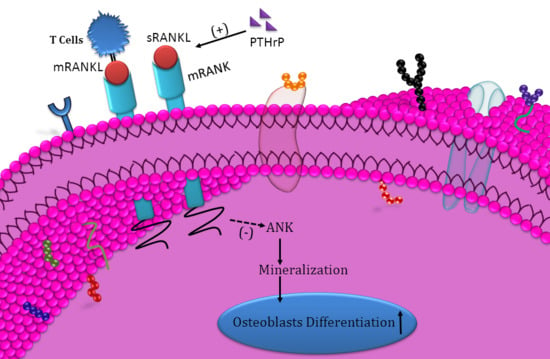
Parathyroid Hormone-Related Protein (PTHrP) Accelerates Soluble RANKL Signals for Downregulation of Osteogenesis of Bone Mesenchymal Stem Cells
Abstract
1. Introduction
2. Methods
2.1. Isolation of Mesenchymal Stem Cells
2.2. Cell Proliferation
2.3. mRNA Expression
2.4. Expression of Membrane RANK Using Confocal Laser Scanning Microscopy
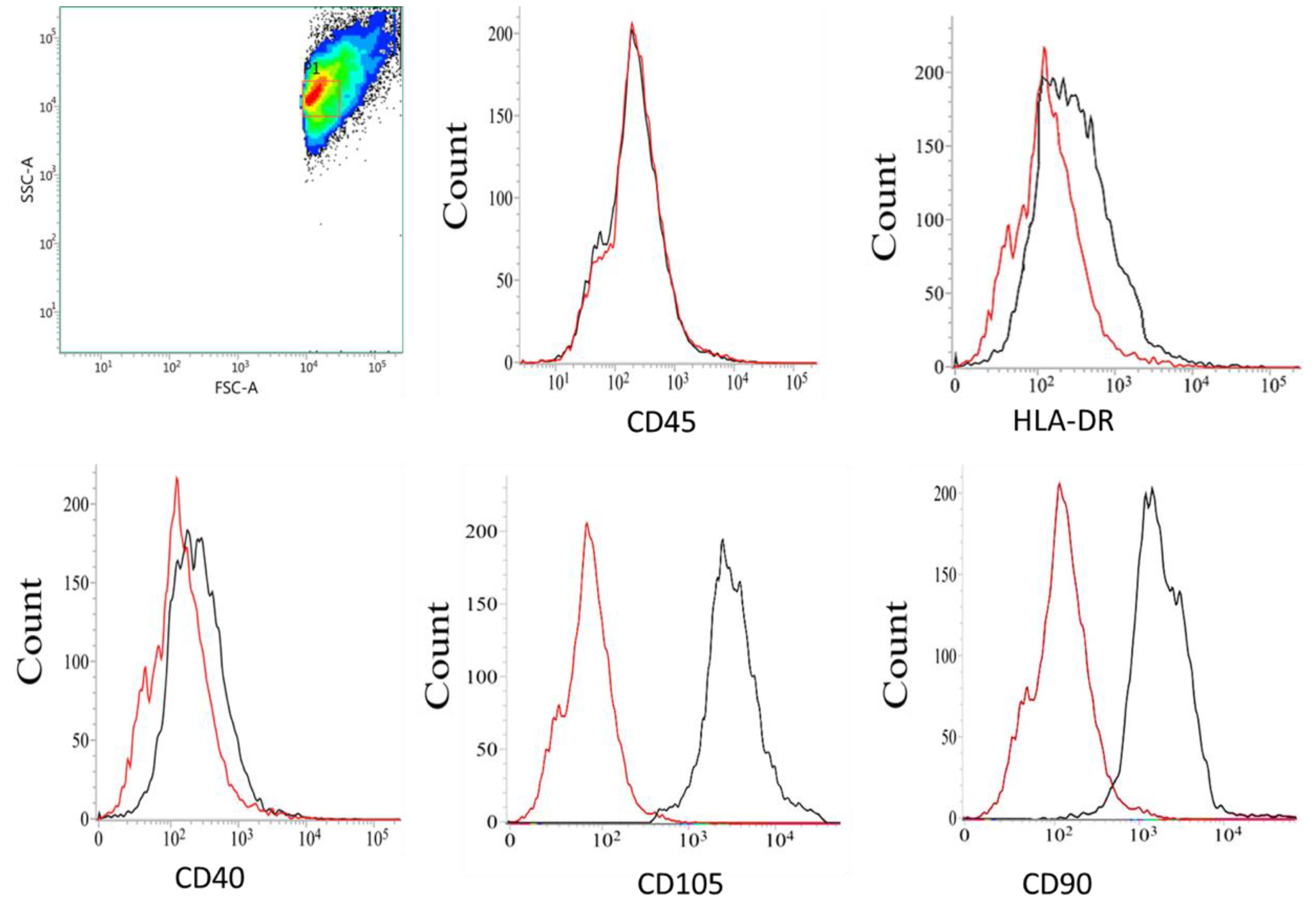
2.5. Immunophenotyping Characterization of MSCs
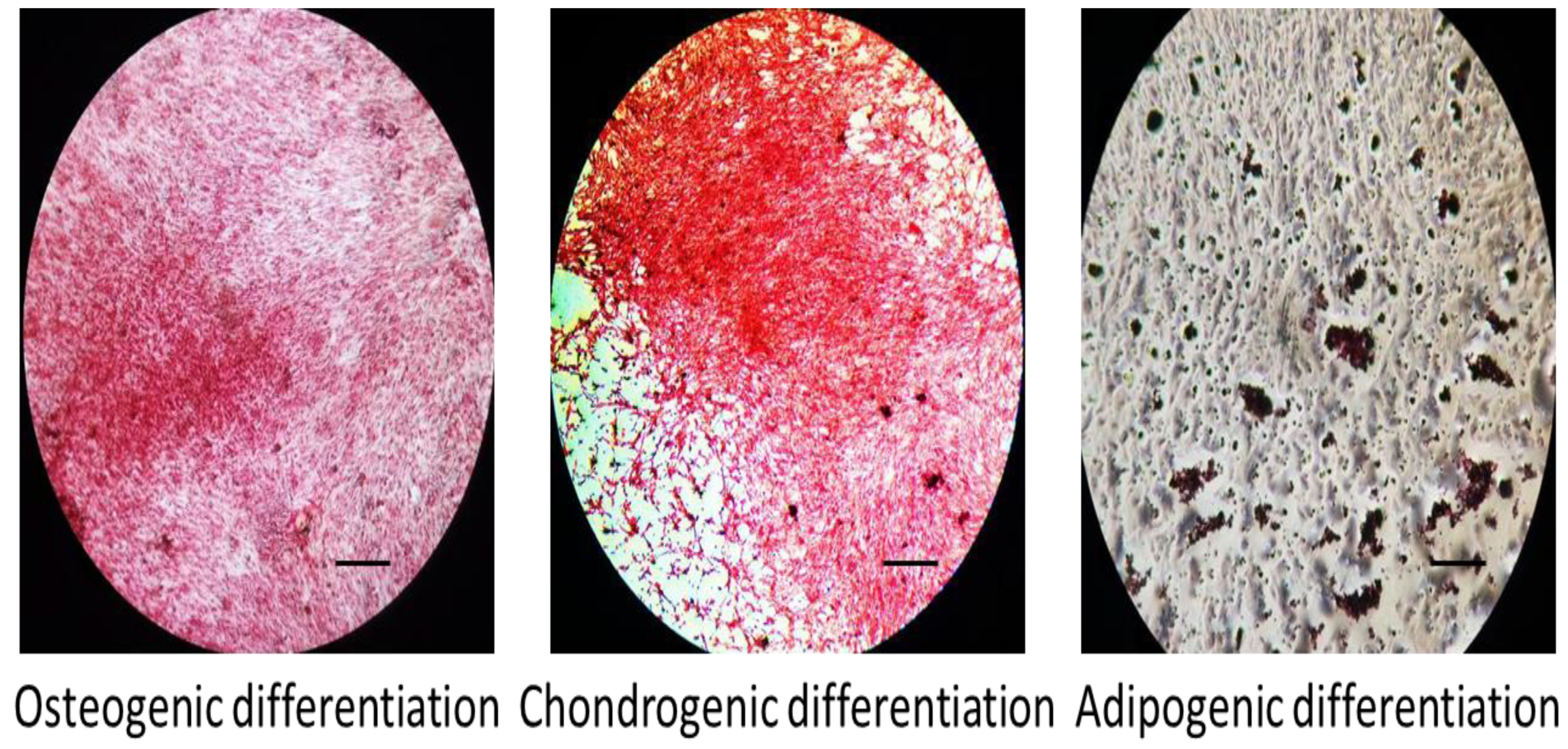
2.6. Tri-Lineage Differentiation Potential of MSCs
2.7. Effect of sRANKL and PTHrP on Osteogenic Differentiation
2.8. The Level of Secretome
2.9. Cellular Alkaline Phosphatase
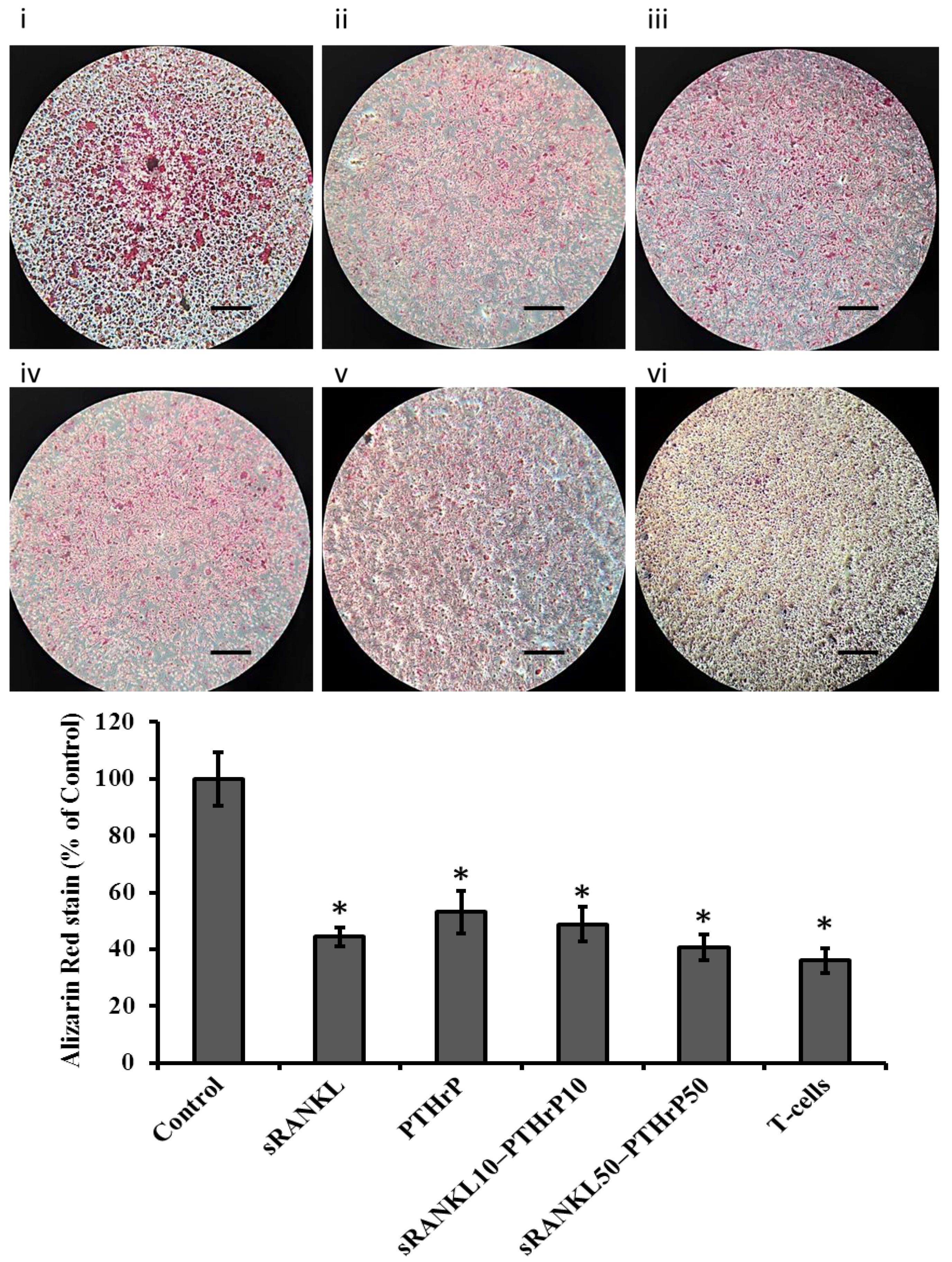
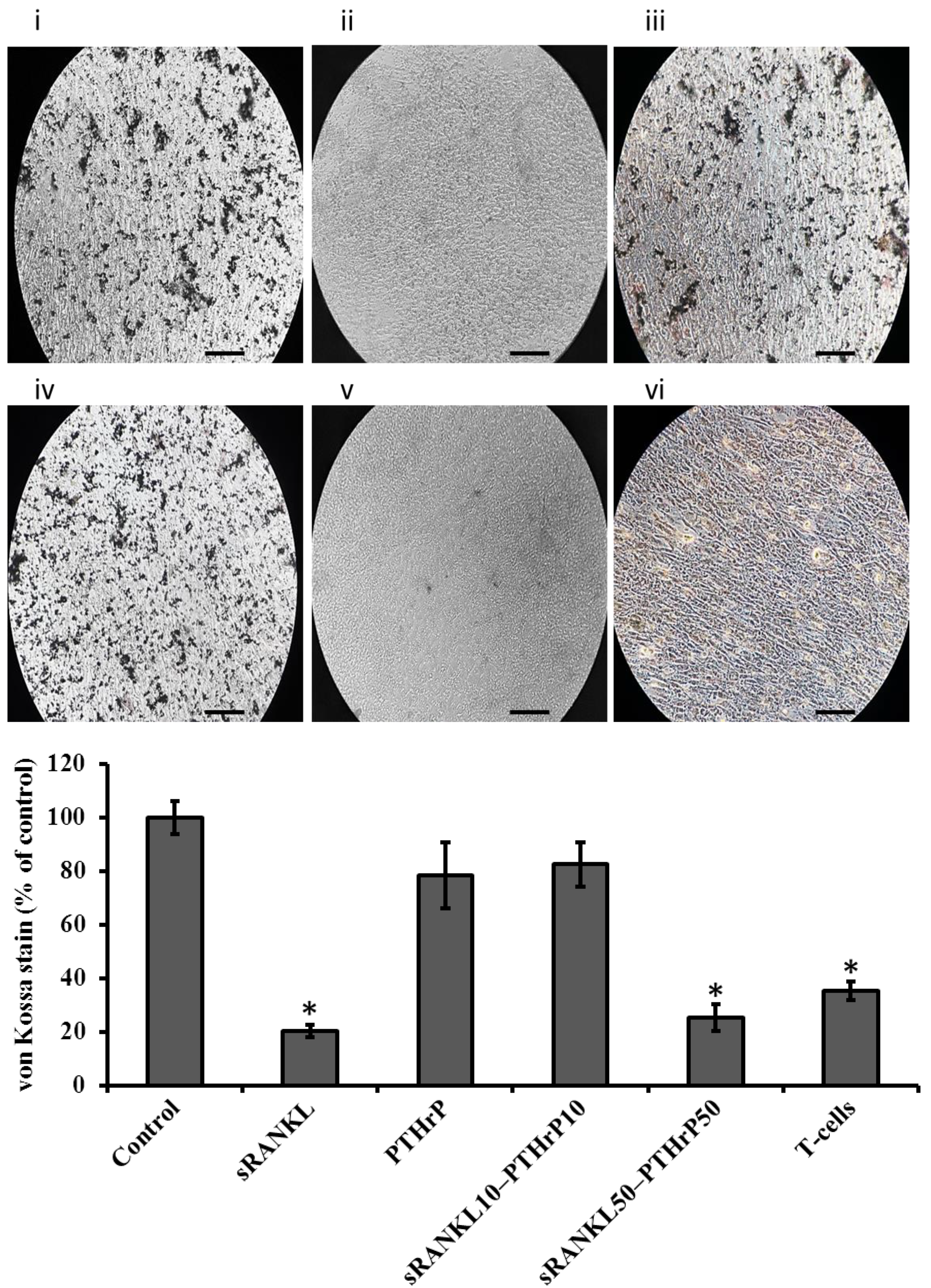
2.10. Cellular Mineral Levels
2.11. Western Blot
2.12. Role of T-Cells in Osteogenesis
2.13. Statistics
3. Results
3.1. Immunophenotyping Characterization of MSCs
3.2. Tri-Lineage Differentiation of MSCs
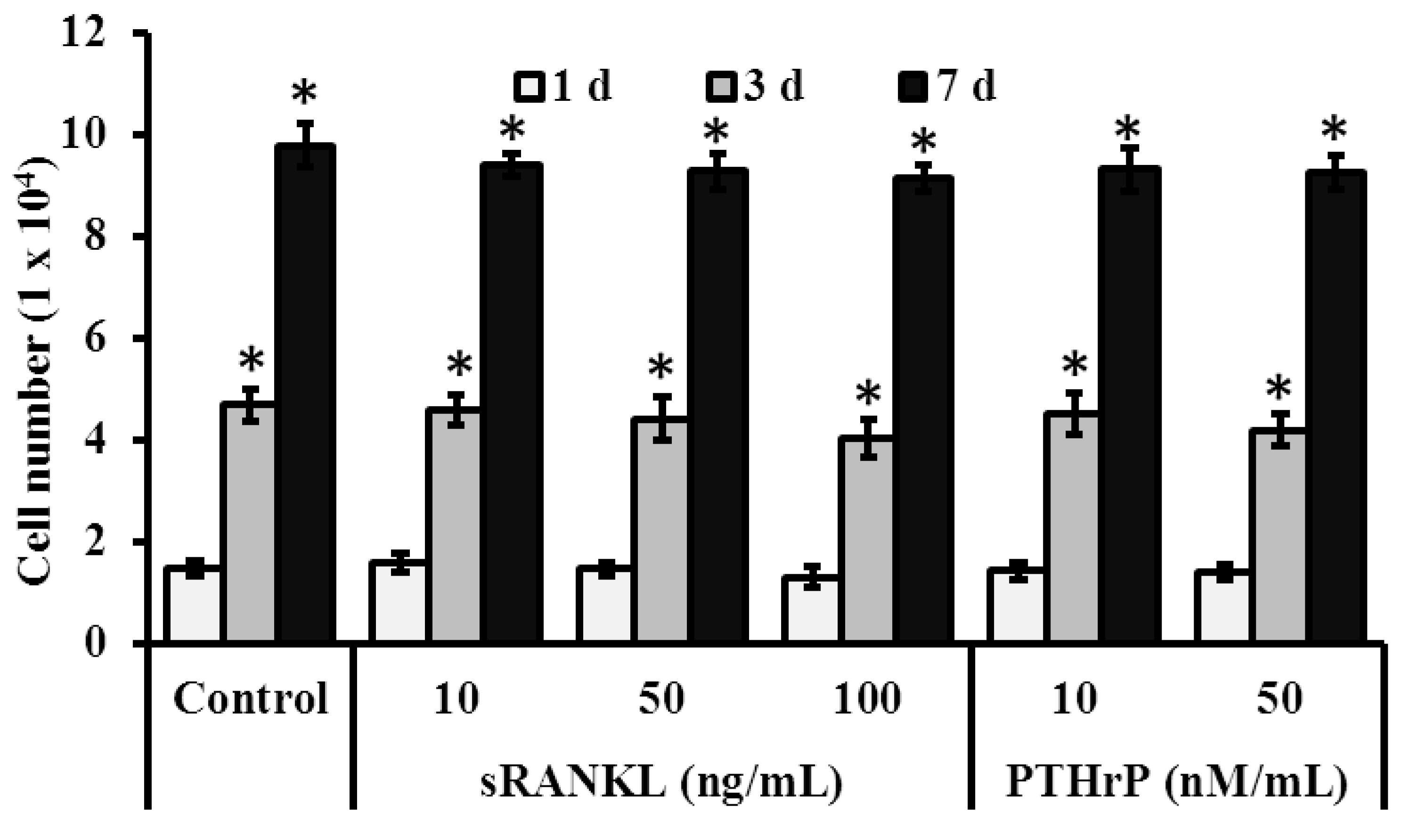
3.3. Role of sRANKL and PTHrP in MSCs Proliferation
3.3.1. Cell Viability
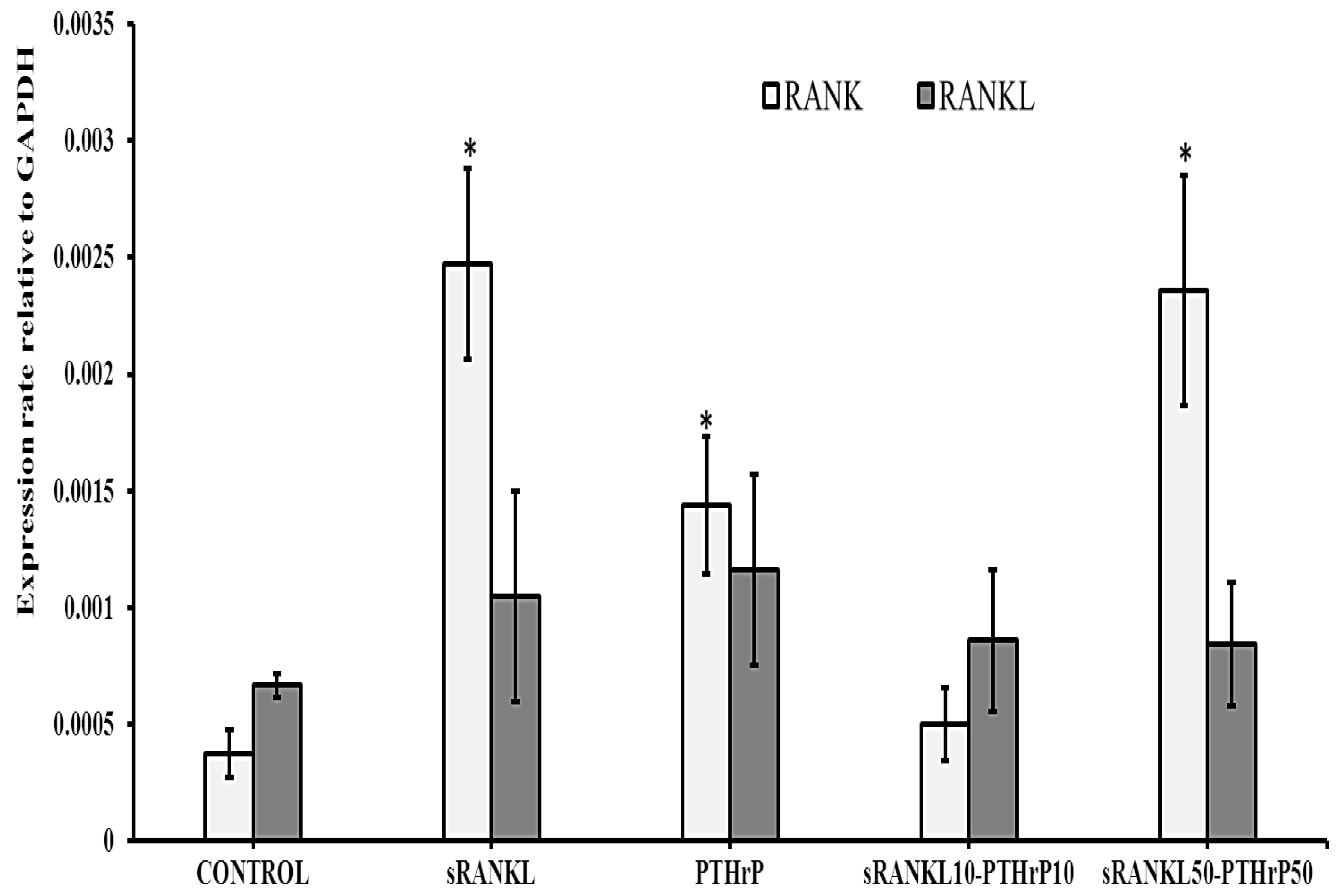
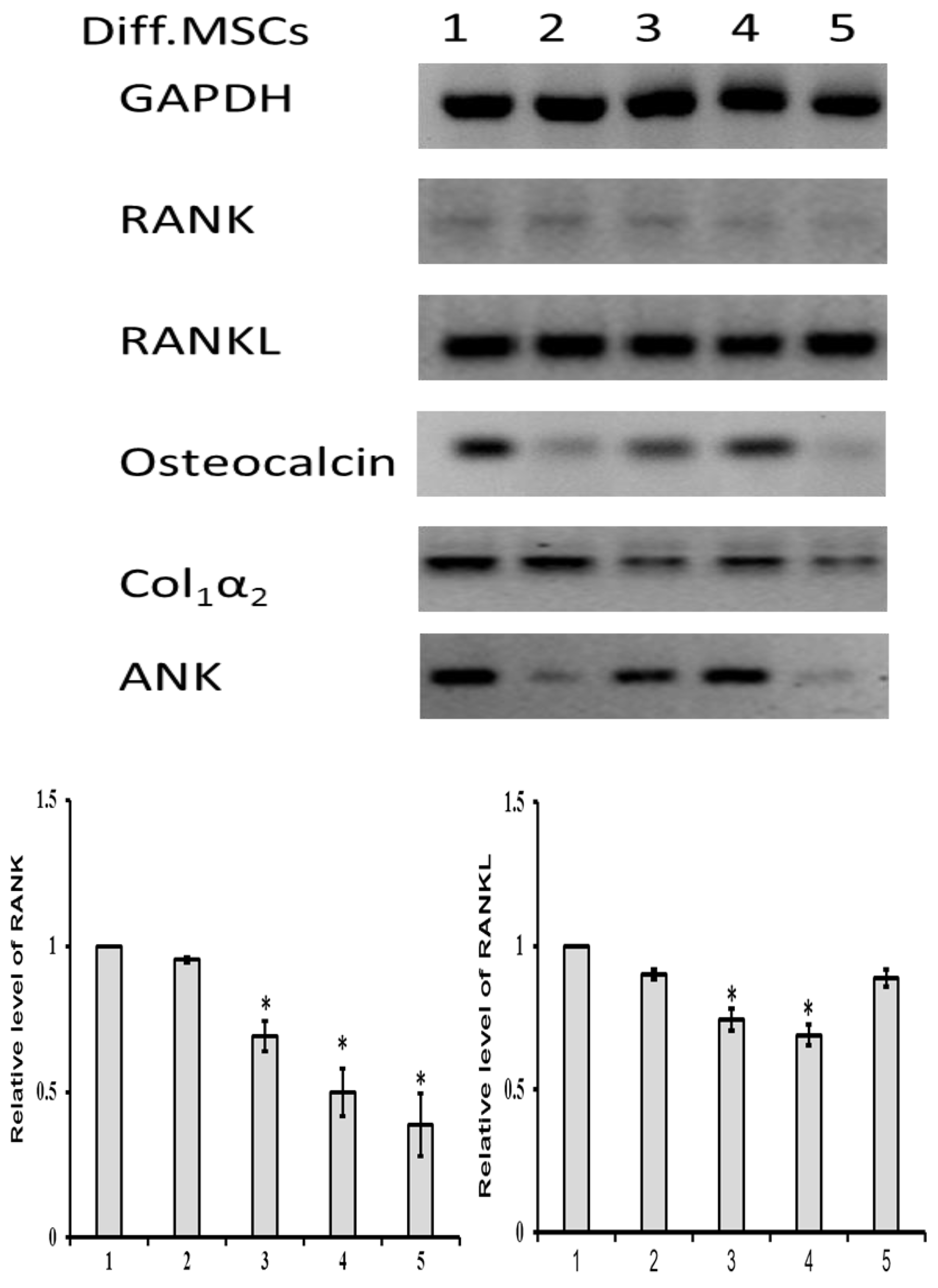
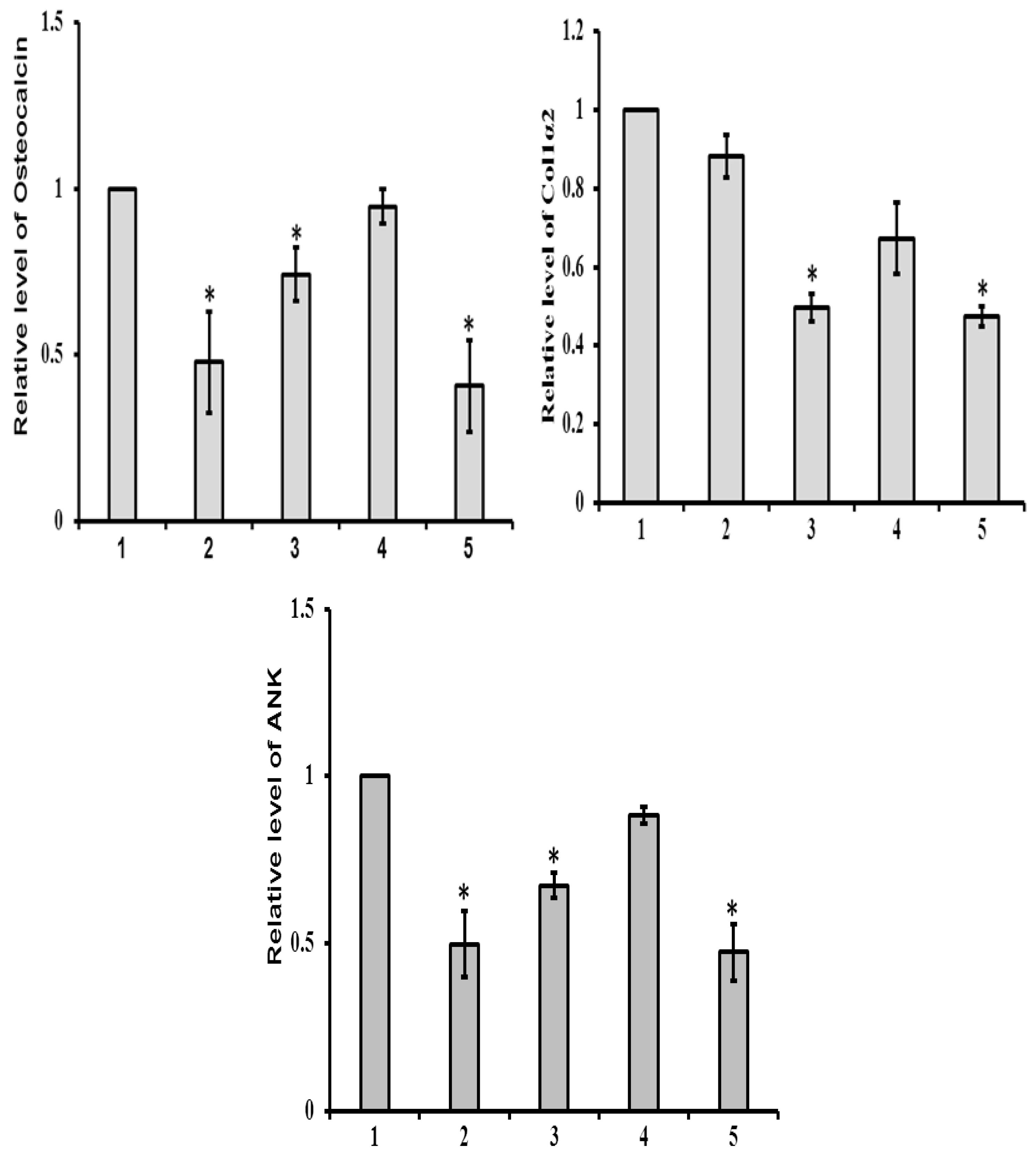
3.3.2. mRNA Expression
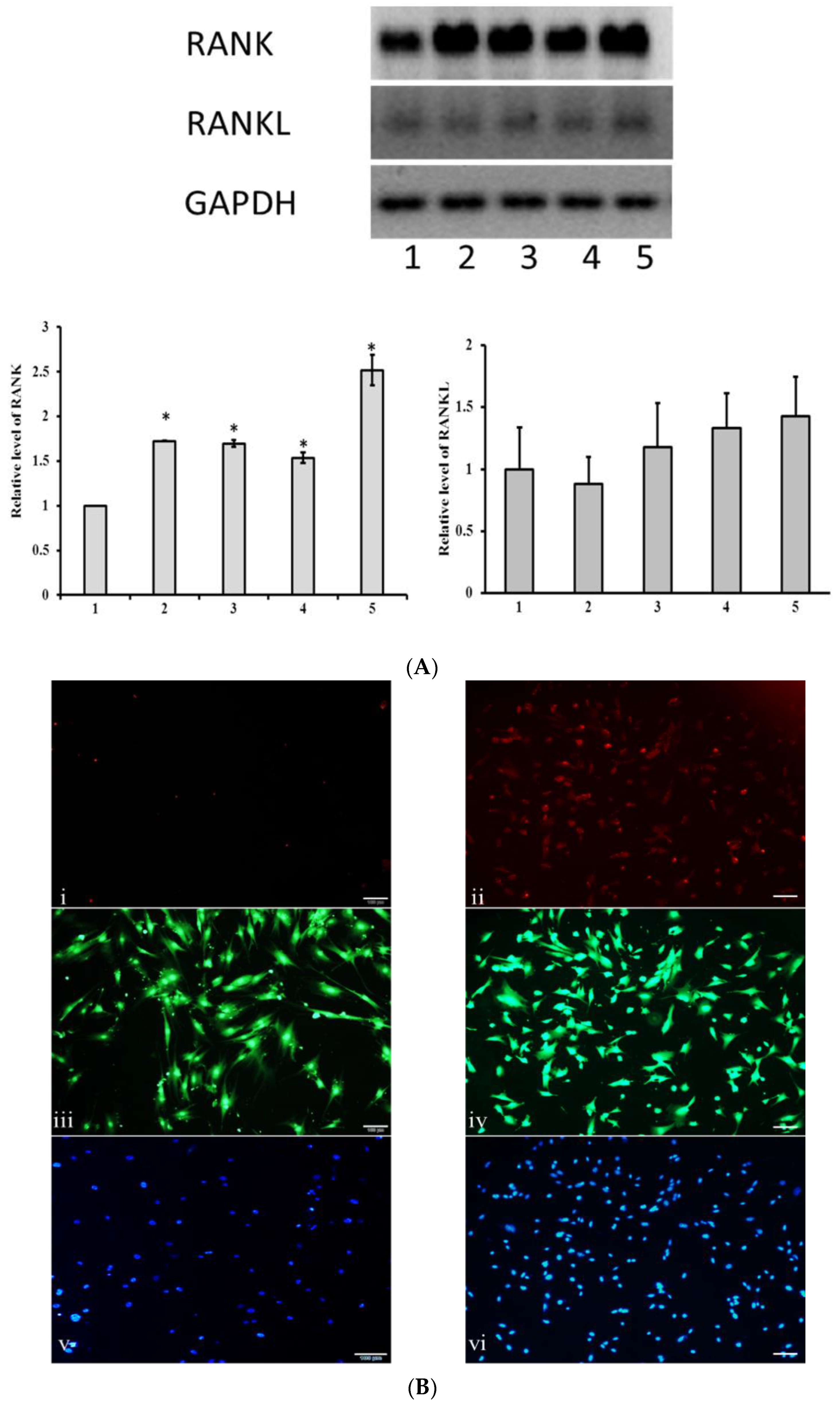
3.4. MSCs Express Membrane RANK
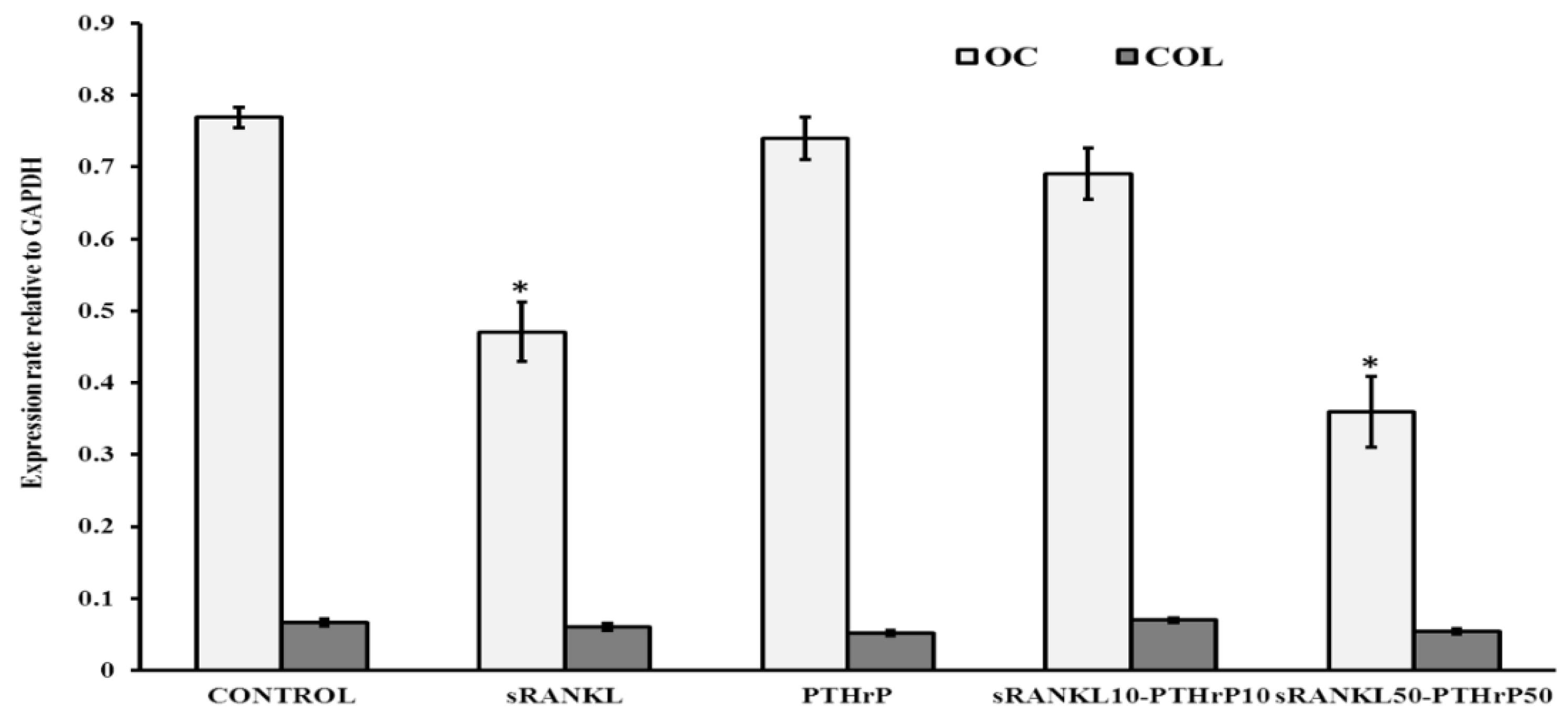
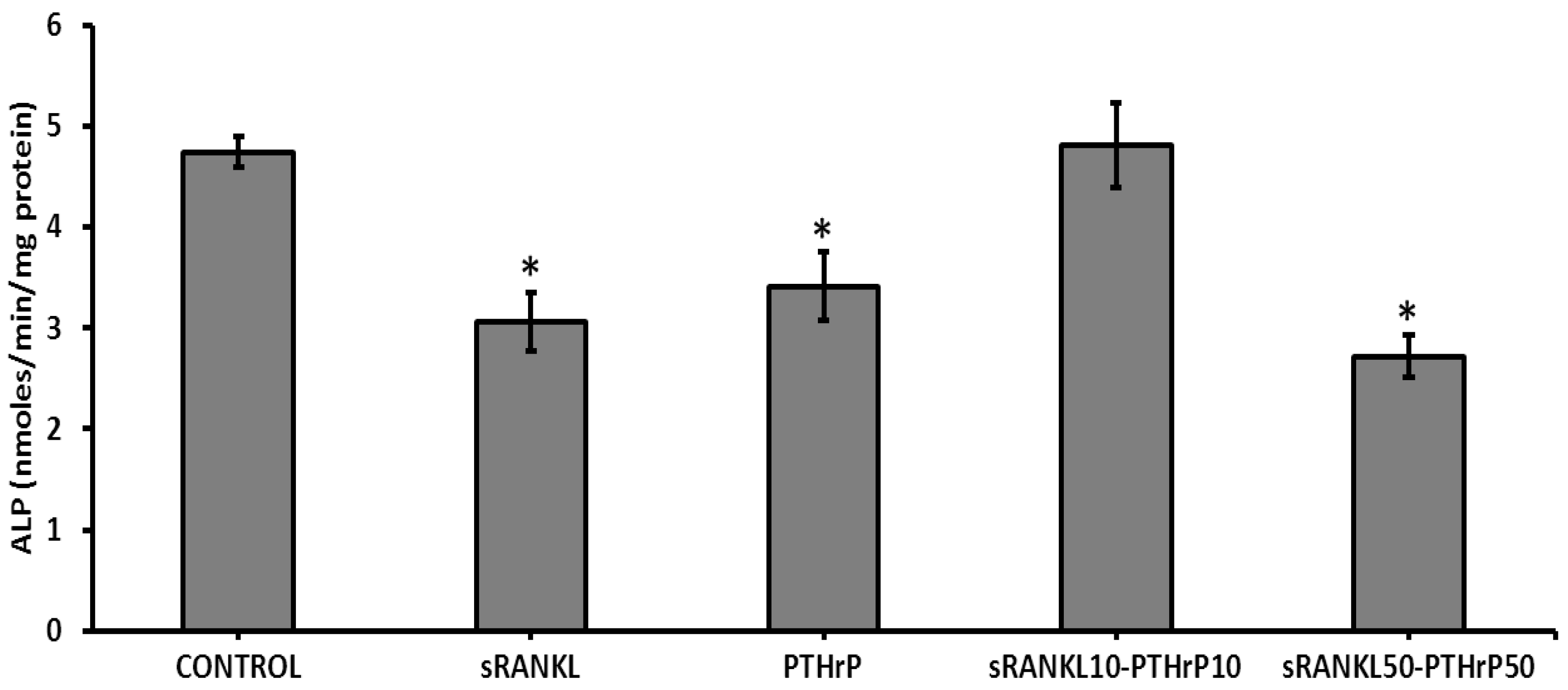
3.5. sRANKL Downregulates Osteogenic Differentiation of MSCs
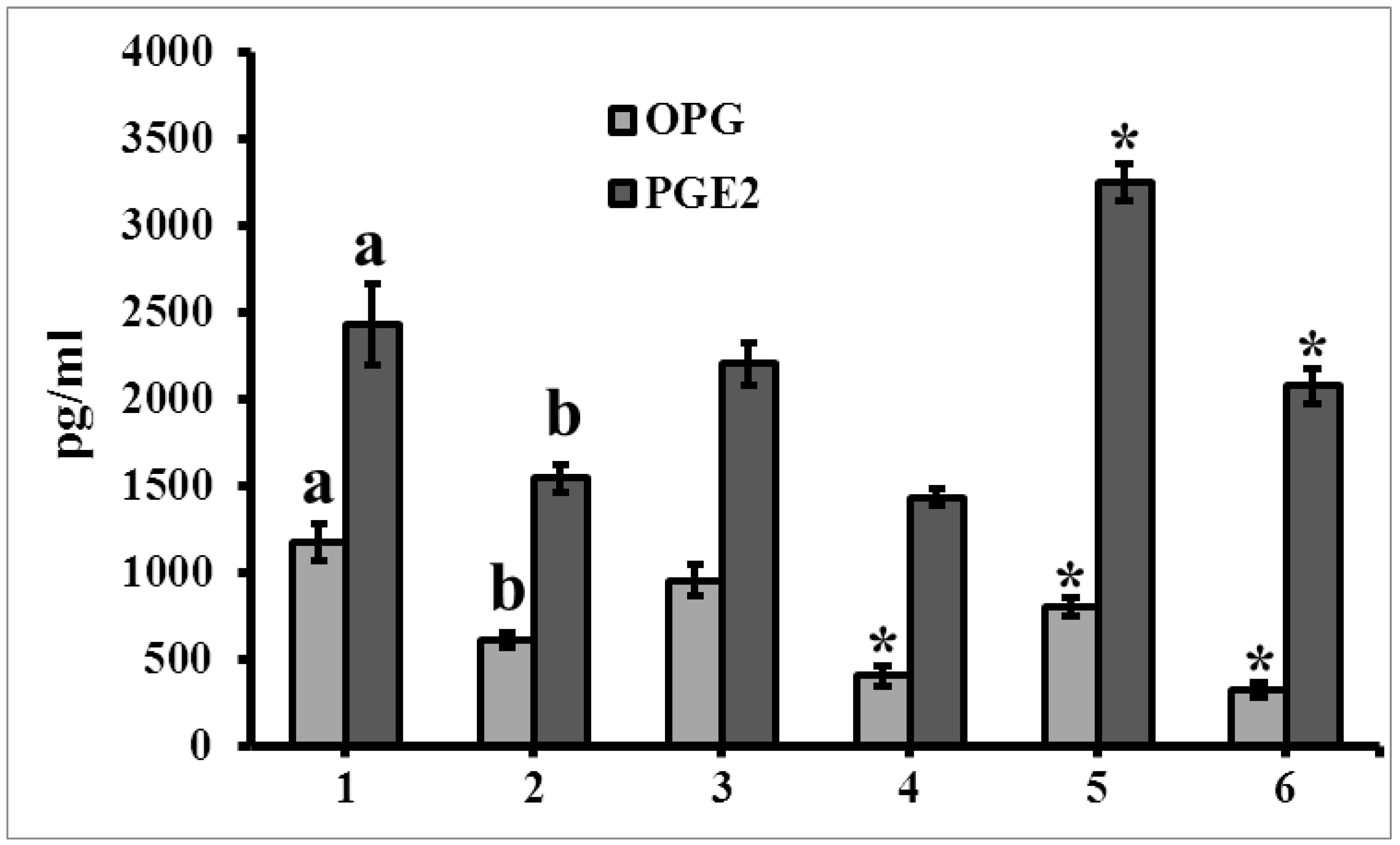
3.6. The Level of Secretome
3.7. T-Cells Downregulate Osteogenesis of MSCs
4. Discussion
5. Conclusions
- Did not affect MSCs proliferation;
