Safety and Efficacy of Medical Cannabis in Fibromyalgia
Abstract
1. Introduction
2. Experimental Section
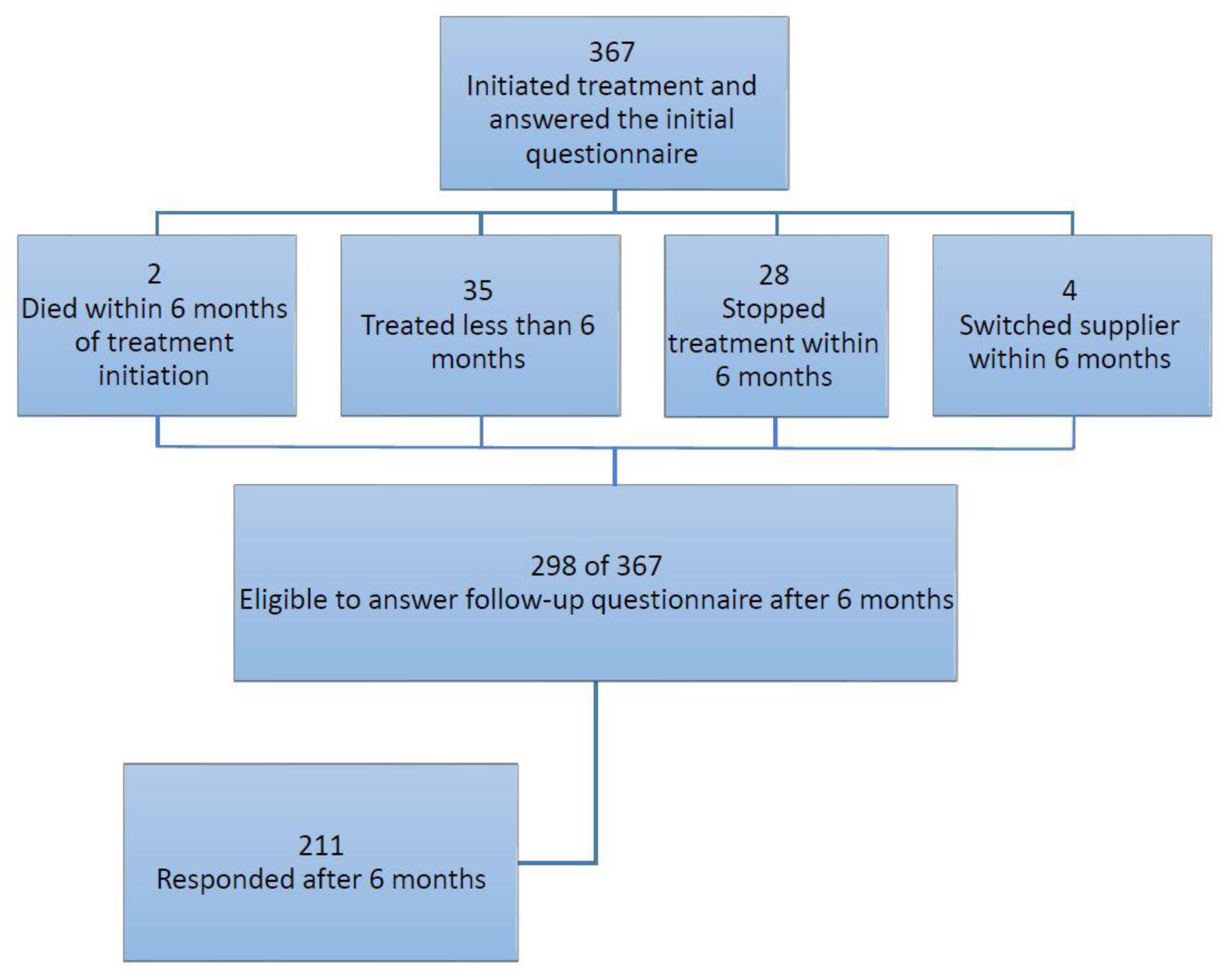
2.1. Study Population
2.2. Data Collection
