Achieving Pain Control in Rheumatoid Arthritis with Baricitinib or Adalimumab Plus Methotrexate: Results from the RA-BEAM Trial
Abstract
1. Introduction
2. Patients and Methods
2.1. Trial Design
2.2. Patients
2.3. Pain Measures
Pain Thresholds
2.4. Outcomes
2.5. Statistical Analyses
3. Results
3.1. Pain Relief
3.2. Remaining Pain
3.3. Relationship between Inflammation and Pain Relief
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Taylor, P.C.; Manger, B.; Alvaro-Gracia, J.; Johnstone, R.; Gomez-Reino, J.; Eberhardt, E.; Wolfe, F.; Schwartzman, S.; Furfaro, N.; Kavanaugh, A. Patient perceptions concerning pain management in the treatment of rheumatoid arthritis. J. Int. Med. Res. 2010, 38, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Boyden, S.D.; Hossain, I.N.; Wohlfahrt, A.; Lee, Y.C. Non-inflammatory causes of pain in patients with rheumatoid arthritis. Curr. Rheumatol. Rep. 2016, 18, 30. [Google Scholar] [CrossRef] [PubMed]
- Altawil, R.; Saevarsdottir, S.; Wedren, S.; Alfredsson, L.; Klareskog, L.; Lampa, J. Remaining Pain in Early Rheumatoid Arthritis Patients Treated with Methotrexate. Arthritis Care Res. 2016, 68, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Ishida, M.; Kuroiwa, Y.; Yoshida, E.; Sato, M.; Krupa, D.; Henry, N.; Ikeda, K.; Kaneko, Y. Residual symptoms and disease burden among patients with rheumatoid arthritis in remission or low disease activity: A systematic literature review. Mod. Rheumatol. 2018, 28, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Meeus, M.; Vervisch, S.; De Clerck, S.; Moorkens, G.; Hans, G.; Nijs, J. Central sensitization in patients with rheumatoid arthritis: A systematic literature review. Semin. Arthritis Rheum. 2012, 41, 556–567. [Google Scholar] [CrossRef]
- McWilliams, D.F.; Walsh, D.A. Pain mechanisms in rheumatoid arthritis. Clin. Exp. Rheumatol. 2017, 35 (Suppl. 107), 94–101. [Google Scholar]
- Taylor, P.C.; Keystone, E.C.; van der Heijde, D.; Weinblatt, M.E.; del Carmen Morales, L.; Reyes Gonzaga, J.; Yakushin, S.; Ishii, T.; Emoto, K.; Beattie, S.; et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N. Engl. J. Med. 2017, 376, 652–662. [Google Scholar] [CrossRef]
- Keystone, E.C.; Taylor, P.C.; Tanaka, Y.; Gaich, C.; DeLozier, A.M.; Dudek, A.; Zamora, J.V.; Cobos, J.A.C.; Rooney, T.; de Bono, S.; et al. Patient-reported outcomes from a phase 3 study of baricitinib versus placebo or adalimumab in rheumatoid arthritis: Secondary analyses from the RA-BEAM study. Ann. Rheum. Dis. 2017, 76, 1853–1861. [Google Scholar] [CrossRef]
- Dworkin, R.H.; Turk, D.C.; Wyrwich, K.W.; Beaton, D.; Cleeland, C.S.; Farrar, J.T.; Haythornthwaite, J.A.; Jensen, M.P.; Kerns, R.D.; Ader, D.N.; et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J. Pain 2008, 9, 105–121. [Google Scholar] [CrossRef]
- IMMPACT: Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials. Available online: http://www.immpact.org/ (accessed on 24 April 2019).