- Increased RANK expression in MSCs;
- Downregulated osteogenesis of MSCs;
- Decreased mineral deposition of differentiated MSCs;
- Downregulated ANK protein expression;
- Regulated by PTHrP;
- Expression increased in differentiated MSCs;
- mRANKL from T-cells downregulate osteogenesis.
- Although we identified the downregulating osteogenic differentiation of MSCs by sRANKL through decreasing ANK protein expression, the actual mechanism of hematopoietic stem cells, osteoclasts and immune cells in downregulating osteogenesis through RANKL need to be verified.
- In the present in vitro study, cells were cultured in 2D culture systems, which do not mimic the actual in vivo environment, thus further in vitro studies are needed using a 3D culture system.
- This study lacks the actual paracrine signaling mechanism of PTHrP on RANK towards osteogenesis, and more research is needed to investigate certain hypotheses such as how the PTHrP upregulates RANKL’s action through scrutinizing the major communication between them and also the need to confirm these effects using in vivo studies.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kartsogiannis, V.; Zhou, H.; Horwood, N.J.; Thomas, R.J.; Hards, D.K.; Quinn, J.M.; Niforas, P.; Ng, K.W.; Martin, T.J.; Gillespie, M.T. Loalization of RANKL (receptor activator of NFkB ligand) mRNA and protein in skeletal and extraskeletal tissues. Bone 1999, 25, 525–534. [Google Scholar] [CrossRef]
