Serologic and Histologic Predictors of Long-Term Renal Outcome in Biopsy-Confirmed IgA Nephropathy (Haas Classification): An Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection and Outcome Assessment
2.3. Statistical Methods
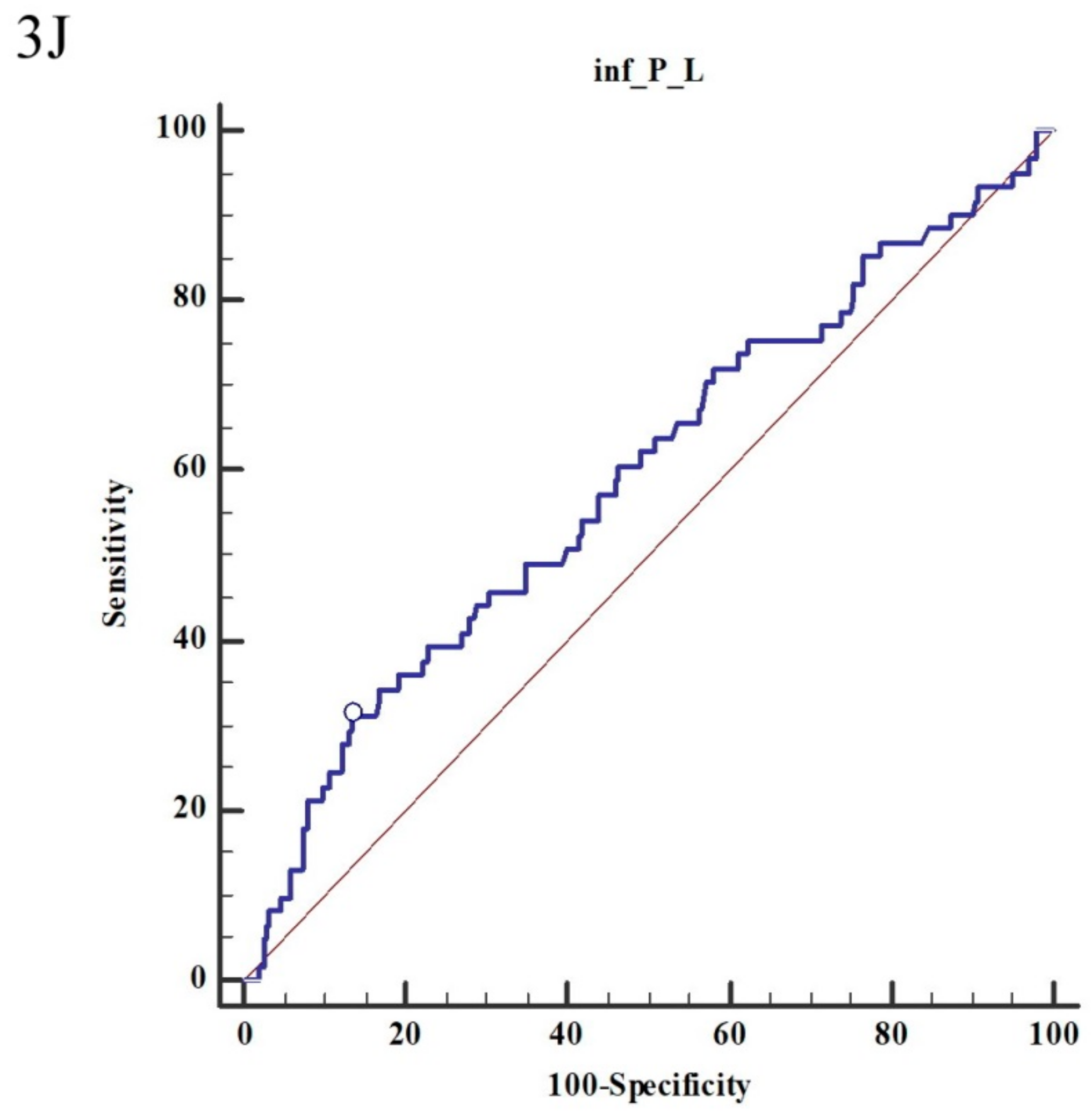
3. Results
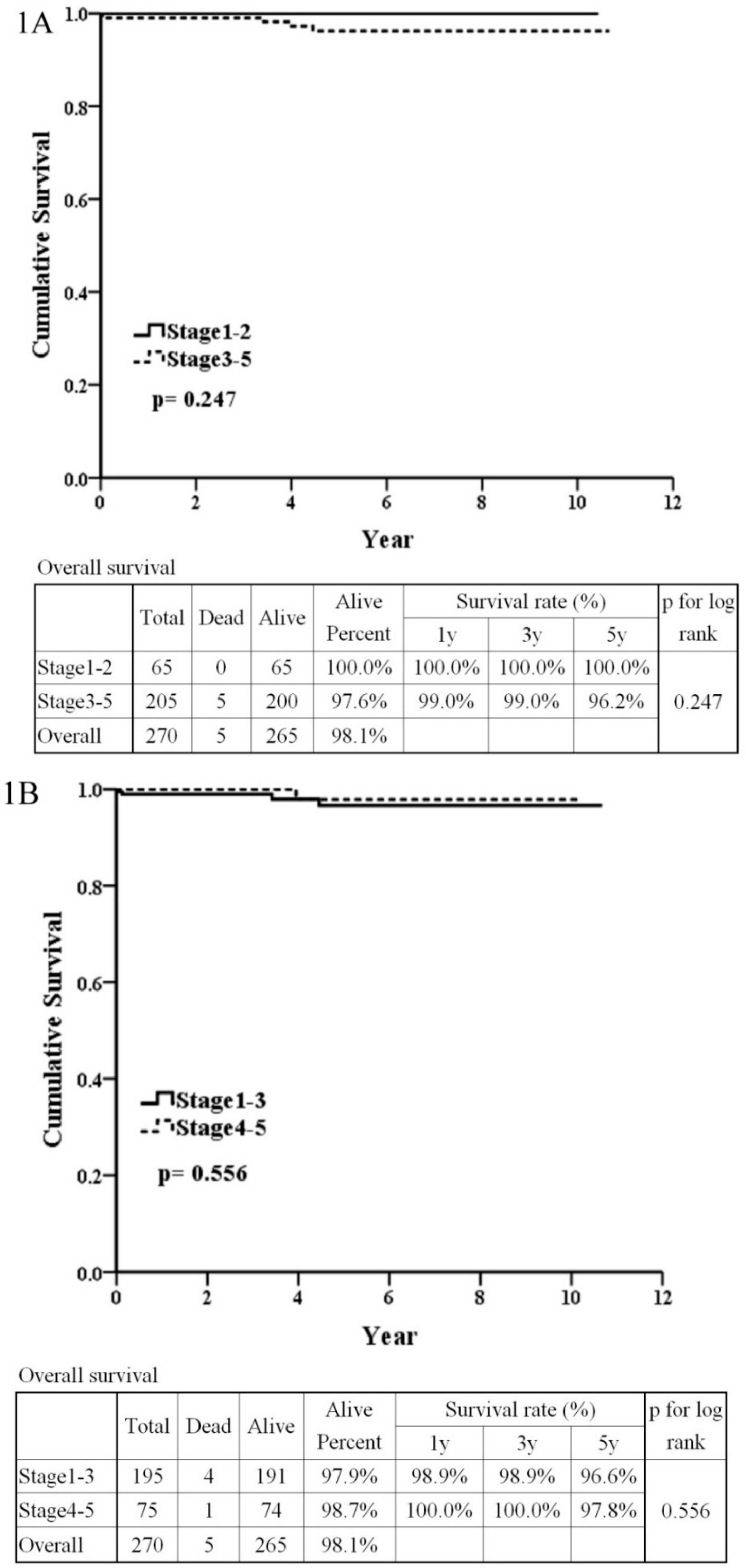
3.1. Pathology Regarded as an Important Influencing Factor According to the Haas Classification
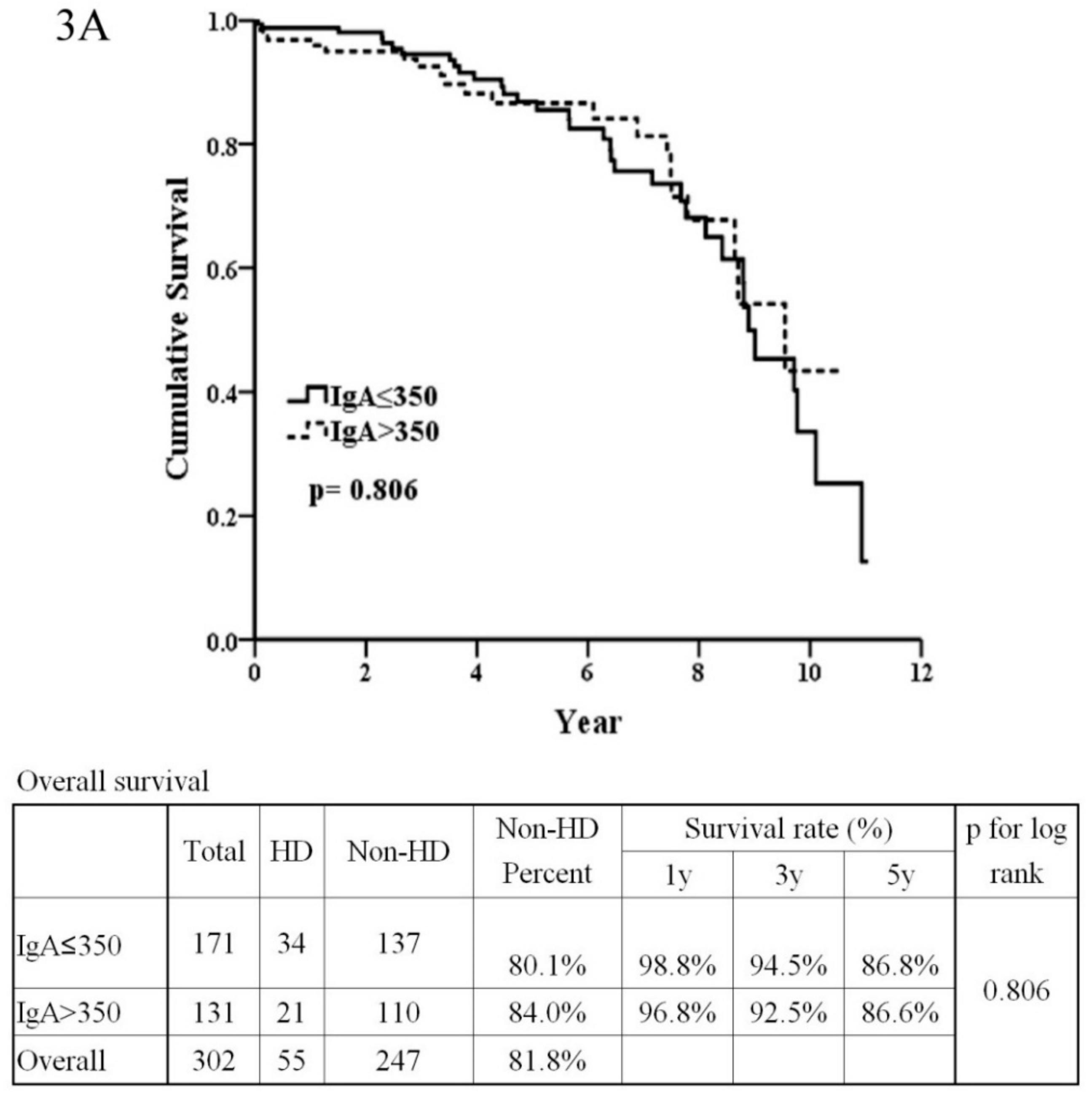
3.2. The Patients’ Outcome Analysis
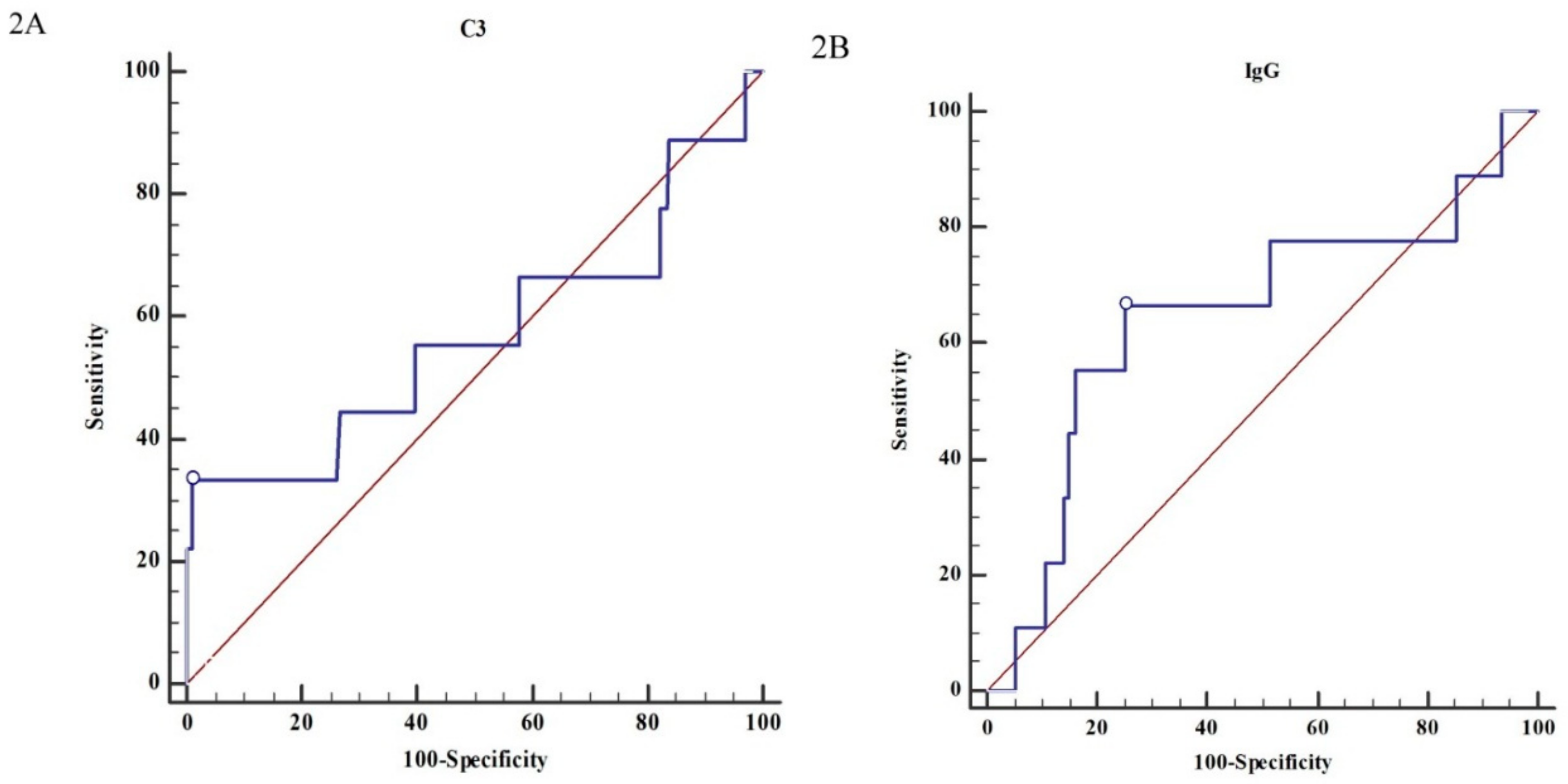
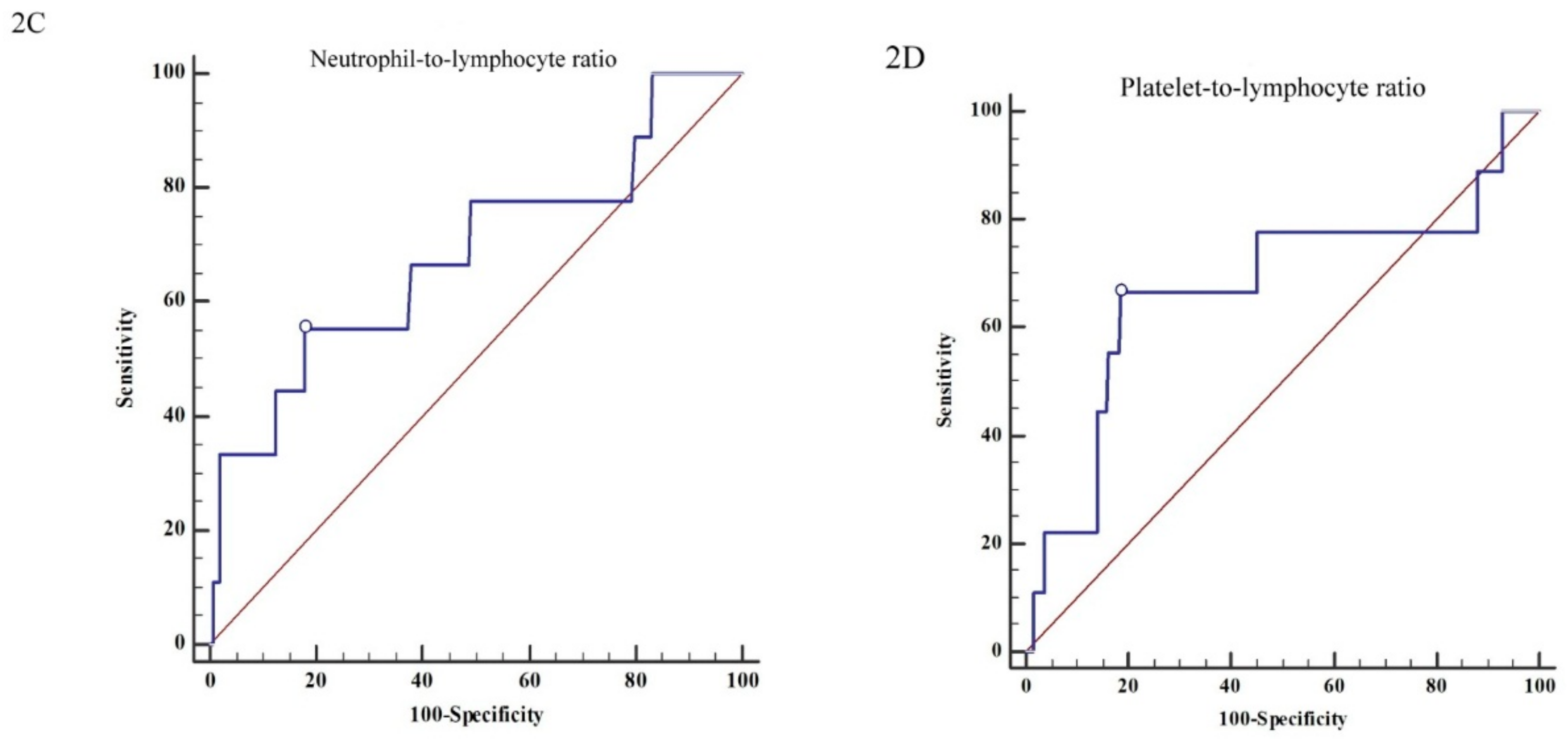
3.3. The Renal Outcome Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| IgAN | Immunoglobulin A nephropathy |
| ESRD | End-stage renal disease |
| SBP | Systolic blood pressure |
| DBP | Diastolic blood pressure |
| BUN | Blood urea nitrogen |
| eGFR | Estimated glomerular filtration rate |
| WBC | White blood cell |
| RBC | Red blood cell |
| GOT | Glutamate oxaloacetate transaminase |
| GPT | Glutamate-pyruvate transaminase |
| LDL | Low-density lipoprotein |
| HDL | High-density lipoprotein |
| NLR | Neutrophil-to-lymphocyte ratio |
| PLR | Platelet-to-lymphocyte ratio |
| ANA | Anti-nuclear Ab |
| anti-dsDNA | Anti-double stranded DNA |
| ANCAs | Anti-neutrophil cytoplasmic antibodies |
| MPO | Proteinase 3 (PR3) and myeloperoxidase |
| ACEis | Angiotensin-converting enzyme inhibitors |
| ARBs | Angiotensin II receptor blockers |
References
- Walsh, M.; Sar, A.; Lee, D.; Yilmaz, S.; Benediktsson, H.; Manns, B.; Hemmelgarn, B. Histopathologic features aid in predicting risk for progression of IgA nephropathy. Clin. J. Am. Soc. Nephrol. 2010, 5, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Gonzalez-Burchard, G.; Broyer, M.; Dommergues, J.P.; Foulard, M.; Sorez, J.P.; Habib, R. Berger’s disease in children. Natural history and outcome. Medicine 1985, 64, 157–180. [Google Scholar] [CrossRef] [PubMed]
- Geddes, C.C.; Rauta, V.; Gronhagen-Riska, C.; Bartosik, L.P.; Jardine, A.G.; Ibels, L.S.; Pei, Y.; Cattran, D.C. A tricontinental view of IgA nephropathy. Nephrol. Dial. Transplant. 2003, 18, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Rao, V.M.; Franklin, W.A.; Schiffer, M.S.; Aronson, A.J.; Spargo, B.H.; Katz, A.I. IgA nephropathy: Morphologic predictors of progressive renal disease. Hum. Pathol. 1982, 13, 314–322. [Google Scholar] [CrossRef]
- Haas, M. Histologic subclassification of IgA nephropathy: A clinicopathologic study of 244 cases. Am. J. Kidney Dis. 1997, 29, 829–842. [Google Scholar] [CrossRef]