2.3. Study Outcomes
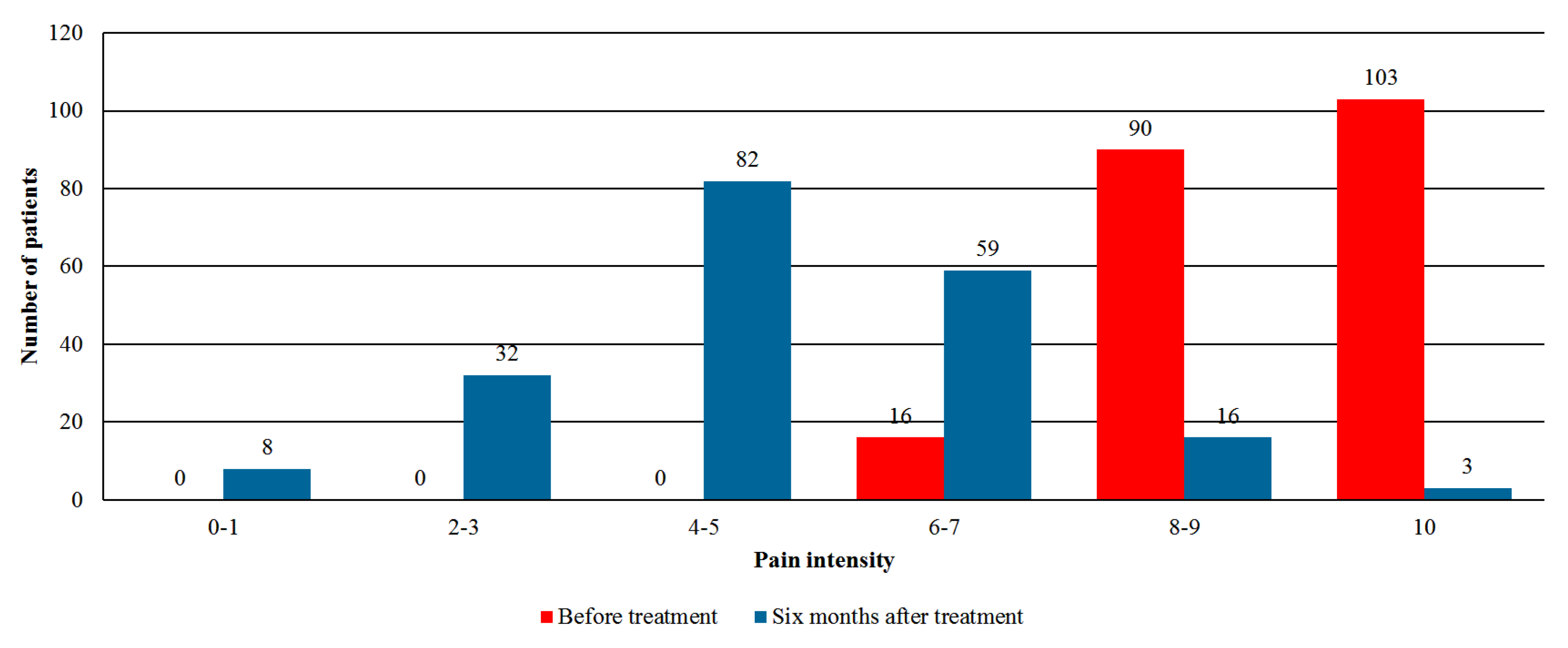
- Pain intensity—assessment by the numeric rating scale (NRS) with an 11-point scale (0 = no pain, 10 = worst pain imaginable).