- Wada, T.; Nakashima, T.; Hiroshi, N.; Penninger, J.M. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol. Med. 2006, 12, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Fata, J.E.; Kong, Y.Y.; Li, J.; Sasaki, T.; Irie-Sasaki, J.; Moorehead, R.A.; Elliott, R.; Scully, S.; Voura, E.B.; Lacey, D.L.; et al. The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell 2000, 103, 41–50. [Google Scholar] [CrossRef]
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef]
- Elango, J.; Sanchez, C.; de Val, J.E.; Henrotin, Y.; Wang, S.; Motaung, K.S.; Guo, R.; Wang, C.; Robinson, J.; Regenstein, J.M.; et al. Cross-talk between primary osteocytes and bone marrow macrophages for osteoclastogenesis upon collagen treatment. Sci. Rep. 2018, 8, 5318. [Google Scholar] [CrossRef]
- Xiong, J.; Onal, M.; Jilka, R.L.; Weinstein, R.S.; Manolagas, S.C.; O’Brien, C.A. Matrix-embedded cells control osteoclast formation. Nat. Med. 2011, 17, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; O’Brien, C.A. Osteocyte RANKL: New Insights into the Control of Bone Remodeling. J. Bone Miner Res. 2012, 27, 499–505. [Google Scholar] [CrossRef]
- Ma, Q.L.; Fang, L.; Jiang, N.; Zhang, L.; Wang, Y.; Zhang, Y.M.; Chen, L.H. Bone mesenchymal stem cell secretion of sRANKL/OPG/M-CSF in response to macrophage-mediated inflammatory response influences osteogenesis on nanostructured Ti surfaces. Biomaterial 2018, 154, 234–247. [Google Scholar] [CrossRef]
- Kim, N.S.; Kim, H.J.; Koo, B.K.; Kwon, M.C.; Kim, Y.W.; Cho, Y.; Yokota, Y.; Penninger, J.M.; Kong, Y.Y. Receptor activator of NF-kappaB ligand regulates the proliferation of mammary epithelial cells via Id2. Mol. Cell Biol. 2006, 26, 1002–1013. [Google Scholar] [CrossRef]