- Working Group of the International IgA Nephropathy Network and the Renal Pathology Society; Roberts, I.S.; Cook, H.T.; Troyanov, S.; Alpers, C.E.; Amore, A.; Barratt, J.; Berthoux, F.; Bonsib, S.; Bruijn, J.A.; et al. The Oxford classification of IgA nephropathy: Pathology definitions, correlations, and reproducibility. Kidney Int. 2009, 76, 546–556. [Google Scholar] [CrossRef]
- Park, K.S.; Han, S.H.; Kie, J.H.; Nam, K.H.; Lee, M.J.; Lim, B.J.; Kwon, Y.E.; Kim, Y.L.; An, S.Y.; Kim, C.H.; et al. Comparison of the Haas and the Oxford classifications for prediction of renal outcome in patients with IgA nephropathy. Hum. Pathol. 2014, 45, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.; Reich, H.N. Morphologic markers of progressive immunoglobulin A nephropathy. Adv. Chron. Kidney Dis. 2012, 19, 107–113. [Google Scholar] [CrossRef]
- Soares, M.F.; Roberts, I.S. IgA nephropathy: An update. Curr. Opin. Nephrol. Hypertens. 2017, 26, 165–171. [Google Scholar] [CrossRef]
- Barbour, S.; Reich, H. An update on predicting renal progression in IgA nephropathy. Curr. Opin. Nephrol. Hypertens. 2018, 27, 214–220. [Google Scholar] [CrossRef]
- Levey, A.S.; Coresh, J.; Greene, T.; Stevens, L.A.; Zhang, Y.L.; Hendriksen, S.; Kusek, J.W.; Van Lente, F.; Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann. Intern. Med. 2006, 145, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, H.; Barratt, J.; Cattran, D.C.; Cook, H.T.; Coppo, R.; Haas, M.; Liu, Z.H.; Roberts, I.S.; Yuzawa, Y.; Zhang, H.; et al. Oxford Classification of IgA nephropathy 2016: An update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017, 91, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Barratt, J.; Feehally, J.; Smith, A.C. Pathogenesis of IgA nephropathy. Semin. Nephrol. 2004, 24, 197–217. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Lim, B.J.; Han, I.M.; Han, S.G.; Kwon, Y.E.; Park, K.S.; Lee, M.J.; Oh, H.J.; Park, J.T.; Han, S.H.; et al. Glomerular IgG deposition predicts renal outcome in patients with IgA nephropathy. Mod. Pathol. 2016, 29, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Audard, V.; Lang, P.; Sahali, D. Minimal change nephrotic syndrome: New insights into disease pathogenesis. Med. Sci. 2008, 24, 853–858. [Google Scholar] [CrossRef]
- Pereira Wde, F.; Brito-Melo, G.E.; Guimaraes, F.T.; Carvalho, T.G.; Mateo, E.C.; Simoes e Silva, A.C. The role of the immune system in idiopathic nephrotic syndrome: A review of clinical and experimental studies. Inflamm. Res. 2014, 63, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Roos, A.; Rastaldi, M.P.; Calvaresi, N.; Oortwijn, B.D.; Schlagwein, N.; van Gijlswijk-Janssen, D.J.; Stahl, G.L.; Matsushita, M.; Fujita, T.; van Kooten, C.; et al. Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J. Am. Soc. Nephrol. 2006, 17, 1724–1734. [Google Scholar] [CrossRef]