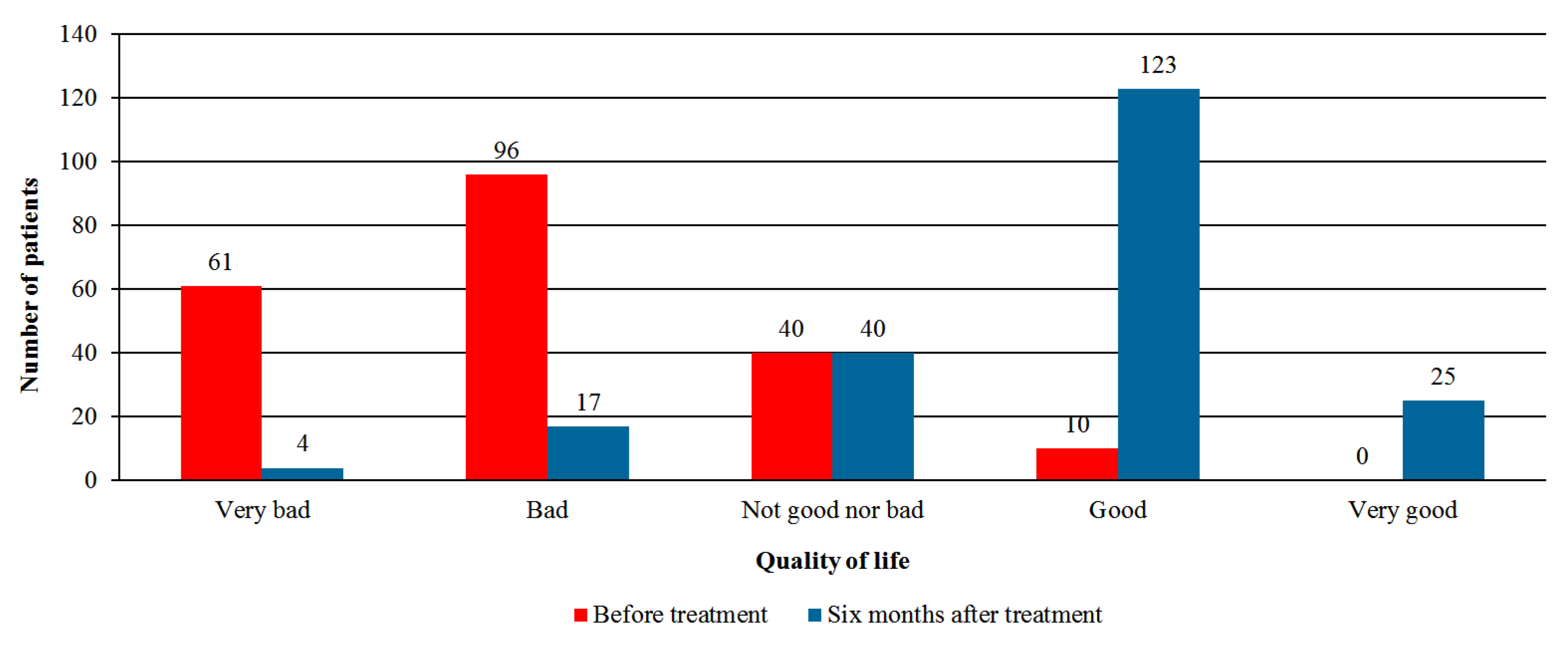
- Quality of life—global assessment by the patient using the Likert scale with five options: very good, good, neither good nor bad, bad, or very bad.