- Roodman, G.D. Cell biology of the osteoclast. Exp. Hematol. 1999, 27, 1229–1241. [Google Scholar] [CrossRef]
- Yasuda, H.; Shima, N.; Nakagawa, N.; Yamaguchi, K.; Kinosaki, M.; Mochizuki, S.; Tomoyasu, A.; Yano, K.; Goto, M.; Murakami, A.; et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. USA 1998, 95, 3597–3602. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Tatsuhiko, S.; Tamio, S.; Hiroyuki, O.; Masafumi, T. Involvement of p38 Mitogen-activated Protein Kinase Signaling Pathway in Osteoclastogenesis Mediated by Receptor Activator of NF-κB Ligand (RANKL). J. Biol. Chem. 2000, 275, 31155–31161. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, J.; Takaesu, G.; Akatsuka, H.; Sakurai, H.; Ninomiya-Tsuji, L.; Matsumoto, K.; Sakurai, N. Receptor activator of NF-kappaB ligand (RANKL) activates TAK1 mitogen-activated protein kinase kinase kinase through a signaling complex containing RANK, TAB2, and TRAF6. Mol. Cell Biol. 2002, 22, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Reeve, J. PTH: A future role in the management of osteoporosis? J. Bone Miner. Res. 1996, 11, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Moseley, J.M.; Gillespie, M.T. Parathyroid hormone-related protein. Crit. Rev. Clin. Lab. Sci. 1995, 32, 299–343. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N. Subchapter 26B-Parathyroid hormone-related protein. In Handbook of Hormones Comparative Endocrinology for Basic and Clinical Research; Takei, Y., Ando, H., Tsutsui, K., Eds.; Academic Press: Cambrige, MA, USA, 2016; pp. 227–229. [Google Scholar]
