Prognostic Value of Incomplete Revascularization after Percutaneous Coronary Intervention Following Acute Coronary Syndrome: Focus on CKD Patients
Abstract
1. Introduction
2. Methods
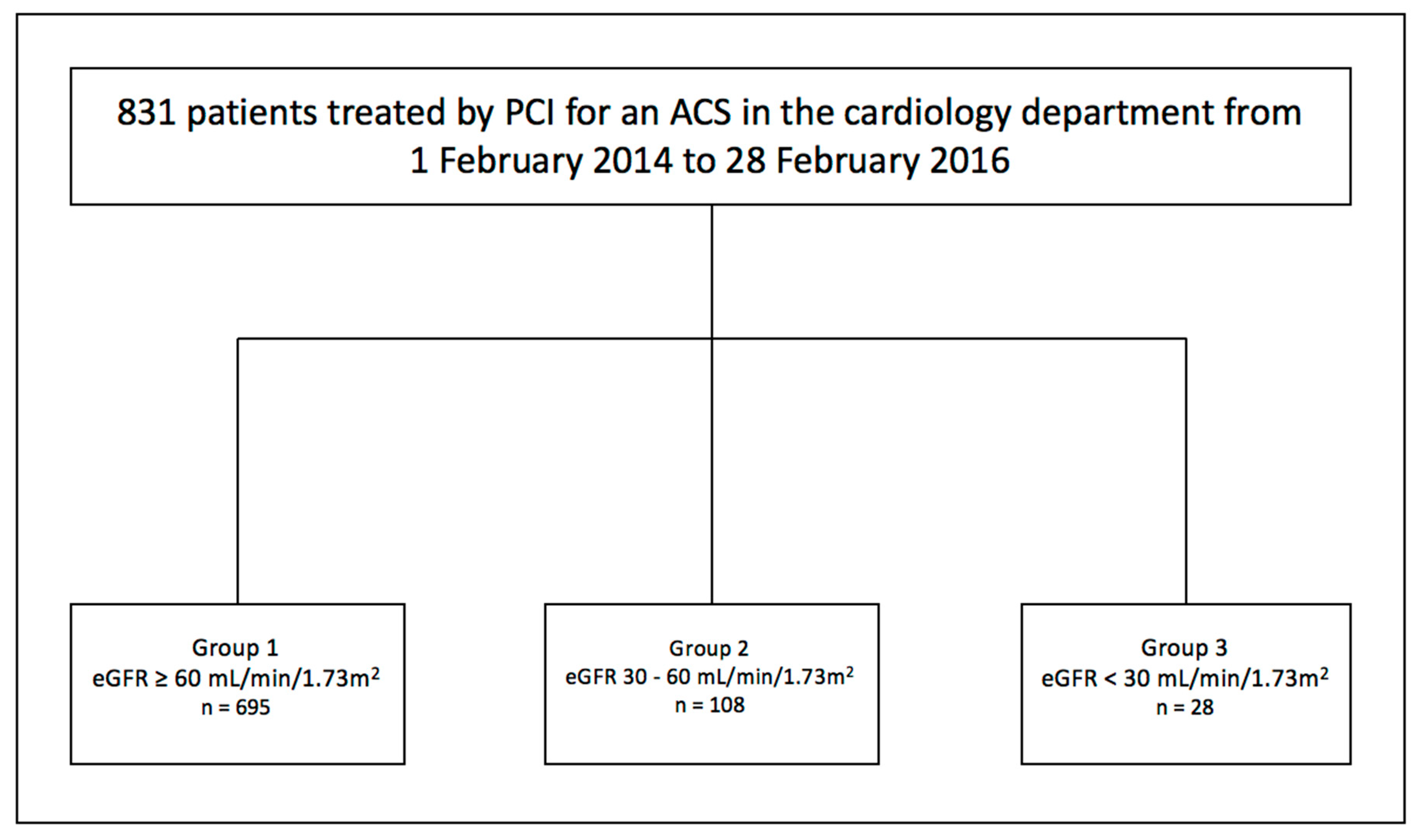
2.1. Patients
2.2. Chronic Kidney Disease Staging
2.3. Collection of Data
2.4. Calculation of SYNTAX Score (SS), Residual SYNTAX Score (rSS) and SYNTAX Index Revascularization (SRI)
2.5. Study Endpoints
2.6. Statistical Analysis
3. Results
3.1. Patient’s Baseline Characteristics
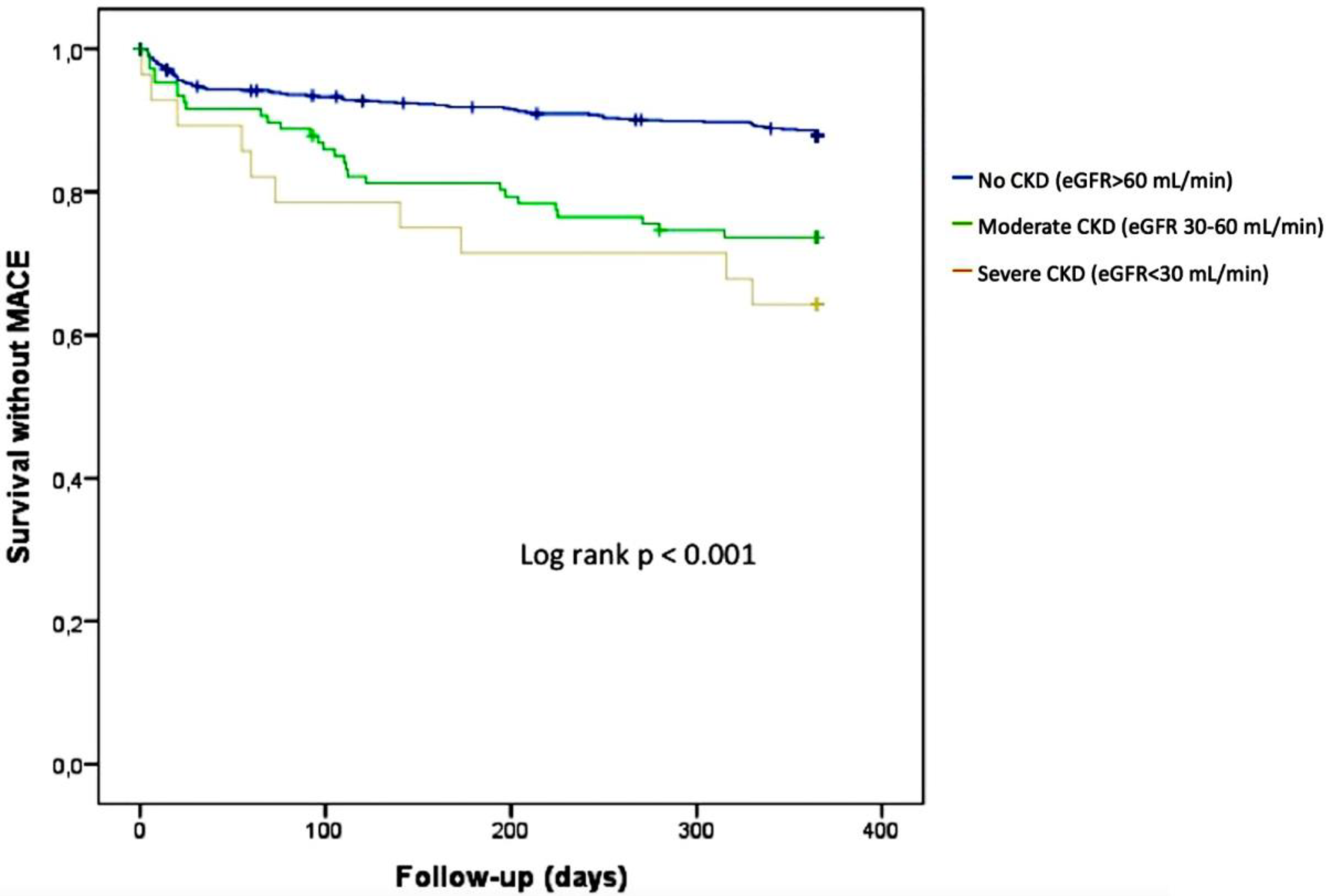
3.2. Impact of CKD on Cardiovascular Outcomes
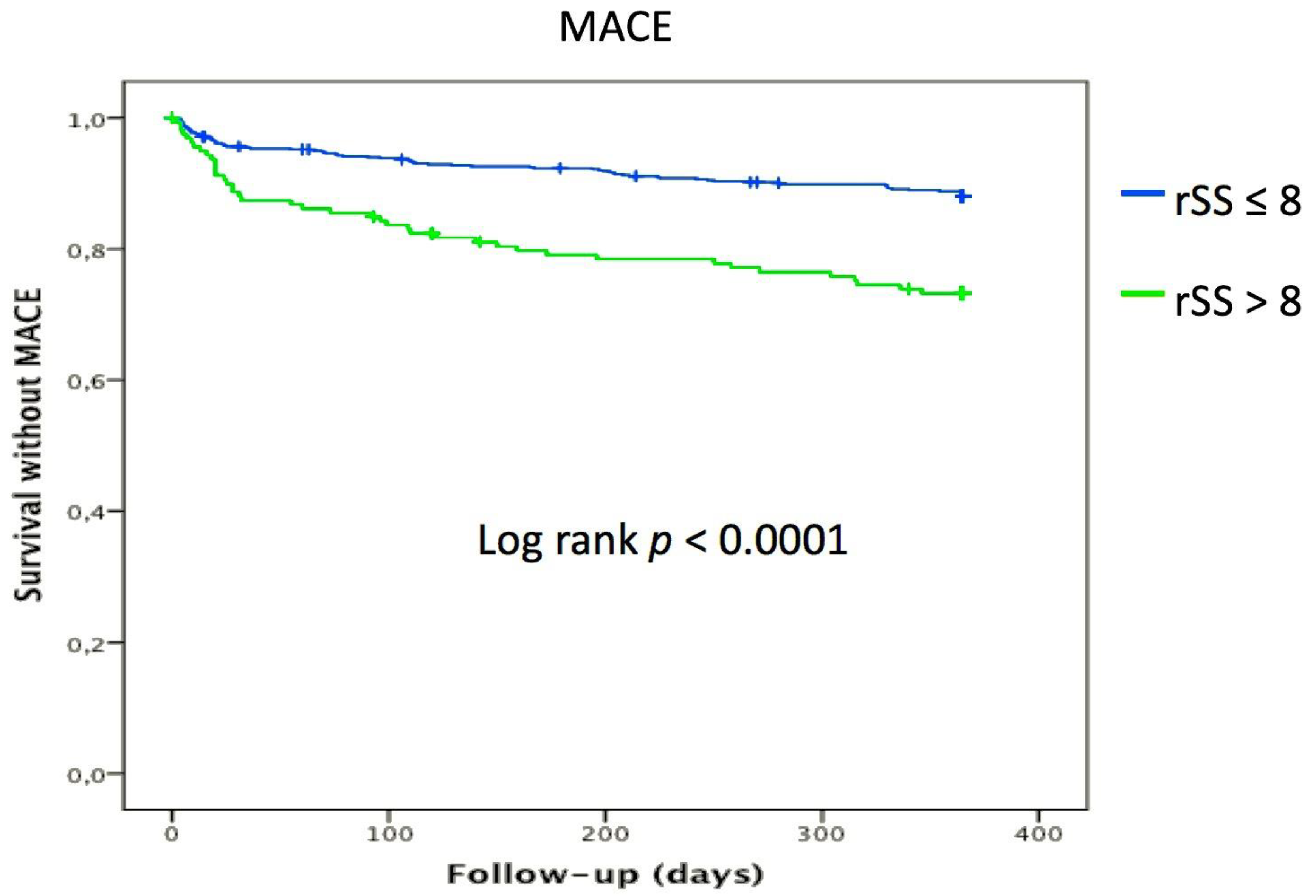
3.3. Impact of Incomplete Revascularization (rSS > 8) on Cardiovascular Outcomes
3.4. Predictors of rSS > 8 (Incomplete Revascularization)
3.5. Predictors of Cardiac Death and MACE
4. Discussion
4.1. Impact of CKD on Adverse Cardiovascular Outcomes Following PCI
4.2. Impact of Incomplete Revascularization on Adverse Cardiovascular Outcomes Following PCI
4.3. Study Limitations
5. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
References
- Khan, R.; Al-Hawwas, M.; Hatem, R.; Azzalini, L.; Fortier, A.; Joliecoeur, E.M.; Tanguay, J.F.; Lavoie-L’allier, P.; Ly, H.Q. Prognostic impact of the residual SYNTAX score on in-hospital outcomes in patients undergoing primary percutaneous coronary intervention. Catheter. Cardiovasc. Interv. 2016, 88, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Hoebers, L.P.; Vis, M.M.; Claessen, B.E.; Van der Schaaf, R.J.; Kikkert, W.J.; Baan, J., Jr.; de Winter, R.J.; Piek, J.J.; Tijssen, J.G.; Dangas, G.D.; et al. The impact of multivessel disease with and without a co-existing chronic total occlusion on short- and long-term mortality in ST-elevation myocardial infarction patients with and without cardiogenic shock. Eur. J. Heart Fail. 2013, 15, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Van der Schaaf, R.J.; Claessen, B.E.; Vis, M.M.; Hoebers, L.P.; Koch, K.T.; Baan, J., Jr.; Meuwissen, M.; Engstrom, A.E.; Kikkert, W.J.; Tijjsen, J.G.; et al. Effect of multivessel coronary disease with or without concurrent chronic total occlusion on one-year mortality in patients treated with primary percutaneous coronary intervention for cardiogenic shock. Am. J. Cardiol. 2010, 105, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Genereux, P.; Palmerini, T.; Caixeta, A.; Rosner, G.; Green, P.; Dressler, O.; Xu, K.; Parise, H.; Mehran, R.; Serruys, P.W.; et al. Quantification and impact of untreated coronary artery disease after percutaneous coronary intervention: The residual SYNTAX (Synergy Between PCI with Taxus and Cardiac Surgery) score. J. Am. Coll. Cardiol. 2012, 59, 2165–2174. [Google Scholar] [CrossRef] [PubMed]
