.54 (0.44–0.67), respectively). |
| Brewer et al. [126] | 2013 | Prospective | 723 | Hydrophilic statins were associated with significantly improved progression-free survival compared with no statin (HR = 0.49 (0.28–0.84)) in inflammatory breast cancer patients. |
| Ahern et al. [125] | 2011 | Prospective | 18,769 | Significant reduction in breast cancer recurrence among patients using simvastatin after 10 y of follow up (adjusted HR = 0.70 (0.57–0.86)). |
| Cauley et al. [106] | 2003 | Prospective | 7528 | Older women who used statins had a reduced risk of breast cancer (RR = 0.28 (0.09–0.86), adjusted for age and body weight) compared with nonusers. |
| Shaitelman et al. [120] | 2017 | Retrospective | 869 | Statin use was significantly associated with overall survival (HR = 0.10 (0.01–0.76)) in triple-negative breast cancer. |
| Smith et al. [121] | 2017 | Retrospective | 6314 | Prediagnostic statin use was associated with breast cancer-specific mortality (HR = 0.81 (0.68–0.96)). This reduction was greatest in statin users with ER-positive tumors (HR = 0.69 (0.55–0.85)). |
| Anothaisintawee et al. [107] | 2016 | Retrospective | 15,718 | Using lipophilic statins, but not hydrophilic statins, could significantly reduce the risk of breast cancer (risk difference = –0.0034 (–0.006,–0.001) lipophilic statin users vs. nonusers). |
| Mc Menamin et al. [139] | 2016 | Retrospective | 15,140 | There was no evidence of an association between statin use and breast cancer-specific death. |
| Sakellaki et al. [117] | 2016 | Retrospective | 610 | Statins may be linked to a favorable outcome in early breast cancer patients, especially in younger age groups (HR = 0.58 (0.36–0.94)). |
| Chae et al. [118] | 2011 | Retrospective | 703 | Significant reduction in breast cancer recurrence among patients who used statins (HR = 0.43 (0.26–0.70)). No association was found regarding overall survival. |
| Schairer et al. [110] | 2018 | Case–control | Total: 228,973 Cases: 30,004 Controls: 198,969 | Statin use did not significantly affect breast cancer risk. |
| McDougall et al. [114] | 2013 | Case–control | Total: 2886 Cases: 916 IDC + 1068 ILC Controls: 902 | Current users of statins for ≥10 y had increased risk of IDC (OR = 1.83 (1.14–2.93)) and ILC (OR = 1.97 (1.25–3.12)) compared with never users of statins. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cedó, L.; Reddy, S.T.; Mato, E.; Blanco-Vaca, F.; Escolà-Gil, J.C. HDL and LDL: Potential New Players in Breast Cancer Development. J. Clin. Med. 2019, 8, 853. https://doi.org/10.3390/jcm8060853
Cedó L, Reddy ST, Mato E, Blanco-Vaca F, Escolà-Gil JC. HDL and LDL: Potential New Players in Breast Cancer Development. Journal of Clinical Medicine. 2019; 8(6):853. https://doi.org/10.3390/jcm8060853
Chicago/Turabian StyleCedó, Lídia, Srinivasa T. Reddy, Eugènia Mato, Francisco Blanco-Vaca, and Joan Carles Escolà-Gil. 2019. "HDL and LDL: Potential New Players in Breast Cancer Development" Journal of Clinical Medicine 8, no. 6: 853. https://doi.org/10.3390/jcm8060853
APA StyleCedó, L., Reddy, S. T., Mato, E., Blanco-Vaca, F., & Escolà-Gil, J. C. (2019). HDL and LDL: Potential New Players in Breast Cancer Development. Journal of Clinical Medicine, 8(6), 853. https://doi.org/10.3390/jcm8060853