- Kim, S.J.; Koo, H.M.; Lim, B.J.; Oh, H.J.; Yoo, D.E.; Shin, D.H.; Lee, M.J.; Doh, F.M.; Park, J.T.; Yoo, T.H.; et al. Decreased circulating C3 levels and mesangial C3 deposition predict renal outcome in patients with IgA nephropathy. PLoS ONE 2012, 7, e40495. [Google Scholar] [CrossRef]
- Yang, X.; Wei, R.B.; Wang, Y.; Su, T.Y.; Li, Q.P.; Yang, T.; Huang, M.J.; Li, K.Y.; Chen, X.M. Decreased Serum C3 Levels in Immunoglobulin A (IgA) Nephropathy with Chronic Kidney Disease: A Propensity Score Matching Study. Med. Sci. Monit. 2017, 23, 673–681. [Google Scholar] [CrossRef][Green Version]
- Onda, K.; Ohsawa, I.; Ohi, H.; Tamano, M.; Mano, S.; Wakabayashi, M.; Toki, A.; Horikoshi, S.; Fujita, T.; Tomino, Y. Excretion of complement proteins and its activation marker C5b-9 in IgA nephropathy in relation to renal function. BMC Nephrol. 2011, 12, 64. [Google Scholar] [CrossRef]
- Heybeli, C.; Unlu, M.; Yildiz, S.; Cavdar, C.; Sarioglu, S.; Camsari, T. IgA nephropathy: Association of C4d with clinical and histopathological findings and possible role of IgM. Ren. Fail. 2015, 37, 1464–1469. [Google Scholar] [CrossRef]
- Roos, A.; Bouwman, L.H.; van Gijlswijk-Janssen, D.J.; Faber-Krol, M.C.; Stahl, G.L.; Daha, M.R. Human IgA activates the complement system via the mannan-binding lectin pathway. J. Immunol. 2001, 167, 2861–2868. [Google Scholar] [CrossRef]
- Tamhane, U.U.; Aneja, S.; Montgomery, D.; Rogers, E.K.; Eagle, K.A.; Gurm, H.S. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am. J. Cardiol. 2008, 102, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, R.; Chen, W.; Xu, X.; Dong, S.; Fan, H.; Liu, C. Prognostic significance of neutrophil to lymphocyte ratio in patients with gallbladder carcinoma. HPB 2016, 18, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Stotz, M.; Gerger, A.; Eisner, F.; Szkandera, J.; Loibner, H.; Ress, A.L.; Kornprat, P.; AlZoughbi, W.; Seggewies, F.S.; Lackner, C.; et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br. J. Cancer 2013, 109, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Personeni, N.; Giordano, L.; Abbadessa, G.; Porta, C.; Borbath, I.; Daniele, B.; Van Laethem, J.L.; Van Vlierberghe, H.; Trojan, J.; De Toni, E.N.; et al. Prognostic value of the neutrophil-to-lymphocyte ratio in the ARQ 197-215 second-line study for advanced hepatocellular carcinoma. Oncotarget 2017, 8, 14408–14415. [Google Scholar] [CrossRef] [PubMed]