- Wells, G.A.; Boers, M.; Shea, B.; Brooks, P.M.; Simon, L.S.; Strand, C.V.; Aletaha, D.; Anderson, J.J.; Bombardier, C.; Dougados, M.; et al. Minimal disease activity for rheumatoid arthritis: A preliminary definition. J. Rheumatol. 2005, 32, 2016–2024. [Google Scholar]
- Wolfe, F.; Michaud, K. Assessment of pain in rheumatoid arthritis: Minimal clinically significant difference, predictors, and the effect of anti-tumor necrosis factor therapy. J. Rheumatol. 2007, 34, 1674–1683. [Google Scholar] [PubMed]
- Tubach, F.; Ravaud, P.; Martin-Mola, E.; Awada, H.; Bellamy, N.; Bombardier, C.; Felson, D.T.; Hajjaj-Hassouni, N.; Hochberg, M.; Logeart, I.; et al. Minimum clinically important improvement and patient acceptable symptom state in pain and function in rheumatoid arthritis, ankylosing spondylitis, chronic back pain, hand osteoarthritis, and hip and knee osteoarthritis: Results from a prospective multinational study. Arthritis Care Res. 2012, 64, 1699–1707. [Google Scholar]
- Gooley, T.A.; Leisenring, W.; Crowley, J.; Storer, B.E. Estimation of failure probabilities in the presence of competing risks: New representations of old estimators. Stat. Med. 1999, 18, 695–706. [Google Scholar] [CrossRef]