- Everhart-Caye, M.; Inzucchi, S.E.; Guinness-Henry, J.M.; Mitnick, A.; Stewart, A.F. Parathyroid hormone (PTH)-related protein(1-36) is equipotent to PTH(1-34) in humans. J. Clin. Endocrinol. Metabol. 1996, 81, 199–208. [Google Scholar]
- Henry, J.G.; Mitnick, M.; Dann, P.R.; Stewart, A.F. Parathyroid hormone-related protein-(1–36) is biologically active when administered subcutaneously to humans. J. Clin. Endocrinol. Metabol. 1997, 82, 900–906. [Google Scholar] [CrossRef]
- Plotkin, H.; Gundberg, C.; Mitnick, M.; Stewart, A.F. Dissociation of bone formation from resorption during 2-Week treatment with human parathyroid hormone-related peptide-(1–36) in humans: Potential as an anabolic therapy for osteoporosis. J. Clin. Endocrinol. Metabol. 1998, 83, 2786–2791. [Google Scholar]
- Zhang, M.; Xie, R.; Hou, W.; Wang, B.; Shen, R.; Wang, X.; Wang, Q.; Zhu, T.; Jonason, J.H.; Chen, D. PTHrP prevents chondrocyte premature hypertrophy by inducing cyclin-D1-dependent Runx2 and Runx3 phosphorylation, ubiquitylation and proteasomal degradation. J. Cell Sci. 2009, 122, 1382–1389. [Google Scholar] [CrossRef]
- Chen, X.; Zhi, X.; Wang, J.; Su, J. RANKL signaling in bone marrow mesenchymal stem cells negatively regulates osteoblastic bone formation. Bone Res. 2018, 276, 34. [Google Scholar] [CrossRef]
- Zhu, H.; Guo, Z.K.; Jiang, X.X.; Li, H.; Wang, X.Y.; Yao, H.Y.; Zhang, Y.; Mao, N. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat. Protoc. 2010, 5, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhang, N.; Marsano, A.; Vunjak-Novakovic, G.; Zhang, Y.; Lopez, M.J. In vitro mesenchymal trilineage differentiation and extracellular matrix production by adipose and bone marrow derived adult equine multipotent stromal cells on a collagen scaffold. Stem Cell Rev. 2013, 9, 858–872. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Schmitt, J.F.; Lee, E.H. Immunohistochemical analysis of human mesenchymal stem cells differentiating into chondrogenic, osteogenic, and adipogenic lineages. Methods Mol Biol. 2011, 698, 353–366. [Google Scholar] [PubMed]
- Bills, C.E.; Eisenberg, H.; Pallante, S.L. Complexes of organic acids with calcium phosphate: The Von Kossa stain as a clue to the composition of bone mineral. Johns Hopkins Med. J. 1974, 128, 194–207. [Google Scholar] [PubMed]
- Kim, N.; Odgren, P.R.; Kim, D.K.; Marks, S.C.J.; Choi, Y. Diverse roles of the tumor necrosis factor family member TRANCE in skeletal physiology revealed by TRANCE deficiency and partial rescue by a lymphocyte-expressed TRANCE transgene. Proc. Natl. Acad. Sci. USA 2000, 97, 10905–10910. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Matsuyama, T.; Hosokawa, Y.; Makihira, S.; Seki, M.; Karimbux, N.Y.; Goncalves, R.B.; Valverde, P.; Dibart, S.; Li, Y.P.; et al. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am. J. Pathol. 2006, 169, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Onal, M.; Xiong, J.; Chen, X.; Thostenson, J.D.; Almeida, M.; Manolagas, S.C.; O’Brien, C.A. Receptor Activator of Nuclear Factor κB Ligand (RANKL) Protein Expression by B Lymphocytes Contributes to Ovariectomy-induced Bone Loss. J. Biol. Chem. 2012, 287, 29851–29860. [Google Scholar] [CrossRef] [PubMed]