- Forget, P.; Khalifa, C.; Defour, J.P.; Latinne, D.; Van Pel, M.C.; De Kock, M. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res. Notes 2017, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Kucuk, H.; Goker, B.; Varan, O.; Dumludag, B.; Haznedaroglu, S.; Ozturk, M.A.; Tufan, A.; Emiroglu, T.; Erten, Y. Predictive value of neutrophil/lymphocyte ratio in renal prognosis of patients with granulomatosis with polyangiitis. Ren. Fail. 2017, 39, 273–276. [Google Scholar] [CrossRef]
- Ayna, A.B.; Ermurat, S.; Coskun, B.N.; Harman, H.; Pehlivan, Y. Neutrophil to Lymphocyte Ratio and Mean Platelet Volume as Inflammatory Indicators in Systemic Lupus Erythematosus Nephritis. Arch. Rheumatol. 2017, 21, 5. [Google Scholar]
- Nagy, G.R.; Kemeny, L.; Bata-Csorgo, Z. Neutrophil-to-lymphocyte ratio: A biomarker for predicting systemic involvement in adult IgA vasculitis patients. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1033–1037. [Google Scholar] [CrossRef]
- Gayret, O.B.; Erol, M.; Tekin Nacaroglu, H. The Relationship of Neutrophil-Lymphocyte Ratio and Platelet-Lymphocyte Ratio with Gastrointestinal Bleeding in Henoch-Schonlein Purpura. Iran J. Pediatr. 2016, 26, e8191. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.N. Neutrophils in IgA nephropathy. Nephrology 1996, 2, 4. [Google Scholar] [CrossRef]
- Lai, K.N.; Shute, J.K.; Lindley, I.J.; Lai, F.M.; Yu, A.W.; Li, P.K.; Lai, C.K. Neutrophil attractant protein-1 interleukin 8 and its autoantibodies in IgA nephropathy. Clin. Immunol. Immunopathol. 1996, 80, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Azab, B.; Shah, N.; Akerman, M.; McGinn, J.T., Jr. Value of platelet/lymphocyte ratio as a predictor of all-cause mortality after non-ST-elevation myocardial infarction. J. Thromb. Thrombolysis 2012, 34, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, A.; Yuksel, M.; Oylumlu, M.; Polat, N.; Akyuz, A.; Acet, H.; Aydin, M.; Ulgen, M.S. The Utility of the Platelet-Lymphocyte Ratio for Predicting No Reflow in Patients With ST-Segment Elevation Myocardial Infarction. Clin. Appl. Thromb./Hemost. 2015, 21, 223–228. [Google Scholar] [CrossRef]