- Fine, J.P.; Gray, R.J. A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- Preacher, K.J.; Hayes, A.F. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav. Res. Methods 2008, 40, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Busch-Dienstfertig, M.; González-Rodríguez, S. IL-4, JAK-STAT signaling, and pain. JAK-STAT 2013, 2, e27638. [Google Scholar] [CrossRef] [PubMed]
- Knopp, K.L.; Kato, A.; Wall, T.M.; McDermott, J.S.; Nisenbaum, E.S.; Adams, B.L.; Johnson, M. SAT0051 Baricitinib improves joint mobility after injury in a rodent forced-ambulation model. Ann. Rheum. Dis. 2019, 78, A1089. [Google Scholar]
- Cook, A.D.; Pobjoy, J.; Steidl, S.; Durr, M.; Braine, E.L.; Turner, A.L.; Lacey, D.C.; Hamilton, J.A. Granulocyte-macrophage colony-stimulating factor is a key mediator in experimental osteoarthritis pain and disease development. Arthritis Res. Ther. 2012, 14, R199. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, E.; Rivat, C.; Pommier, B.; Mauborgne, A.; Pohl, M. JAK/STAT3 pathway is activated in spinal cord microglia after peripheral nerve injury and contributes to neuropathic pain development in rat. J. Neurochem. 