- Rifas, L.; Arackal, S.; Weitzmann, M.N. Inflammatory T cells rapidly induce differentiation of human bone marrow stromal cells into mature osteoblasts. J. Cell Biochem. 2003, 188, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Josien, R.; Wong, B.R.; Li, H.L.; Steinman, R.M.; Choi, Y. TRANCE, a TNF family member, is differentially expressed on T cell subsets and induces cytokine production in dendritic cells. J. Immunol. 1999, 162, 2562–2568. [Google Scholar]
- Kong, Y.Y.; Feige, U.; Sarosi, I.; Bolon, B.; Tafuri, A.; Morony, S.; Capparelli, C.; Li, J.; Elliott, R.; McCabe, S.; et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 1999, 402, 304–509. [Google Scholar] [CrossRef]
- Anderson, D.M.; Maraskovsky, E.; Billingsley, W.L.; Dougall, W.C.; Tometsko, M.E.; Roux, E.R.; Teepe, M.C.; DuBose, R.F.; Cosman, D.; Galibert, L. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 1997, 390, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.R.; Rho, J.; Arron, J.; Robinson, E.; Orlinick, J.; Chao, M.; Kalachikov, S.; Cayani, E.; Bartlett, F.S., 3rd; Frankel, W.N.; et al. TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J. Biol. Chem. 1997, 272, 25190–25194. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.M.; Johnson, M.D.; Kingsley, D.M. Role of the mouse ank gene in control of tissue calcification and arthritis. Science 2000, 289, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Nuernberg, P.; Thiele, H.; Chandler, D.; Hohne, W.; Cunningham, M.L.; Ritter, H.; Leschik, G.; Uhlmann, K.; Mischung, C.; Harrop, K.; et al. Heterozygous mutations in ANKH, the human ortholog of the mouse progressive ankylosis gene, result in craniometaphyseal dysplasia. Nat. Genet. 2001, 28, 37–41. [Google Scholar] [CrossRef]