- Toprak, E.; Bozkurt, M.; Dincgez Cakmak, B.; Ozcimen, E.E.; Silahli, M.; Ender Yumru, A.; Caliskan, E. Platelet-to-lymphocyte ratio: A new inflammatory marker for the diagnosis of preterm premature rupture of membranes. J. Turk. Ger. Gynecol. Assoc. 2017, 18, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Tatar, E.; Mirili, C.; Isikyakar, T.; Yaprak, M.; Guvercin, G.; Ozay, E.; Asci, G. The association of neutrophil/lymphocyte ratio and platelet/lymphocyte ratio with clinical outcomes in geriatric patients with stage 3-5 chronic kidney disease. Acta Clinica Belgica 2016, 71, 221–226. [Google Scholar] [CrossRef]
- Ahbap, E.; Sakaci, T.; Kara, E.; Sahutoglu, T.; Koc, Y.; Basturk, T.; Sevinc, M.; Akgol, C.; Kayalar, A.O.; Ucar, Z.A.; et al. Neutrophil-to-lymphocyte ratio and platelet-tolymphocyte ratio in evaluation of inflammation in end-stage renal disease. Clin. Nephrol. 2016, 85, 199–208. [Google Scholar] [CrossRef]
- Taymez, D.G.; Ucar, E.; Turkmen, K.; Ucar, R.; Afsar, B.; Gaipov, A.; Turk, S. The Predictive Value of Platelet/Lymphocyte Ratio in Hemodialysis Patients With Erythropoietin Resistance. Ther. Apher. Dial. 2016, 20, 118–121. [Google Scholar] [CrossRef]
- Borissoff, J.I.; Spronk, H.M.; ten Cate, H. The hemostatic system as a modulator of atherosclerosis. N. Engl. J. Med. 2011, 364, 1746–1760. [Google Scholar] [CrossRef]
- Harden, P.N.; Geddes, C.; Rowe, P.A.; McIlroy, J.H.; Boulton-Jones, M.; Rodger, R.S.; Junor, B.J.; Briggs, J.D.; Connell, J.M.; Jardine, A.G. Polymorphisms in angiotensin-converting-enzyme gene and progression of IgA nephropathy. Lancet 1995, 345, 1540–1542. [Google Scholar] [CrossRef]
- Yoshida, H.; Mitarai, T.; Kawamura, T.; Kitajima, T.; Miyazaki, Y.; Nagasawa, R.; Kawaguchi, Y.; Kubo, H.; Ichikawa, I.; Sakai, O. Role of the deletion of polymorphism of the angiotensin converting enzyme gene in the progression and therapeutic responsiveness of IgA nephropathy. J. Clin. Investig. 1995, 96, 2162–2169. [Google Scholar] [CrossRef] [PubMed]
- Hunley, T.E.; Julian, B.A.; Phillips, J.A., 3rd; Summar, M.L.; Yoshida, H.; Horn, R.G.; Brown, N.J.; Fogo, A.; Ichikawa, I.; Kon, V. Angiotensin converting enzyme gene polymorphism: Potential silencer motif and impact on progression in IgA nephropathy. Kidney Int. 1996, 49, 571–577. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, N.; Hou, P.; Lv, J.; Moldoveanu, Z.; Li, Y.; Kiryluk, K.; Gharavi, A.G.; Novak, J.; Zhang, H. The level of galactose-deficient IgA1 in the sera of patients with IgA nephropathy is associated with disease progression. Kidney Int. 2012, 82, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Berthoux, F.; Mohey, H.; Laurent, B.; Mariat, C.; Afiani, A.; Thibaudin, L. Predicting the risk for dialysis or death in IgA nephropathy. J. Am. Soc. Nephrol. 2011, 22, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Bartosik, L.P.; Lajoie, G.; Sugar, L.; Cattran, D.C. Predicting progression in IgA nephropathy. Am. J. Kidney Dis. 2001, 38, 728–735. [Google Scholar] [CrossRef]