2008, 107, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Wigerblad, G.; Bas, D.B.; Fernades-Cerqueira, C.; Krishnamurthy, A.; Nandakumar, K.S.; Rogoz, K.; Kato, J.; Sandor, K.; Su, J.; Jimenez–Andrade, J.M.; et al. Autoantibodies to citrullinated proteins induce joint pain independent of inflammation via chemokine-dependent mechanism. Ann. Rheum. Dis. 2016, 74, 730–738. [Google Scholar] [CrossRef]
- Augustsson, J.; Neovius, M.; Cullinane-Carli, C.; Eksborg, S.; van Vollenhoven, R.F. Patients with rheumatoid arthritis treated with tumour necrosis factor antagonists increase their participation in the workforce: Potential for significant long-term indirect cost gains (data from a population-based registry). Ann. Rheum. Dis. 2010, 69, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.C.; Moore, A.; Vasilescu, R.; Alvir, J.; Tarallo, M. A structured literature review of the burden of illness and unmet needs in patients with rheumatoid arthritis: A current perspective. Rheumatol. Int. 2016, 36, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Michaud, K.; Pope, J.; Emery, P.; Zhu, B.; Gaich, C.; DeLozier, A.M.; Zhang, X.; Dickson, C.; Smolen, J.S. Relative impact of pain and fatigue on work productivity in patients with rheumatoid arthritis from the RA-BEAM baricitinib trial. Rheumatol. Ther. 2019, in press. [Google Scholar]