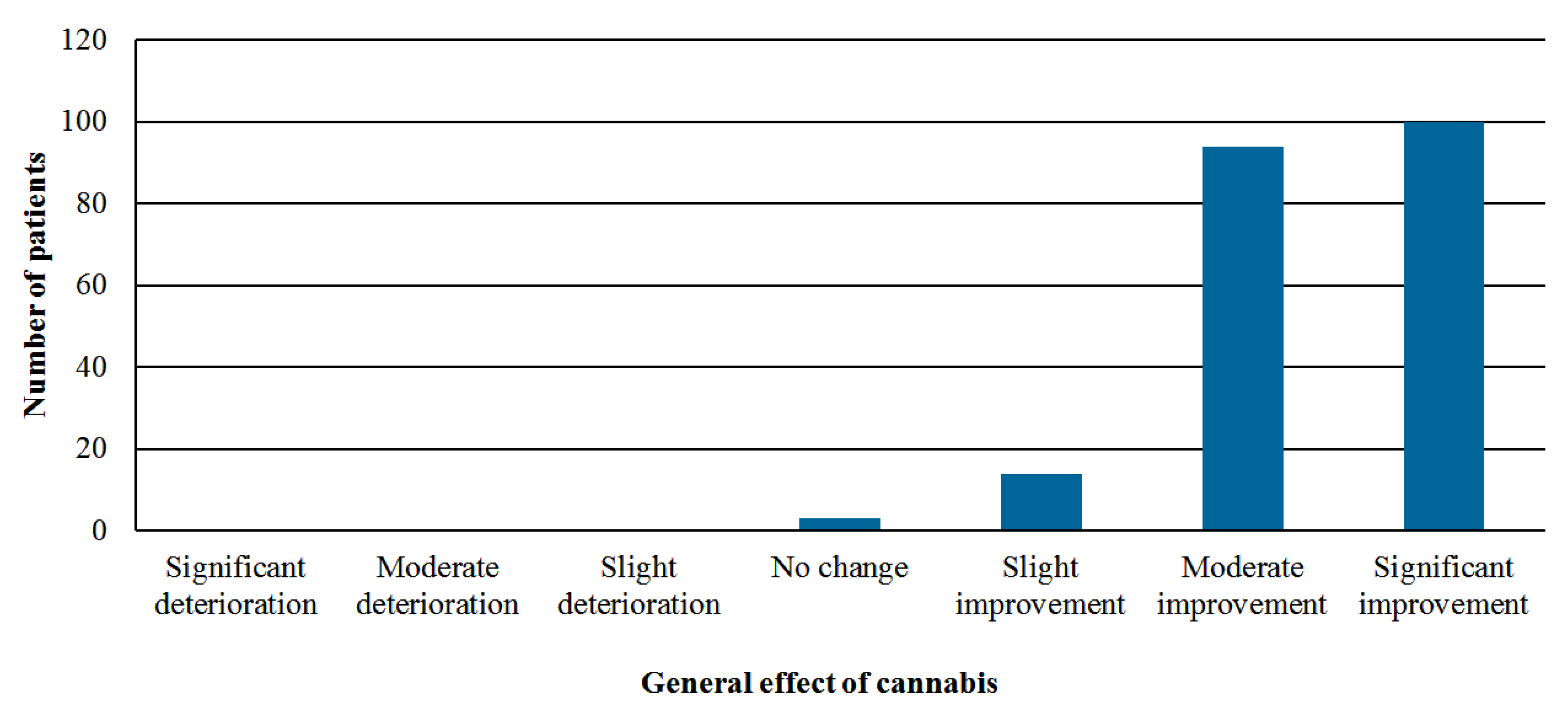
- Perception of the general effect of cannabis—global assessment by using the Likert scale with seven options: significant improvement, moderate improvement, slight improvement, no change, slight deterioration, moderate deterioration, or significant deterioration.
2.4. Statistical Analysis
3. Results
3.1. Cohort Characteristics
3.2. Safety Analysis
3.3. Effectiveness Analysis
3.4. Additional Regimens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Phillips, K.; Clauw, D.J. Central pain mechanisms in the rheumatic diseases: Future directions. Arthritis Rheumatol. 2013, 65, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Aaron, L.A.; Buchwald, D. A review of the evidence for overlap among unexplained clinical conditions. Ann. Intern. Med. 2001, 134, 868–881. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.; Lahr, B.D.; Wolfe, F.; Clauw, D.J.; Whipple, M.O.; Oh, T.H.; Barton, D.L.; St. Sauver, J. Prevalence of fibromyalgia: A population-based study in Olmsted County, Minnesota, utilizing the Rochester Epidemiology Project. Arthritis Care Res. 2013, 65, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Ross, K.; Anderson, J.; Russell, I.J.; Hebert, L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheumatol. 1995, 38, 19–28. [Google Scholar] [CrossRef]
- Macfarlane, G.J.; Kronisch, C.; Dean, L.E.; Atzeni, F.; Häuser, W.; Fluß, E.; Choy, E.; Kosek, E.; Amris, K.; Branco, J.; et al. EULAR revised recommendations for the management of fibromyalgia. Ann. Rheum. Dis. 2017, 76, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Fitzcharles, M.A.; Faregh, N.; Ste-Marie, P.A.; Shir, Y. Opioid use in fibromyalgia is associated with negative health related measures in a prospective cohort study. Pain Res. Treat. 2013, 2013, 898493. [Google Scholar] [CrossRef] [PubMed]
- Schleider, L.B.; Mechoulam, R.; Lederman, V.; Hilou, M.; Lencovsky, O.; Betzalel, O.; Shbiro, L.; Novack, V. Prospective analysis of safety and efficacy of medical cannabis in large unselected population of patients with cancer. Eur. J. Intern. Med. 2018, 49, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Abuhasira, R.; Schleider, L.B.; Mechoulam, R.; Novack, V. Epidemiological characteristics, safety and efficacy of medical cannabis in the elderly. Eur. J. Intern. Med. 2018, 49, 44–50. [Google Scholar] [CrossRef]