- Kim, H.J.; Minashima, T.; McCarthy, E.F.; Winkles, J.A.; Kirsch, T. Progressive Ankylosis Protein (ANK) in Osteoblasts and Osteoclasts Controls Bone Formation and Bone Remodeling. J. Bone Miner. Res. 2010, 25, 1771–1783. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elango, J.; Rahman, S.U.; Henrotin, Y.; de Val, J.E.M.S.; Bao, B.; Wang, S.; Li, B.; Wu, W. Parathyroid Hormone-Related Protein (PTHrP) Accelerates Soluble RANKL Signals for Downregulation of Osteogenesis of Bone Mesenchymal Stem Cells. J. Clin. Med. 2019, 8, 836. https://doi.org/10.3390/jcm8060836
Elango J, Rahman SU, Henrotin Y, de Val JEMS, Bao B, Wang S, Li B, Wu W. Parathyroid Hormone-Related Protein (PTHrP) Accelerates Soluble RANKL Signals for Downregulation of Osteogenesis of Bone Mesenchymal Stem Cells. Journal of Clinical Medicine. 2019; 8(6):836. https://doi.org/10.3390/jcm8060836
Chicago/Turabian StyleElango, Jeevithan, Saeed Ur Rahman, Yves Henrotin, José Eduardo Maté Sánchez de Val, Bin Bao, Shujun Wang, Bailin Li, and Wenhui Wu. 2019. "Parathyroid Hormone-Related Protein (PTHrP) Accelerates Soluble RANKL Signals for Downregulation of Osteogenesis of Bone Mesenchymal Stem Cells" Journal of Clinical Medicine 8, no. 6: 836. https://doi.org/10.3390/jcm8060836
APA StyleElango, J., Rahman, S. U., Henrotin, Y., de Val, J. E. M. S., Bao, B., Wang, S., Li, B., & Wu, W. (2019). Parathyroid Hormone-Related Protein (PTHrP) Accelerates Soluble RANKL Signals for Downregulation of Osteogenesis of Bone Mesenchymal Stem Cells. Journal of Clinical Medicine, 8(6), 836. https://doi.org/10.3390/jcm8060836