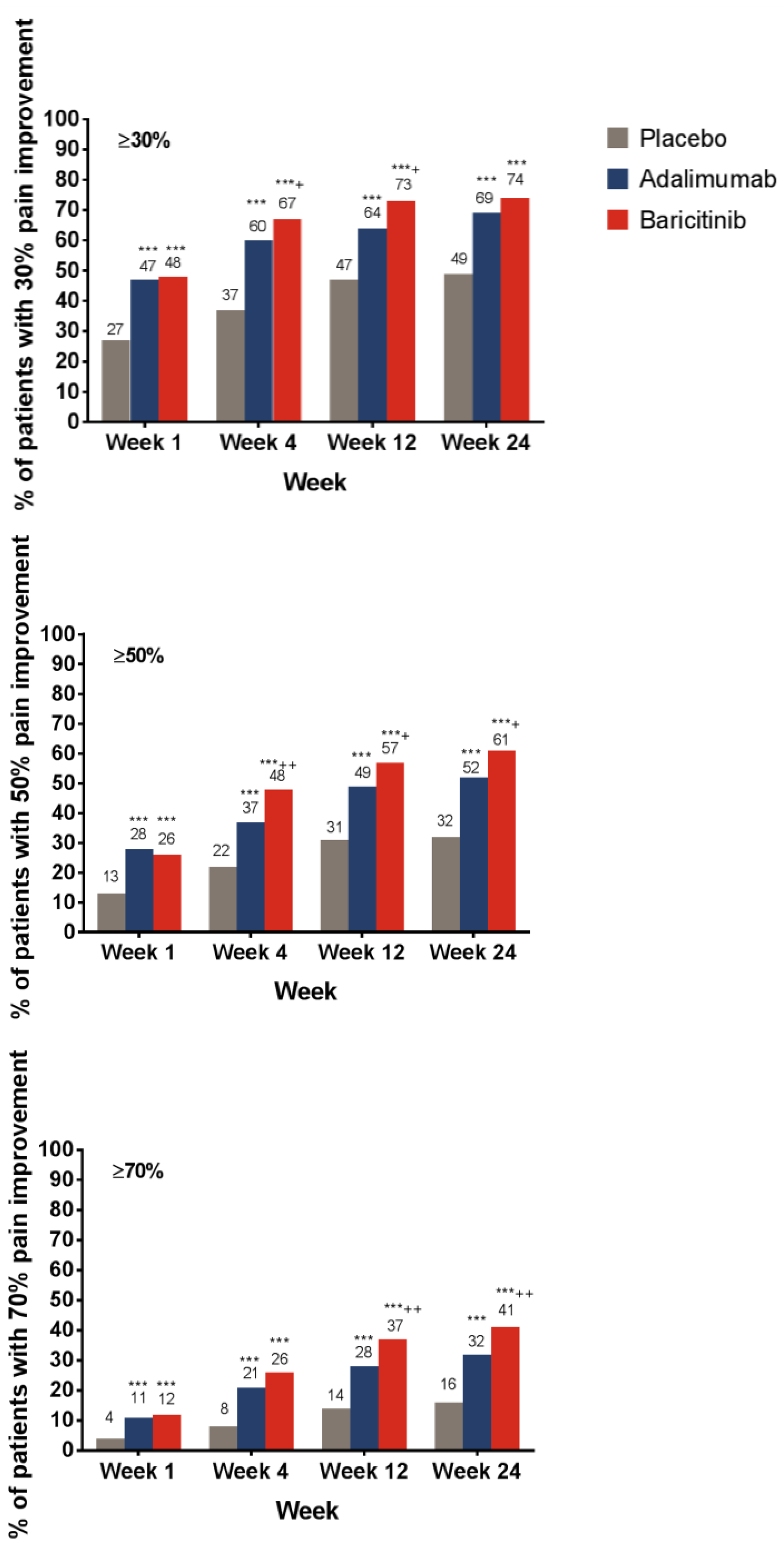
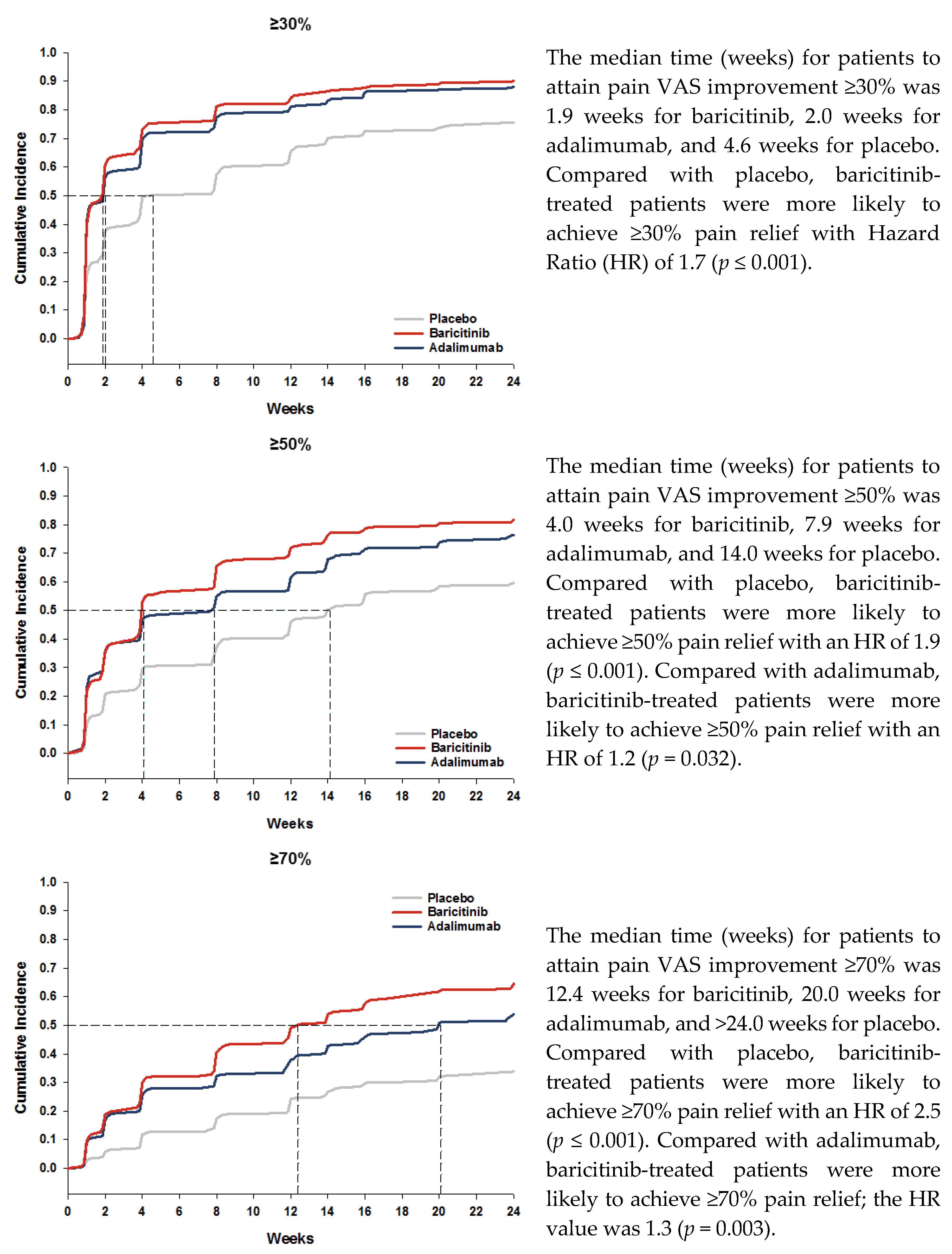
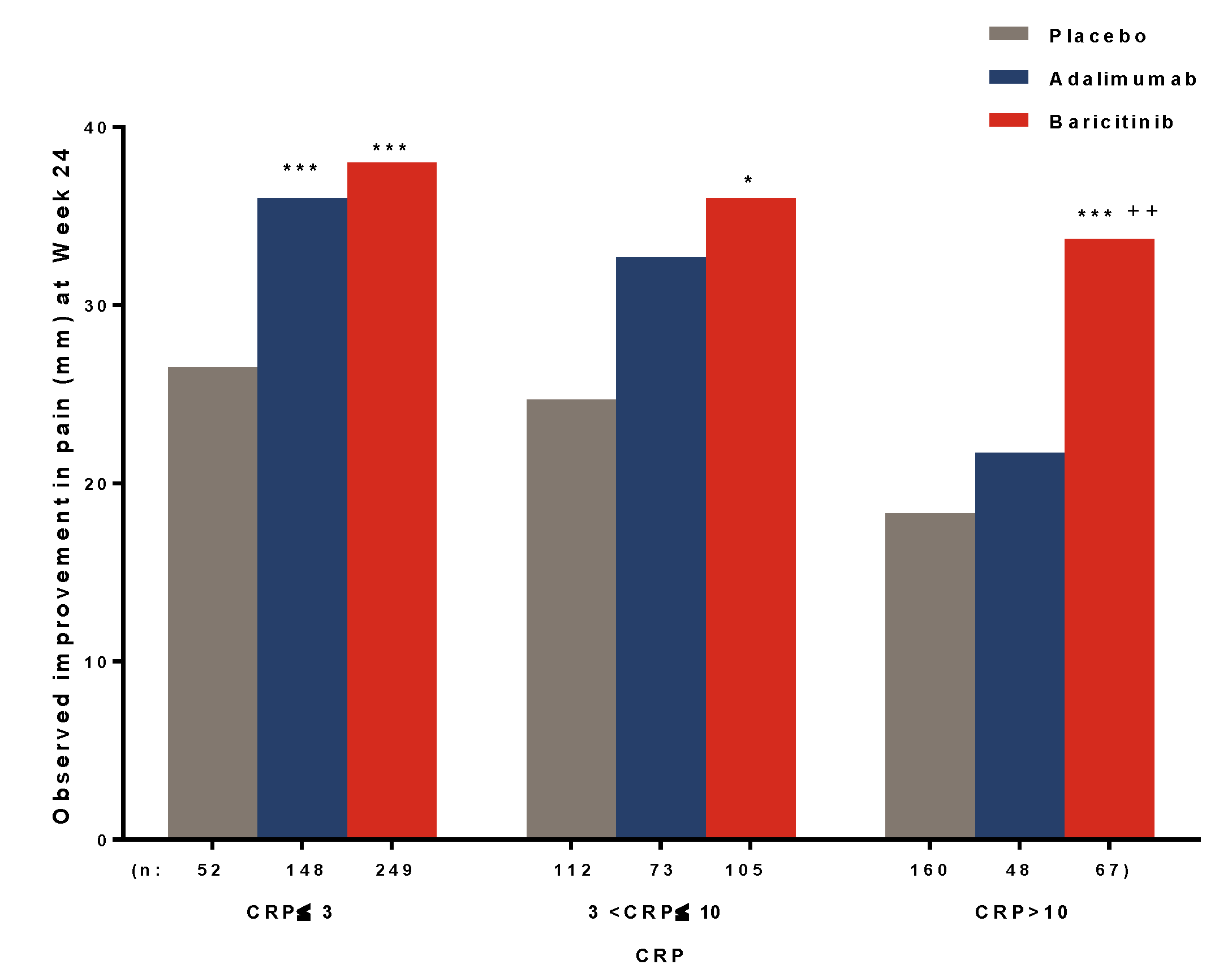
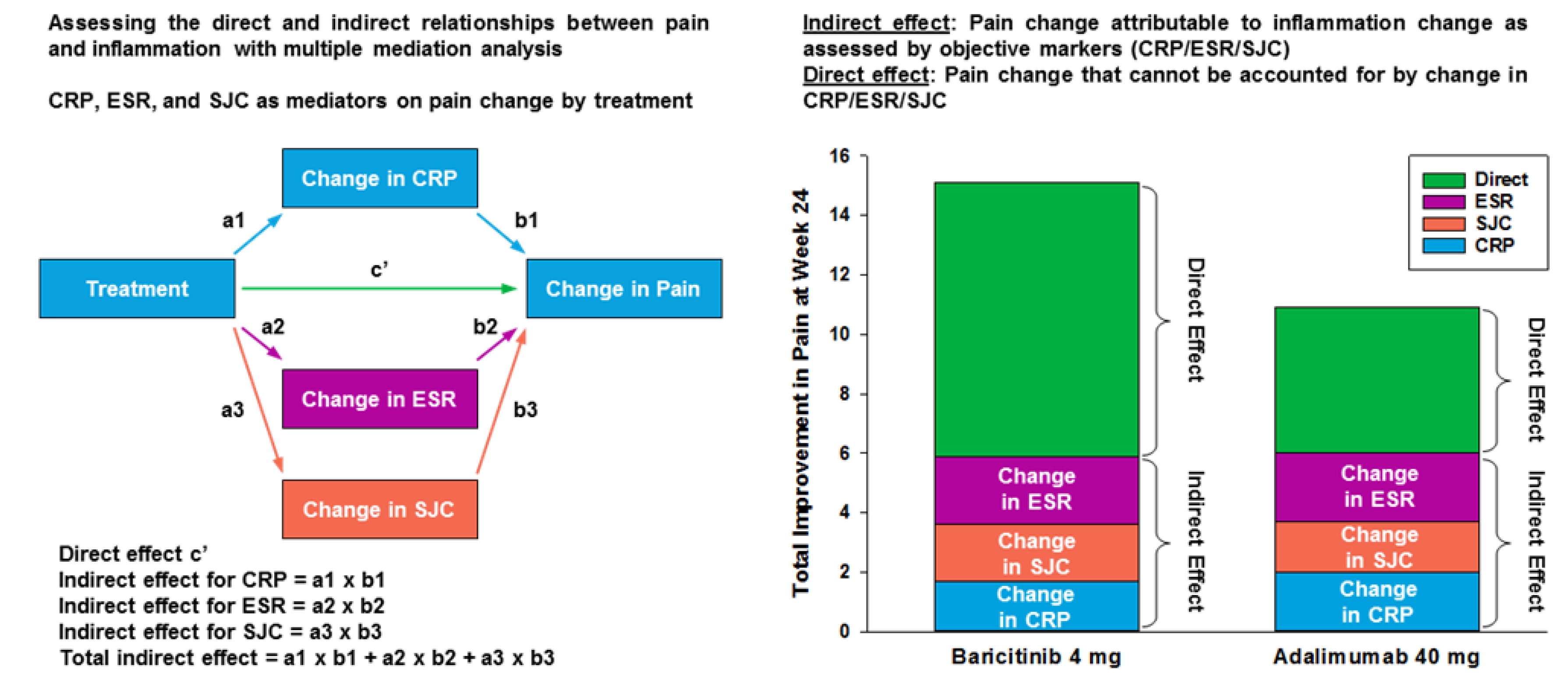
| Threshold of Remaining Pain at Each Time Point (Week) | Placebo n (%) | Adalimumab n (%) | Baricitinib n (%) |
|---|---|---|---|
| ≤40 mm | |||
| 1 | 150 (31) | 132 (41) *** | 208 (43) *** |
| 4 | 193 (40) | 169 (52) *** | 298 (62) ***,††† |
| 12 | 225 (46) | 202 (62) *** | 335 (69) ***,† |
| 24 | 236 (49) | 218 (66) *** | 351 (73) ***,† |
| ≤20 mm | |||
| 1 | 51 (11) | 64 (20) *** | 90 (19) *** |
| 4 | 80 (17) | 93 (28) *** | 158 (33) *** |
| 12 | 103 (21) | 120 (37) *** | 209 (43) ***,† |
| 24 | 105 (22) | 121 (37) *** | 239 (49) ***,††† |
| ≤10 mm | |||
| 1 | 20 (4) | 32 (10) *** | 40 (8) *** |
| 4 | 29 (6) | 49 (15) *** | 88 (18) *** |
| 12 | 52 (11) | 63 (19) *** | 124 (26) ***,† |
| 24 | 56 (12) | 86 (26) *** | 144 (30) *** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taylor, P.C.; Lee, Y.C.; Fleischmann, R.; Takeuchi, T.; Perkins, E.L.; Fautrel, B.; Zhu, B.; Quebe, A.K.; Gaich, C.L.; Zhang, X.; et al. Achieving Pain Control in Rheumatoid Arthritis with Baricitinib or Adalimumab Plus Methotrexate: Results from the RA-BEAM Trial. J. Clin. Med. 2019, 8, 831. https://doi.org/10.3390/jcm8060831