- Reich, H.N.; Troyanov, S.; Scholey, J.W.; Cattran, D.C.; Toronto Glomerulonephritis Registry. Remission of proteinuria improves prognosis in IgA nephropathy. J. Am. Soc. Nephrol. 2007, 18, 3177–3183. [Google Scholar] [CrossRef]
- Syrjanen, J.; Mustonen, J.; Pasternack, A. Hypertriglyceridaemia and hyperuricaemia are risk factors for progression of IgA nephropathy. Nephrol. Dial. Transplant. 2000, 15, 34–42. [Google Scholar] [CrossRef]
- Yamamoto, R.; Nagasawa, Y.; Shoji, T.; Iwatani, H.; Hamano, T.; Kawada, N.; Inoue, K.; Uehata, T.; Kaneko, T.; Okada, N.; et al. Cigarette smoking and progression of IgA nephropathy. Am. J. Kidney Dis. 2010, 56, 313–324. [Google Scholar] [CrossRef]

| Total (n = 272) | Stage 1–2 (n = 66) | Stage 3–5 (n = 206) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| n | ±SD or % | n | ±SD or % | n | ±SD or % | ||
| Male | 151 | 55.5% | 34 | 51.5% | 117 | 56.8% | 0.543 |
| Age (years old) | 39.25 | ±15.39 | 40.21 | ±15.50 | 38.95 | ±15.38 | 0.498 |
| Crescent | 44 | 16.2% | 3 | 4.5% | 41 | 19.9% | 0.006 ** |
| SBP (mmHg) | 131.53 | ±18.49 | 131.74 | ±21.27 | 131.47 | ±17.57 | 0.833 |
| DBP (mmHg) | 83.69 | ±13.95 | 82.48 | ±14.50 | 84.07 | ±13.78 | 0.411 |
| Body height (cm) | 164.35 | ±9.11 | 163.38 | ±9.68 | 164.69 | ±8.92 | 0.497 |
| Body weight (kg) | 65.43 | ±12.71 | 65.90 | ±13.61 | 65.28 | ±12.44 | 0.493 |
| BUN (mg/dL) | 26.90 | ±23.43 | 19.49 | ±12.85 | 29.23 | ±25.47 | <0.001 ** |
| Creatinine (mg/dL) | 2.01 | ±2.31 | 1.23 | ±0.67 | 2.26 | ±2.58 | 0.001 ** |
| eGFR (min/min/1.732 m2) | 64.65 | ±40.07 | 73.96 | ±32.79 | 61.69 | ±41.76 | 0.006 ** |
| Blood WBC (/cumm) | 7853.44 | ±2368.22 | 7930.89 | ±2638.71 | 7828.63 | ±2281.19 | 0.612 |
| Blood RBC (/cumm) | 4.19 | ±0.84 | 4.37 | ±0.72 | 4.13 | ±0.86 | 0.063 |
| Hemoglobin (g/dL) | 12.49 | ±2.17 | 13.12 | ±1.81 | 12.28 | ±2.24 | 0.008 ** |
| Neutrophil (%) | 63.78 | ±10.03 | 63.31 | ±9.34 | 63.92 | ±10.25 | 0.607 |
| Lymphocyte (%) | 26.40 | ±8.99 | 27.27 | ±9.16 | 26.12 | ±8.94 | 0.426 |
| Platelet (103/cumm) | 253.80 | ±93.91 | 253.91 | ±60.35 | 253.76 | ±102.48 | 0.562 |
| Uric acid (mg/dL) | 7.35 | ±2.12 | 6.81 | ±2.01 | 7.51 | ±2.13 | 0.016 * |
| Na (meq/L) | 140.08 | ±3.14 | 140.33 | ±3.23 | 140.00 | ±3.11 | 0.408 |
| K (meq/L) | 4.25 | ±0.53 | 4.15 | ±0.41 | 4.28 | ±0.56 | 0.145 |
| Ca (mg/dL) | 8.54 | ±1.02 | 8.83 | ±0.81 | 8.45 | ±1.07 | 0.001 ** |
| P (mg/dL) | 3.90 | ±1.05 | 3.58 | ±0.59 | 3.99 | ±1.14 | 0.037 |