- Loutfi, M.; Ayad, S.; Sobhy, M. Impact of the Residual SYNTAX Score on Outcomes of Revascularization in Patients with ST-Segment Elevation Myocardial Infarction and Multivessel Disease. Clin. Med. Insights Cardiol. 2016, 10, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Morel, O.; El Ghannudi, S.; Jesel, L.; Radulescu, B.; Meyer, N.; Wiesel, M.L.; Caillard, S.; Campia, U.; Moulin, B.; Gachet, C.; et al. Cardiovascular mortality in chronic kidney disease patients undergoing percutaneous coronary intervention is mainly related to impaired P2Y12 inhibition by clopidogrel. J. Am. Coll. Cardiol. 2011, 57, 399–408. [Google Scholar] [CrossRef]
- El Ghannudi, S.; Ohlmann, P.; Meyer, N.; Wiesel, M.L.; Radulescu, B.; Chauvin, M.; Bareiss, P.; Gachet, C.; Morel, O. Impact of P2Y12 inhibition by clopidogrel on cardiovascular mortality in unselected patients treated by percutaneous coronary angioplasty: A prospective registry. JACC Cardiovasc. Interv. 2010, 3, 648–656. [Google Scholar] [CrossRef]
- Coskun, U.; Orta Kilickesmez, K.; Abaci, O.; Kocas, C.; Bostan, C.; Yildiz, A.; Baskurt, M.; Arat, A.; Ersanli, M.; Gurmen, T. The relationship between chronic kidney disease and SYNTAX score. Angiology 2011, 62, 504–508. [Google Scholar] [CrossRef]
- Yan, L.Q.; Guo, L.J.; Zhang, F.C.; Gao, W. The relationship between kidney function and angiographically-derived SYNTAX score. Can. J. Cardiol. 2011, 27, 768–772. [Google Scholar] [CrossRef]
- Tsai, T.T.; Messenger, J.C.; Brennan, J.M.; Patel, U.D.; Dai, D.; Piana, R.N.; Anstrom, K.J.; Eisenstein, E.L.; Dokholyan, R.S.; Peterson, E.D.; et al. Safety and efficacy of drug-eluting stents in older patients with chronic kidney disease: A report from the linked CathPCI Registry-CMS claims database. J. Am. Coll. Cardiol. 2011, 58, 1859–1869. [Google Scholar] [CrossRef] [PubMed]
- Latif, F.; Kleiman, N.S.; Cohen, D.J.; Pencina, M.J.; Yen, C.H.; Cutlip, D.E.; Moliterno, D.J.; Nassif, D.; Lopez, J.J.; Saucedo, J.F. In-hospital and 1-year outcomes among percutaneous coronary intervention patients with chronic kidney disease in the era of drug-eluting stents: A report from the EVENT (Evaluation of Drug Eluting Stents and Ischemic Events) registry. JACC Cardiovasc. Interv. 2009, 2, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Machecourt, J.; Danchin, N.; Lablanche, J.M.; Fauvel, J.M.; Bonnet, J.L.; Marliere, S.; Foote, A.; Quesada, J.L.; Eltchaninoff, H.; Vanzetto, G. Risk factors for stent thrombosis after implantation of sirolimus-eluting stents in diabetic and nondiabetic patients: The EVASTENT Matched-Cohort Registry. J. Am. Coll. Cardiol. 2007, 50, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Akashi, N.; Sakakura, K.; Watanabe, Y.; Noguchi, M.; Taniguchi, Y.; Yamamoto, K.; Wada, H.; Momomura, S.I.; Fujita, H. The comparison of clinical outcomes in patients with acute myocardial infarction and advanced chronic kidney disease on chronic hemodialysis versus off hemodialysis. Heart Vessel. 