Taylor PC, Lee YC, Fleischmann R, Takeuchi T, Perkins EL, Fautrel B, Zhu B, Quebe AK, Gaich CL, Zhang X, et al. Achieving Pain Control in Rheumatoid Arthritis with Baricitinib or Adalimumab Plus Methotrexate: Results from the RA-BEAM Trial. Journal of Clinical Medicine. 2019; 8(6):831. https://doi.org/10.3390/jcm8060831
Chicago/Turabian StyleTaylor, Peter C., Yvonne C. Lee, Roy Fleischmann, Tsutomu Takeuchi, Elizabeth L. Perkins, Bruno Fautrel, Baojin Zhu, Amanda K. Quebe, Carol L. Gaich, Xiang Zhang, and et al. 2019. "Achieving Pain Control in Rheumatoid Arthritis with Baricitinib or Adalimumab Plus Methotrexate: Results from the RA-BEAM Trial" Journal of Clinical Medicine 8, no. 6: 831. https://doi.org/10.3390/jcm8060831
APA StyleTaylor, P. C., Lee, Y. C., Fleischmann, R., Takeuchi, T., Perkins, E. L., Fautrel, B., Zhu, B., Quebe, A. K., Gaich, C. L., Zhang, X., Dickson, C. L., Schlichting, D. E., Patel, H., Durand, F., & Emery, P. (2019). Achieving Pain Control in Rheumatoid Arthritis with Baricitinib or Adalimumab Plus Methotrexate: Results from the RA-BEAM Trial. Journal of Clinical Medicine, 8(6), 831. https://doi.org/10.3390/jcm8060831