| Mg (mg/dL) | 2.34 | ±0.44 | 2.16 | ±0.45 | 2.39 | ±0.43 | 0.264 |
| Albumin (g/dL) | 3.75 | ±0.60 | 3.96 | ±0.62 | 3.68 | ±0.59 | <0.001 ** |
| Total protein (gm/dL) | 6.62 | ±0.85 | 6.84 | ±0.77 | 6.55 | ±0.86 | 0.001 ** |
| GOT (U/L) | 24.34 | ±20.42 | 27.57 | ±21.79 | 23.34 | ±19.94 | 0.124 |
| GPT (U/L) | 23.42 | ±19.99 | 26.44 | ±25.30 | 22.47 | ±17.98 | 0.385 |
| Total cholesterol (mg/dL) | 196.28 | ±51.81 | 195.98 | ±68.29 | 196.38 | ±45.44 | 0.397 |
| Triglyceride (mg/dL) | 137.67 | ±90.43 | 129.84 | ±79.09 | 140.09 | ±93.73 | 0.272 |
| LDL (mg/dL) | 118.67 | ±40.84 | 106.41 | ±30.79 | 122.69 | ±42.95 | 0.005 ** |
| HDL (mg/dL) | 56.39 | ±19.80 | 57.67 | ±23.21 | 55.95 | ±18.56 | 0.668 |
| Fasting glucose (mg/dL) | 92.72 | ±17.36 | 96.06 | ±25.13 | 91.73 | ±14.20 | 0.147 |
| Postprandial glucose (mg/dL) | 129.11 | ±68.81 | 102.00 | ±4.24 | 132.50 | ±72.49 | 1.000 |
| HbA1c (%) | 5.58 | ±0.87 | 5.72 | ±1.02 | 5.54 | ±0.82 | 0.289 |
| IgG (mg/dL) | 1103.04 | ±337.82 | 1185.85 | ±341.74 | 1074.34 | ±332.63 | 0.897 |
| IgA (mg/dL) | 361.21 | ±136.63 | 361.40 | ±135.43 | 361.14 | ±137.40 | 0.556 |
| IgM (mg/dL) | 114.52 | ±53.12 | 118.97 | ±56.25 | 113.01 | ±52.10 | 0.930 |
| IgE (mg/dL) | 208.68 | ±358.03 | 203.06 | ±428.18 | 210.49 | ±336.55 | 0.068 |
| C3 (mg/dL) | 110.75 | ±24.27 | 115.54 | ±23.51 | 109.15 | ±24.36 | 0.061 |
| C4 (mg/dL) | 29.82 | ±10.77 | 27.92 | ±10.46 | 30.46 | ±10.82 | 0.398 |
| Negative for HBV | 215 | 79.0% | 52 | 78.8% | 163 | 79.1% | 0.428 |
| Negative for HCV | 244 | 89.7% | 61 | 92.4% | 183 | 88.8% | 0.249 |
| ANA (≥1:160) | 22 | 8.4% | 6 | 9.4% | 16 | 8.0% | 0.921 |
| dsDNA | 19.26 | ±17.07 | 24.64 | ±25.93 | 17.46 | ±12.58 | 0.645 |
| ANCA | 5 | 3.2% | 1 | 2.6% | 4 | 3.4% | 1.000 |
| MPO | 11.60 | ±31.83 | 35.40 | ±65.75 | 6.31 | ±18.04 | 0.414 |
| PR3 | 3.67 | ±3.51 | 2.73 | ±2.53 | 3.93 | ±3.76 | 0.614 |
| Urinary albumin (mg/dL) | 64.18 | ±86.65 | 17.24 | ±27.79 | 74.13 | ±91.77 | 0.022 * |
| 24 h proteinuria (g/day) | 2.11 | ±2.15 | 1.25 | ±1.84 | 2.38 | ±2.17 | <0.001 ** |
| Urine creatinine (mg/dL) | 100.48 | ±67.95 | 115.89 | ±71.08 | 95.45 | ±66.31 | <0.001 ** |
| Urine PCR (mg/mg) | 1.91 | ±2.57 | 0.97 | ±1.17 | 2.21 | ±2.82 | 0.021 * |
| Urine ACR (mg/g) | 854.05 | ±1145.90 | 184.75 | ±248.90 | 818.85 | ±955.30 | 0.009 ** |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95%CI | p-Value | Hazard Ratio | 95%CI | p-Value | |
| Sex (male/female) | 1.14 | (0.68–1.91) | 0.631 | |||
| Age, year | 1.03 | (1.01–1.05) | 0.001 ** | |||