2018, 33, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Nakahashi, H.; Kosuge, M.; Sakamaki, K.; Kiyokuni, M.; Ebina, T.; Hibi, K.; Tsukahara, K.; Iwahashi, N.; Kuji, S.; Oba, M.S.; et al. Combined impact of chronic kidney disease and contrast-induced nephropathy on long-term outcomes in patients with ST-segment elevation acute myocardial infarction who undergo primary percutaneous coronary intervention. Heart Vessel. 2017, 32, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Shroff, G.R.; Herzog, C.A. Coronary Revascularization in Patients with CKD Stage 5D: Pragmatic Considerations. J. Am. Soc. Nephrol. 2016, 27, 3521–3529. [Google Scholar] [CrossRef] [PubMed]
- Morel, O.; Muller, C.; Jesel, L.; Moulin, B.; Hannedouche, T. Impaired platelet P2Y12 inhibition by thienopyridines in chronic kidney disease: Mechanisms, clinical relevance and pharmacological options. Nephrol. Dial. Transplant. 2013, 28, 1994–2002. [Google Scholar] [CrossRef]
- Muller, C.; Caillard, S.; Jesel, L.; El Ghannudi, S.; Ohlmann, P.; Sauleau, E.; Hannedouche, T.; Gachet, C.; Moulin, B.; Morel, O. Association of estimated GFR with platelet inhibition in patients treated with clopidogrel. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2012, 59, 777–785. [Google Scholar] [CrossRef]
- Chertow, G.M.; Normand, S.L.; McNeil, B.J. “Renalism”: Inappropriately low rates of coronary angiography in elderly individuals with renal insufficiency. J. Am. Soc. Nephrol. 2004, 15, 2462–2468. [Google Scholar] [CrossRef]
- Chung, J.W.; Park, K.H.; Lee, M.H.; Park, K.W.; Park, J.S.; Kang, H.J.; Koo, B.K.; Kwon, Y.W.; Kim, H.S. Benefit of complete revascularization in patients with multivessel coronary disease in the drug-eluting stent era. Circ. J. 2012, 76, 1624–1630. [Google Scholar] [CrossRef]
- Yazji, K.; Abdul, F.; Elangovan, S.; Ossei-Gerning, N.; Choudhury, A.; Cockburn, J.; Anderson, R.; Mamas, M.; Kinnaird, T. Comparison of the Effects of Incomplete Revascularization on 12-Month Mortality in Patients <80 Compared With >/=80 Years Who Underwent Percutaneous Coronary Intervention. Am. J. Cardiol. 2016, 118, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Rosner, G.F.; Kirtane, A.J.; Genereux, P.; Lansky, A.J.; Cristea, E.; Gersh, B.J.; Weiz, G.; Parise, H.; Fahy, M.; Mehran, R.; et al. Impact of the presence and extent of incomplete angiographic revascularization after percutaneous coronary intervention in acute coronary syndromes: The Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial. Circulation 2012, 125, 2613–2620. [Google Scholar] [CrossRef] [PubMed]
- Farooq, V.; Serruys, P.W.; Bourantas, C.V.; Zhang, Y.; Muramatsu, T.; Feldman, T.; Holmes, D.R.; Mack, M.; Morice, M.C.; Stahle, E.; et al. Quantification of incomplete revascularization and its association with five-year mortality in the synergy between percutaneous coronary intervention with taxus and cardiac surgery (SYNTAX) trial validation of the residual SYNTAX score. Circulation 2013, 128, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Tonino, P.A.; De Bruyne, B.; Pijls, N.H.; Siebert, U.; Ikeno, F.; van’t Veer, M.; Klauss, V.; Manoharan, G.; Engstrom, T.; Oldroyd, K.G.; et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N. Engl. J. Med. 2009, 360, 213–224. [Google Scholar] [CrossRef] [PubMed]
- De Bruyne, B. Multivessel disease: From reasonably incomplete to functionally complete revascularization. Circulation 2012, 125, 2557–2559. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kobayashi, Y.; Nam, C.W.; Tonino, P.A.; Kimura, T.; De Bruyne, B.; Pijls, N.H.; Fearon, W.F. The Prognostic Value of Residual Coronary Stenoses After Functionally Complete Revascularization. J. Am. Coll. Cardiol. 2016, 67, 1701–1711. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.; Sandoval, Y.; Roukoz, H.; Adabag, S.; Canoniero, M.; Yannopoulos, D.; Brilakis, E.S. Outcomes after complete versus incomplete revascularization of patients with multivessel coronary artery disease: A meta-analysis of 89,883 patients enrolled in randomized clinical trials and observational studies. J. Am. Coll. Cardiol. 2013, 62, 1421–1431. [Google Scholar] [CrossRef]
| Variables | eGFR ≥ 60 n = 695 | eGFR 30-59 n = 108 | eGFR < 30 n = 28 | p Value |
|---|---|---|---|---|
| Age | 61.88 (±13.15) | 76.79 (±12.49) | 76.43 (±12.55) | <0.001 |
| Gender (female) | 153 (22%) | 38 (35.2%) | 10 (35.7%) | 0.004 |
| STEMI | 392 (56.4%) | 50 (46.3%) | 15 (53.6%) | 0.161 |
| NSTEMI | 268 (38.6%) | 52 (48.1%) | 12 (42.9%) | 0.159 |
| Unstable angina | 35 (5%) | 6 (5.6%) | 1 (3.6%) | 0.911 |
| Diabetes mellitus | 144 (20.7%) | 44 (40.7%) | 12 (42.9%) | <0.001 |
| Hypertension | 382 (55%) | 89 (82.4%) | 23 (82.1%) | <0.001 |
| Current smoking | 299 (43%) | 19 (17.6%) | 4 (14.3%) | <0.001 |
| Dyslipidemia | 314 (45.2%) | 66 (61.1%) | 20 (71.4%) | <0.001 |
| Family history of CAD | 160 (23%) | 8 (7.4%) | 4 (14.3%) | 0.001 |
| Prior STEMI | 68 (9.80%) | 14 (13%) | 7 (25%) | 0.028 |
| Prior NSTEMI | 44 (6.3%) | 17 (15.7%) | 6 (21.4%) | <0.001 |
| Prior angioplasty | 102 (14.7%) | 24 (22.2%) | 10 (35.7%) | 0.003 |
| Prior Stroke | 35 (5%) | 12 (11.1%) | 6 (21.4%) | <0.001 |
| Atrial fibrillation | 40 (5.8%) | 17 (15.9%) | 7 (25%) | <0.001 |
| PAD | 41 (5.90%) | 17 (15.9%) | 6 (21.4%) | <0.001 |
| Obesity | 188 (27.1%) | 19 (17.6%) | 5 (17.9%) | 0.237 |
| Killip ≥ 2 | 105 (15.1%) | 44 (40.7%) | 14 (50%) | <0.001 |