| Crescent (with/without) | 2.05 | (1.12–3.75) | 0.019 * | |||
| BUN | 1.02 | (1.01–1.02) | <0.001 ** | |||
| Serum creatinine | 1.14 | (1.09–1.20) | <0.001 ** | |||
| eGFR | 0.95 | (0.94–0.97) | <0.001 ** | 0.88 | (0.82–0.95) | 0.001 ** |
| Uric acid | 1.30 | (1.17–1.44) | <0.001 ** | 0.93 | (0.64–1.36) | 0.716 |
| Albumin | 0.44 | (0.31–0.62) | <0.001 ** | 0.80 | (0.22–2.85) | 0.728 |
| Total protein | 0.52 | (0.40–0.68) | <0.001 ** | 0.95 | (0.44–2.08) | 0.903 |
| LDL | 1.00 | (0.99–1.01) | 0.735 | |||
| Urine PCR | 1.13 | (1.08–1.19) | <0.001 ** | |||
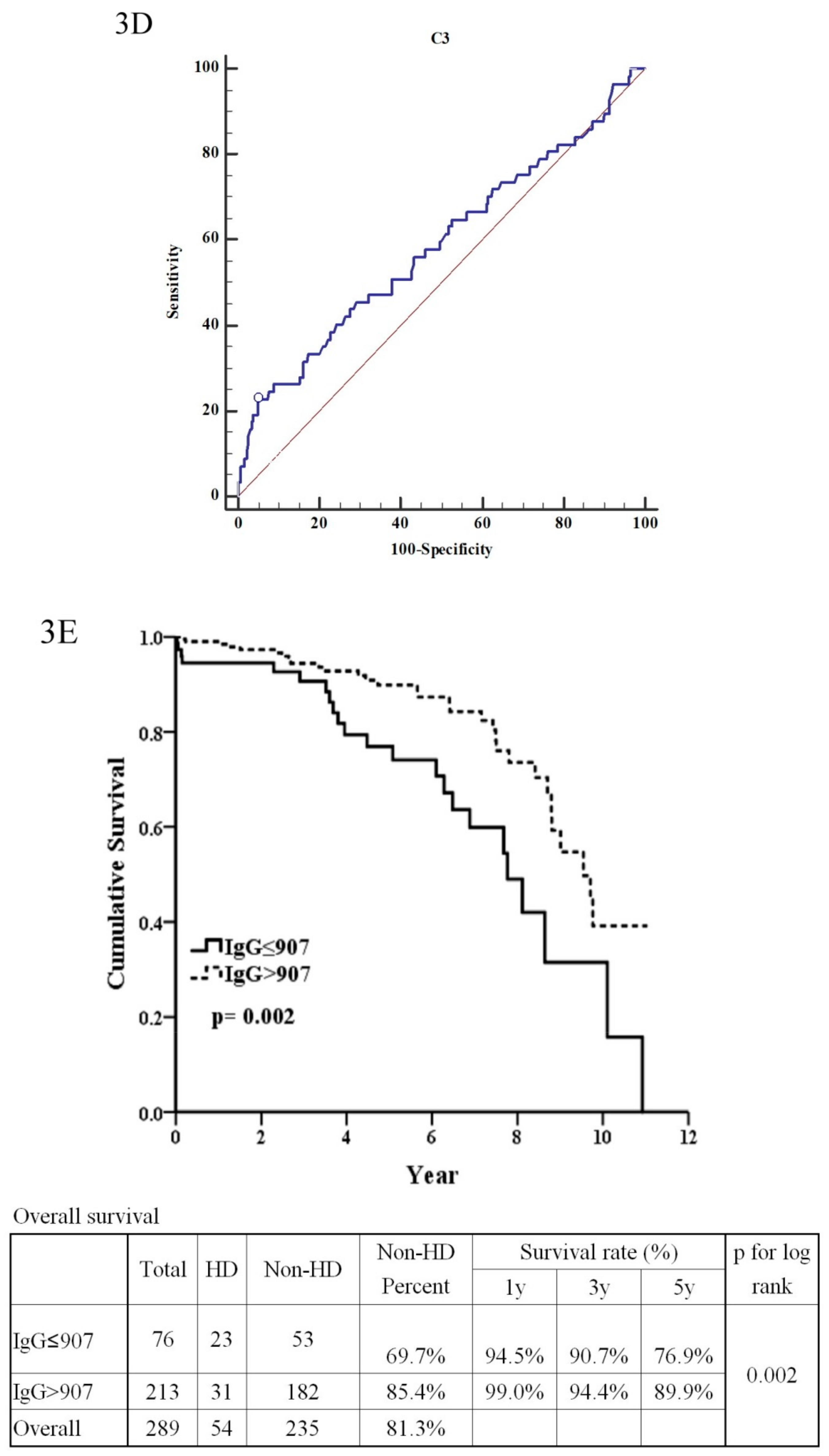
| IgG (≤907:>907) | 2.29 | (1.33–3.96) | 0.003 ** | 0.90 | (0.20–4.14) | 0.897 |
| IgA (>350:≤350) | 1.07 | (0.62–1.85) | 0.806 | |||
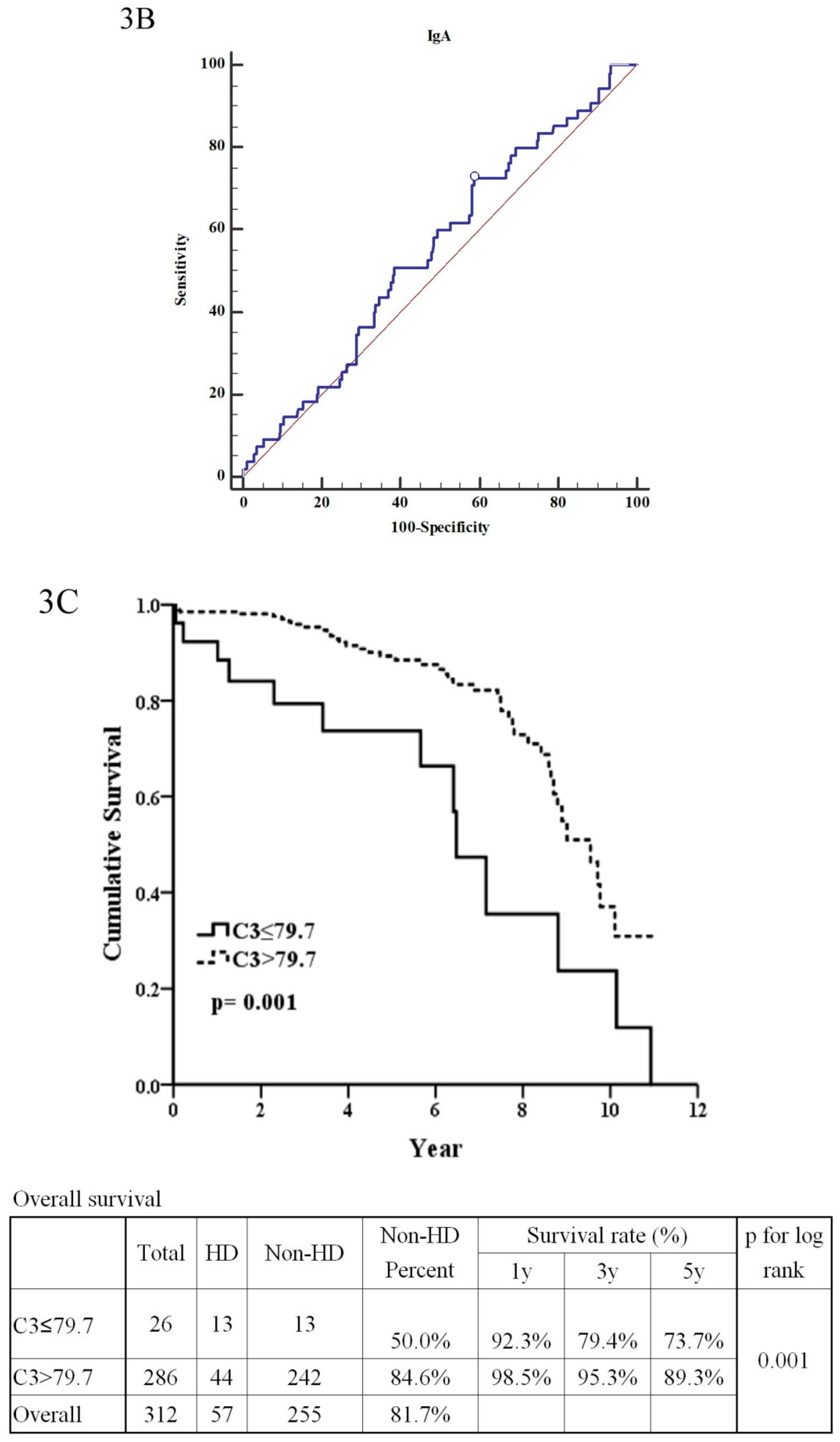
| C3 (>79.7:≤79.7) | 2.76 | (1.46–5.23) | 0.002 ** | 17.27 | (2.62–113.81) | 0.003 ** |
| C4 | 1.02 | (1.00–1.04) | 0.026 * | 1.11 | (1.02–1.21) | 0.014 * |
| Inflammatory marker | ||||||
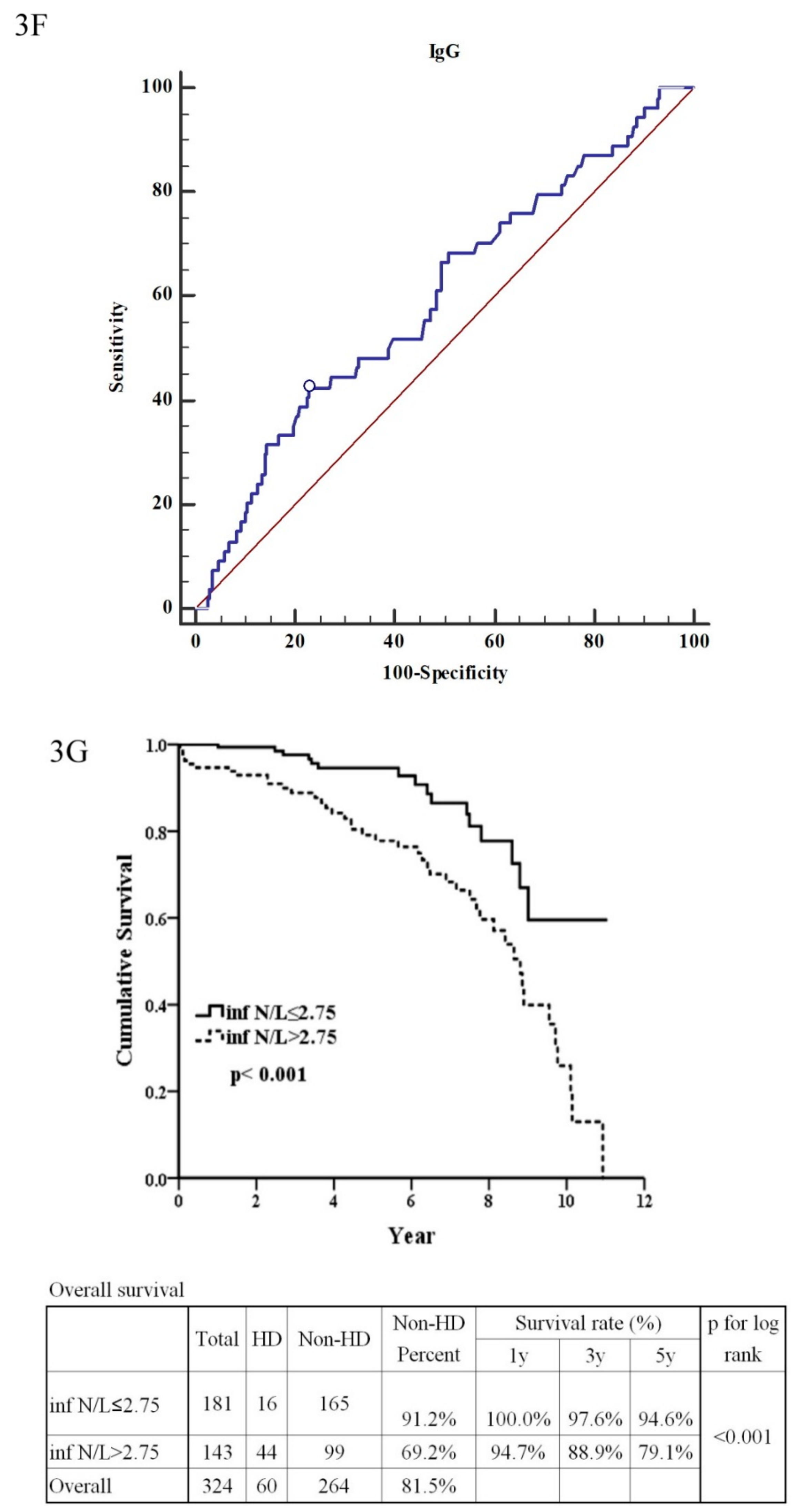
| Neutrophil/Lymphocyte ratio (>2.75:≤2.75) | 2.92 | (1.65–5.19) | <0.001 ** | 0.75 | (0.18–3.09) | 0.690 |
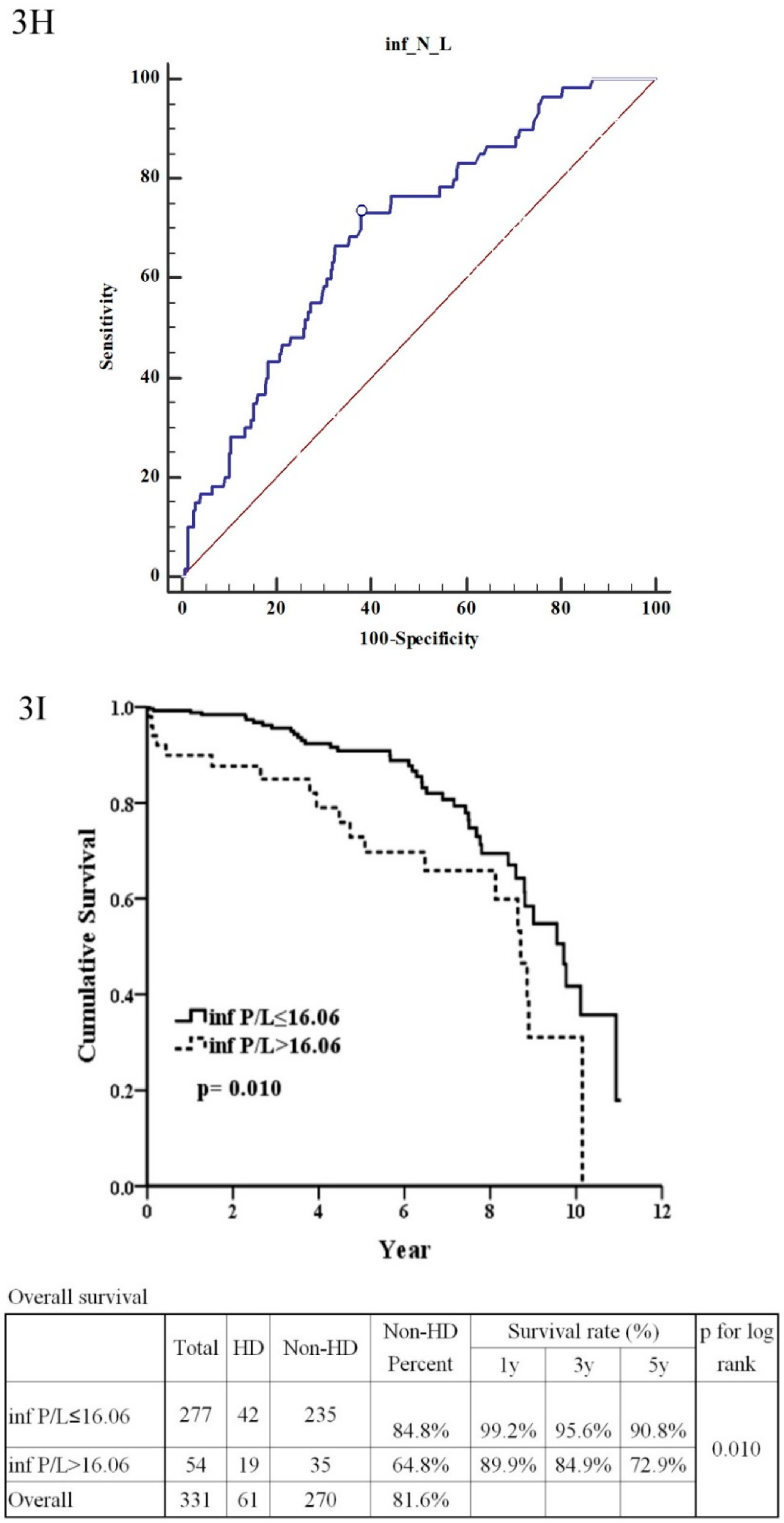
| Platelet/Lymphocyte ratio (≤16.06:>16.06) | 2.02 | (1.17–3.49) | 0.012 * | 1.41 | (0.33–5.91) | 0.641 |
| 24 h proteinuria | 1.12 | (1.05–1.19) | <0.001 ** | |||
| Haas’ classification (stage 1–3:stage 4–5) | 3.67 | (1.89–7.13) | <0.001 ** | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, S.-F.; Wu, M.-J.; Wen, M.-C.; Chen, C.-H. Serologic and Histologic Predictors of Long-Term Renal Outcome in Biopsy-Confirmed IgA Nephropathy (Haas Classification): An Observational Study. J. Clin. Med. 2019, 8, 848. https://doi.org/10.3390/jcm8060848
Tsai S-F, Wu M-J, Wen M-C, Chen C-H. Serologic and Histologic Predictors of Long-Term Renal Outcome in Biopsy-Confirmed IgA Nephropathy (Haas Classification): An Observational Study. Journal of Clinical Medicine. 2019; 8(6):848. https://doi.org/10.3390/jcm8060848
Chicago/Turabian StyleTsai, Shang-Feng, Ming-Ju Wu, Mei-Chin Wen, and Cheng-Hsu Chen. 2019. "Serologic and Histologic Predictors of Long-Term Renal Outcome in Biopsy-Confirmed IgA Nephropathy (Haas Classification): An Observational Study" Journal of Clinical Medicine 8, no. 6: 848. https://doi.org/10.3390/jcm8060848
APA StyleTsai, S.-F., Wu, M.-J., Wen, M.-C., & Chen, C.-H. (2019). Serologic and Histologic Predictors of Long-Term Renal Outcome in Biopsy-Confirmed IgA Nephropathy (Haas Classification): An Observational Study. Journal of Clinical Medicine, 8(6), 848. https://doi.org/10.3390/jcm8060848