| LVEF (%) | 52.51 (±11.44) | 49.22 (±12.87) | 45.25 (±12.21) | <0.001 |
| LVEF ≤ 40% | 125 (18%) | 32 (29.6%) | 12 (42.9%) | <0.001 |
| RV dysfunction | 42 (6%) | 11 (10.2%) | 9 (32.1%) | <0.001 |
| Aspirin | 688 (99%) | 104 (96.3%) | 27 (96.4%) | 0.058 |
| Clopidogrel | 248 (35.7%) | 74 (68.5%) | 25 (89.3%) | <0.001 |
| Ticagrelor | 188 (27.1%) | 18 (16.7%) | 1 (3.6%) | <0.001 |
| Prasugrel | 249 (35.80%) | 12 (11.1%) | 0 (0%) | <0.001 |
| ACE-I | 571 (82.2%) | 67 (62%) | 14 (50%) | <0.001 |
| ARB | 57 (8.2%) | 20 (18.5%) | 4 (14.3%) | <0.001 |
| Statin | 671 (96.5%) | 97 (89.8%) | 24 (85.7%) | 0.002 |
| Betablocker | 645 (92.9%) | 90 (83.3%) | 23 (82.1%) | 0.001 |
| VKA | 67 (9.6%) | 20 (18.5%) | 9 (32.1%) | 0.003 |
| In hospital hemorrhage | 17 (2.4%) | 7 (6.5%) | 3 (10.7%) | 0.007 |
| Acute kidney injury | 54 (7.8%) | 41 (38%) | 14 (50%) | <0.001 |
| Variables | eGFR ≥ 60 n = 695 | eGFR 30-59 n = 108 | eGFR < 30 n = 28 | p Value |
|---|---|---|---|---|
| Creatinine (µmol/L) | 71.31 ± 14.82 | 123.61 ± 25.76 | 262.29 ± 169.98 | <0.001 |
| eGFR (mL/min/1.73 m²) | 85.77 ± 7.75 | 48.48 ± 8.13 | 21.93 ± 8.44 | <0.001 |
| Troponin at admission (µg/L) | 10.01 ± 34.72 | 13.75 ± 36.54 | 15.56 ± 26.44 | 0.436 |
| Troponin peak (µg/L) | 70.93 ± 130.26 | 67.14 ± 123.98 | 75.24 ± 121.28 | 0.942 |
| BNP (µg/L) | 139.87 ± 253.3 | 462.74 ± 640.46 | 639.07 ± 599.53 | <0.001 |
| CRP (mg/L) | 11.69 ± 27.21 | 34.72 ± 57.78 | 72.44 ± 92.99 | <0.001 |
| Total-cholesterol (mg/dL) | 1.76 ± 0.58 | 1.56 ± 0.6 | 1.35 ± 0.69 | <0.001 |
| LDL-C (mg/dL) | 1.09 ± 0.44 | 0.92 ± 0.45 | 0.76 ± 0.45 | <0.001 |
| HDL-C (mg/dL) | 0.39 ± 0.15 | 0.4 ± 0.17 | 0.33 ± 0.16 | 0.065 |
| Triglycerid (mg/dL) | 1.37 ± 0.9 | 1.14 ± 0.66 | 1.14 ± 0.93 | 0.019 |
| Glycated Hemoglobin (%) | 5.55 ± 1.9 | 5.58 ± 2.16 | 5.57 ± 2.09 | 0.994 |
| Hemoglobin (g/dL) | 14.23 ± 1.69 | 13.15 ± 2.07 | 11.32 ± 2.16 | <0.001 |
| White blood cell count (×109/L) | 10.8 ± 4.07 | 11.22 ± 5.87 | 11.92 ± 5.98 | 0.323 |
| Platelets (×109/L) | 242.5 ± 67.17 | 225.36 ± 90.02 | 222.64 ± 72.34 | 0.028 |
| VASP (%) | 23.61 ± 25.53 | 28.47 ± 29.19 | 48.44 ± 33.04 | <0.001 |
| Variables | eGFR ≥ 60 n = 695 | eGFR 30-59 n = 108 | eGFR < 30 n = 28 | p Value |
|---|---|---|---|---|
| One-vessel disease | 340 (48.9%) | 35 (32.4%) | 11 (39.3%) | 0.004 |
| Three-vessel disease | 144 (20.7%) | 31 (28.7%) | 10 (35.7%) | 0.039 |
| LAD | 426 (61.3%) | 75 (69.4%) | 16 (57.1%) | 0.228 |
| Left main | 28 (4%) | 9 (8.3%) | 2 (7.1%) | 0.119 |
| Bifurcation | 28 (4%) | 6 (5.6%) | 1 (3.6%) | 0.752 |
| SYNTAX score (SS) | 15.77 (±9.95) | 19.27 (±10) | 21.34 (±13.91) | <0.001 |
| SS > 22 | 159 (22.8%) | 37 (34.3%) | 11 (39.3%) | 0.007 |
| SS > 32 | 53 (7.6%) | 9 (8.3%) | 7 (25%) | 0.005 |
| Stent implantation | 655 (94.2%) | 105 (97.2%) | 28 (100%) | 0.195 |
| Total stent lenghts (mm) | 29.99 (±20.35) | 32.86 (±21.29) | 34.39 (±19.51) | 0.238 |
| Maximum Stent Diameter per lesion (mm) | 2.91 (±0.82) | 2.95 (±0.65) | 3.18 (±0.39) | 0,199 |
| Thrombectomy | 102 (14.7%) | 10 (9.3%) | 4 (14.3%) | 0.319 |
| Irradiation time (min) | 11.42 (±8.34) | 12.36 (±10.98) | 12.38 (±7) | 0.511 |
| Contrast medium (mL) | 207.7 (±88.23) | 190.25 (±78.64) | 155.04 (±70.79) | 0.002 |
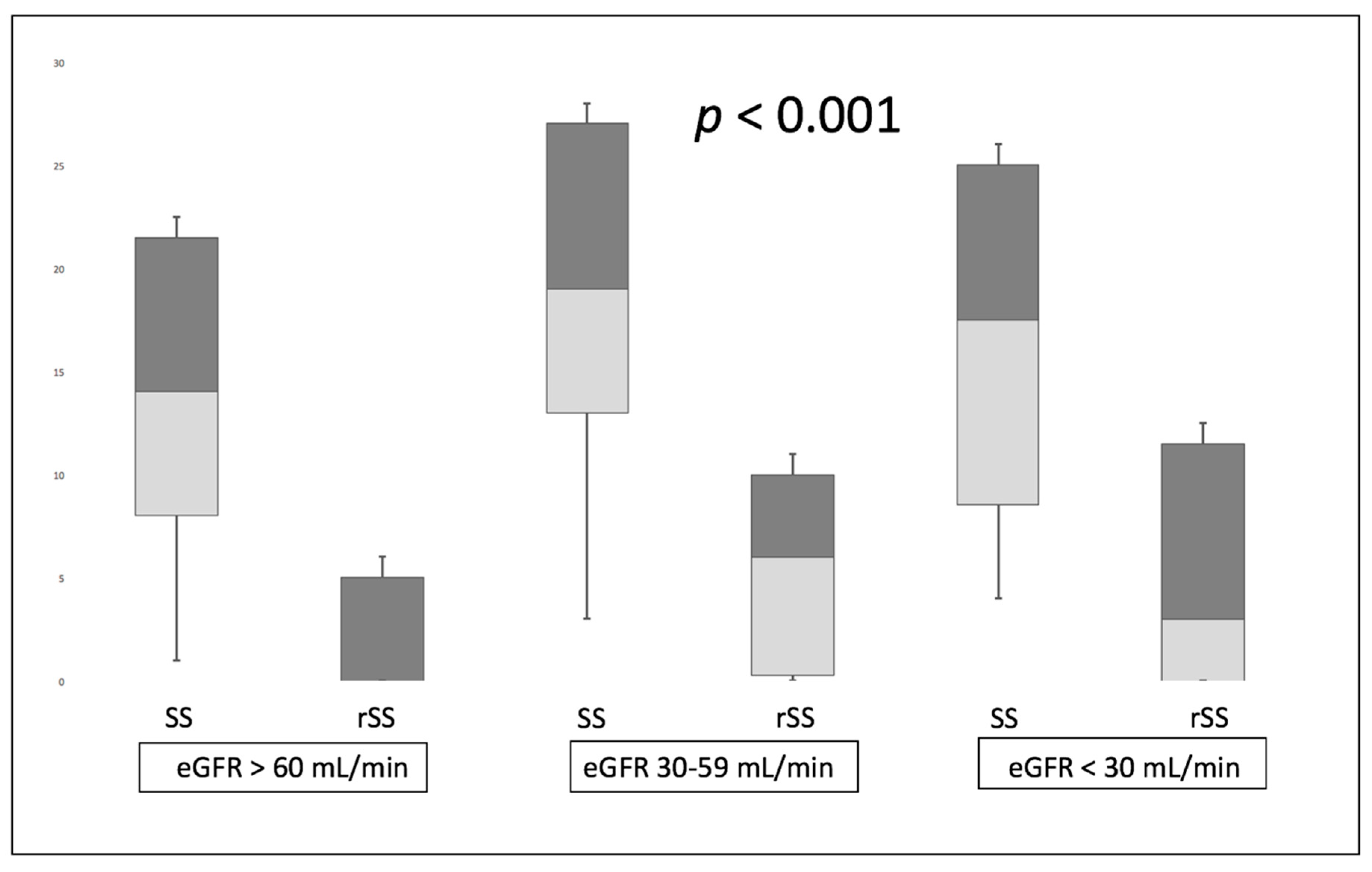
| Residual SYNTAX score (rSS) | 3.69 (±5.73) | 6.67 (±7.36) | 8.64 (±10.01) | <0.001 |
| rSS > 8 | 113 (16.3%) | 36 (33.3%) | 13 (46.4%) | <0.001 |
| SYNTAX index revascularization (%) | 80.34 (±26.32) | 68.9 (±28.52) | 68.99 (±28.31) | <0.001 |
| SRI < 70% | 198 (28.4%) | 54 (50%) | 14 (50%) | <0.001 |
| Staged PCI | 173 (24.9%) | 31 (29%) | 6 (21.4%) | 0.593 |
| Events | eGFR ≥ 60 n = 695 | eGFR 30-59 n = 108 | eGFR < 30 n = 28 | p Value |
|---|---|---|---|---|
| MACE | 83 (12%) | 28 (25.9%) | 10 (35.7%) | <0.001 |
| All cause mortality | 25 (3.6%) | 12 (11.1%) | 7 (25%) | <0.001 |
| Cardiac death | 11 (1.6%) | 7 (6.5%) | 3 (10.7%) | <0.001 |
| STEMI | 7 (1%) | 1 (0.9%) | 0 (0%) | 0.866 |
| NSTEMI | 33 (4.8%) | 8 (7.4%) | 2 (7.1%) | 0.457 |
| Stroke | 9 (1.3%) | 6 (4.6%) | 0 (0%) | 0.036 |
| Stent thrombosis | 12 (1.7%) | 1 (0.9%) | 0 (0%) | 0.657 |
| Hemorrhagic events | 56 (8.3%) | 13 (12%) | 5 (17.9%) | 0.096 |
| Heart Failure | 22 (3.2%) | 17 (15.7%) | 6 (21.4%) | <0.001 |
| Angina | 52 (7.5%) | 9 (8.3%) | 3 (10.7%) | 0.792 |
| Events | rSS ≤ 8 n = 669 (80.5%) | rSS > 8 n = 162 (19.5%) | p Value |
|---|---|---|---|
| MACE | 79 (11.8%) | 42 (25.9%) | <0.001 |
| All cause mortality | 23 (3.4%) | 21 (13%) | <0.001 |
| Cardiac death | 8 (1.2%) | 13 (8%) | <0.001 |
| STEMI | 4 (0.6%) | 4 (2.5%) | 0.029 |
| NSTEMI | 35 (5.2%) | 8 (5%) | 0.893 |
| CABG | 3 (0.4%) | 5 (3.1%) | 0.002 |
| Stroke | 6 (0.9%) | 9 (5.6%) | <0.001 |
| Stent thrombosis | 11 (1.6%) | 2 (1.2%) | 0.703 |
| Angina | 41 (6.1%) | 23 (14.2%) | 0.001 |
| Heart Failure | 24 (3.6%) | 21 (13%) | <0.001 |
| Hemorrhagic events | 55 (8.2%) | 19 (11.7%) | 0.160 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | p Value | HR (95% CI) | P Value |
| Age | 1.04 (1.03–1.06) | <0.001 | 1.03 (1.01–1.04) | <0.001 |
| STEMI | 0.95 (0.67–1.34) | 0.765 | ||
| NSTEMI | 0.82 (0.73–1.48) | 0.819 | ||
| Diabetes mellitus | 2.14 (1.48–3.11) | <0.001 | 1.50 (0.97–2.30) | 0.064 |
| Hypertension | 2.09 (1.43–3.05) | <0.001 | 1.05 (0.66–1.66) | 0.823 |
| Dyslipidemia | 1.58 (1.12–2.25) | 0.009 | 0.92 (0.61–1.14) | 0.732 |
| Current Smoking | 0.63 (0.44–0.91) | 0.014 | ||
| Obesity | 0.93 (0.63–1.38) | 0.732 | ||
| Atrial Fibrillation | 2.01 (1.15–3.53) | 0.014 | 0.99 (0.52–1.92) | 0.978 |
| PAD | 2.01 (1.14–3.49) | 0.015 | 1.00 (0.52–1.92) | 0.983 |
| Prior STEMI | 1.62 (0.98–2.67) | 0.062 | ||
| Prior NSTEMI | 2.35 (1.37–4.02) | 0.002 | 1.31 (0.70–2.45) | 0.383 |
| Previous angioplasty | 1.21 (0.77–1.89) | 0.41 | ||
| CKD (eGFR < 60 mL/min/m2) | 3.10 (2.07–4.67) | <0.001 | 1.75 (1.07–2.86) | 0.024 |
| Three-vessel disease | 5.05 (3.49–7.33) | <0.001 | 4.29 (2.89–6.37) | <0.001 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Events | HR (95% CI) | p Value | HR (95% CI) | p Value |
| Age | 1.09 (1.05–1.14) | <0.001 | 1.04 (0.99–1.09) | 0.064 |
| Gender (female) | 1.27 (0.49–3.27) | 0.620 | ||
| STEMI | 1.07 (0.45–2.56) | 0.866 | ||
| NSTEMI | 1.16 (0.49–2.76) | 0.733 | ||
| Diabete mellitus | 1.29 (0.49–3.31) | 0.602 | ||
| Hypertension | 2.21 (0.81–6.02) | 0.123 | ||
| Dyslipidemia | 1.45 (0.61–3.45) | 0.390 | ||
| Current Smoking | 0.26 (0.07–0.89) | 0.031 | ||
| Family history of CAD | 0.64 (0.19–2.17) | 0.474 | ||
| Obesity | 0.91 (0.33–2.45) | 0.844 | ||
| Atrial Fibrillation | 5.39 (2.07–14.03) | 0.001 | 1.69 (0.59–4.82) | 0.326 |
| PAD | 4.72 (1.83–12.18) | 0.001 | 1.84 (0.63–5.42) | 0.267 |
| CKD (eGFR<60 mL/min) | 4.21 (1.77–9.99) | 0.001 | 0.77 (0.28–2.15) | 0.627 |
| SBP | 0.99 (0.97–1.01) | 0.566 | ||
| DBP | 1.02 (0.98–1.05) | 0.380 | ||
| Killip ≥ 2 | 7.02 (2.91–16.95) | <0.001 | 1.92 (0.67–5.55) | 0.226 |
| LVEF ≤ 40% | 5.65 (2.38–13.41) | <0.001 | 1.60 (0.57–4.52) | 0.372 |
| Acute Kidney Injury | 7.81 (3.31–18.39) | <0.001 | 2.57 (0.94–6.97) | 0.064 |
| Aspirin | 0.004 (0.002–0.01) | <0.001 | ||
| Colpidogrel | 0.87 (0.36–2.11) | 0.762 | ||
| ACE/ARB | 0.08 (0.034–0.191) | <0.001 | ||
| Betablocker | 0.08 (0.03–0.181) | <0.001 | ||
| Statin | 0.03 (0.01–0.08) | <0.001 | ||
| One-vessel disease | 0.26 (0.08–0.76) | 0.014 | ||
| Three-vessel disease | 2.86 (1.20–6.78) | 0.017 | ||
| Left main | 1.06 (0.14–7.89) | 0.956 | ||
| LAD | 2.69 (0.91–7.98) | 0.075 | ||
| SYNTAX score > 22 | 4.29 (1.81–10.17) | 0.001 | ||
| SYNTAX score > 32 | 6.39 (2.58–15.86) | <0.001 | ||
| SRI < 70% | 3.55 (1.47–8.56) | 0.005 | ||
| rSS > 8 | 7.22 (2.99–17.44) | <0.001 | 3.38 (1.28–8.93) | 0.014 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | p Value | HR (95% CI) | p Value |
| Age | 1.03 (1.01–1.04) | <0.001 | 1.01(0.99–1.02) | 0.669 |
| Gender (female) | 1.19 (0.80–1.78) | 0.386 | ||
| STEMI | 0.59 (0.41–0.85) | 0.005 | ||
| NSTEMI | 1.84 (1.29–2.63) | 0.001 | 1.74 (1.20–2.53) | 0.003 |
| Diabete mellitus | 1.68 (1.15–2.44) | 0.007 | 1.10 (0.73–1.67) | 0.645 |
| Hypertension | 1.96 (1.31–2.94) | 0.001 | 1.33 (0.85–2.09) | 0.401 |
| Dyslipidemia | 1.37 (0.95–1.96) | 0.087 | ||
| Current Smoking | 0.74 (0.50–1.08) | 0.116 | ||
| Family history of CAD | 0.99 (0.64–1.54) | 0.976 | ||
| Obesity | 0.95 (0.63–1.43) | 0.817 | ||
| Prior NTSEMI | 2.23 (1.37–3.64) | 0.001 | ||
| Prior STEMI | 1.75 (1.08–2.83) | 0.022 | ||
| Prior unstable angina | 1.84 (0.96–3.51) | 0.031 | ||
| Prior Stroke | 1.92 (1.08–3.41) | 0.026 | ||
| Atrial Fibrillation | 2.15 (1.28–3.59) | 0.003 | 1.17 (0.68–2.04) | 0.574 |
| PAD | 2.13 (1.28–3.51) | 0.003 | 1.11 (0.64–1.92) | 0.710 |
| CKD (eGFR<60 mL/min) | 2.36 (1.59–3.49) | <0.001 | 1.26 (0.78–2.02) | 0.340 |
| Killip ≥ 2 | 2.39 (1.65–3.48) | <0.001 | 1.24 (0.78–1.98) | 0.358 |
| LVEF ≤ 40% | 2.42 (1.67–3.51) | <0.001 | 1.74 (1.11–2.71) | 0.015 |
| Acute Kidney Injury | 2.44 (1.61–3.70) | <0.001 | 1.46 (0.91–2.35) | 0.115 |
| Aspirin | 0.03 (0.01–0.05) | <0.001 | ||
| Colpidogrel | 1.52 (1.06–2.17) | 0.021 | ||
| ACE/ARB | 0.55 (0.38–0.82) | 0.003 | ||
| Betablocker | 0.38 (0.24–0.62) | <0.001 | ||
| Statin | 0.19 (0.12–0.32) | <0.001 | ||
| One-vessel disease | 0.49 (0.33–0.71) | <0.001 | ||
| Three-vessel disease | 2.38 (1.64–3.44) | <0.001 | ||
| Left main | 2.66 (1.49–4.73) | 0.001 | ||
| LAD | 1.95 (1.29–2.95) | 0.002 | ||
| SYNTAX score > 22 | 2.18 (1.51–3.13) | <0.001 | ||
| Syntax score > 32 | 2.67 (1.65–4.32) | <0.001 | ||
| SRI < 70% | 1.46 (1.01–2.11) | 0.040 | ||
| rSS > 8 | 2.46 (1.69–3.58) | <0.001 | 1.63 (1.08–2.46) | 0.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardi, T.; Kayali, A.; Trimaille, A.; Marchandot, B.; Ristorto, J.; Hoang, V.A.; Hess, S.; Kibler, M.; Jesel, L.; Ohlmann, P.; et al. Prognostic Value of Incomplete Revascularization after Percutaneous Coronary Intervention Following Acute Coronary Syndrome: Focus on CKD Patients. J. Clin. Med. 2019, 8, 810. https://doi.org/10.3390/jcm8060810
Cardi T, Kayali A, Trimaille A, Marchandot B, Ristorto J, Hoang VA, Hess S, Kibler M, Jesel L, Ohlmann P, et al. Prognostic Value of Incomplete Revascularization after Percutaneous Coronary Intervention Following Acute Coronary Syndrome: Focus on CKD Patients. Journal of Clinical Medicine. 2019; 8(6):810. https://doi.org/10.3390/jcm8060810
Chicago/Turabian StyleCardi, Thomas, Anas Kayali, Antonin Trimaille, Benjamin Marchandot, Jessica Ristorto, Viet Anh Hoang, Sébastien Hess, Marion Kibler, Laurence Jesel, Patrick Ohlmann, and et al. 2019. "Prognostic Value of Incomplete Revascularization after Percutaneous Coronary Intervention Following Acute Coronary Syndrome: Focus on CKD Patients" Journal of Clinical Medicine 8, no. 6: 810. https://doi.org/10.3390/jcm8060810
APA StyleCardi, T., Kayali, A., Trimaille, A., Marchandot, B., Ristorto, J., Hoang, V. A., Hess, S., Kibler, M., Jesel, L., Ohlmann, P., & Morel, O. (2019). Prognostic Value of Incomplete Revascularization after Percutaneous Coronary Intervention Following Acute Coronary Syndrome: Focus on CKD Patients. Journal of Clinical Medicine, 8(6), 810. https://doi.org/10.3390/jcm8060810