- Pacher, P.; Batkai, S.; Kunos, G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 2006, 58, 389–462. [Google Scholar] [CrossRef]
- Skrabek, R.Q.; Galimova, L.; Ethans, K.; Perry, D. Nabilone for the treatment of pain in fibromyalgia. J. Pain 2008, 9, 164–173. [Google Scholar] [CrossRef]
- Ware, M.A.; Fitzcharles, M.A.; Joseph, L.; Shir, Y. The effects of nabilone on sleep in fibromyalgia: Results of a randomized controlled trial. Anesth. Analg. 2010, 110, 604–610. [Google Scholar] [CrossRef]
- Fitzcharles, M.A.; Ste-Marie, P.A.; Häuser, W.; Clauw, D.J.; Jamal, S.; Karsh, J.; Landry, T.; Leclercq, S.; Mcdougall, J.J.; Shir, Y.; et al. Efficacy, tolerability, and safety of cannabinoid treatments in the rheumatic diseases: A systematic review of randomized controlled trials. Arthritis Care Res. 2016, 68, 681–688. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010, 62, 600–610. [Google Scholar] [CrossRef] [PubMed]
- MacCallum, C.A.; Russo, E.B. Practical considerations in medical cannabis administration and dosing. Eur. J. Intern. Med. 2018, 49, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Fiz, J.; Durán, M.; Capellà, D.; Carbonell, J.; Farré, M. Cannabis use in patients with fibromyalgia: Effect on symptoms relief and health-related quality of life. PLoS ONE 2011, 6, e18440. [Google Scholar] [CrossRef] [PubMed]
- Üçeyler, N.; Sommer, C.; Walitt, B.; Häuser, W. Anticonvulsants for Fibromyalgia; The Cochrane Library: London, UK, 2013. [Google Scholar]
- Lunn, M.; Hughes, R.A.; Wiffen, P.J. Duloxetine for Treating Painful Neuropathy, Chronic Pain or Fibromyalgia; The Cochrane Library: London, UK, 2014. [Google Scholar]
- Sexton, M.; Cuttler, C.; Finnell, J.S.; Mischley, L.K. A cross-sectional survey of medical cannabis users: Patterns of use and perceived efficacy. Cannabis Cannabinoid Res. 2016, 1, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Corroon, J.M., Jr.; Mischley, L.K.; Sexton, M. Cannabis as a substitute for prescription drugs—A cross-sectional study. J. Pain Res. 2017, 10, 989–998. [Google Scholar] [CrossRef]
- Piper, B.J.; DeKeuster, R.M.; Beals, M.L.; Cobb, C.M.; Burchman, C.A.; Perkinson, L.; Lynn, S.T.; Nichols, S.D.; Abess, A.T. Substitution of medical cannabis for pharmaceutical agents for pain, anxiety, and sleep. J. Psychopharmacol. 2017, 31, 569–575. [Google Scholar] [CrossRef]
- Ricardo Buenaventura, M.; Rajive Adlaka, M.; Nalini Sehgal, M. Opioid complications and side effects. Pain Physician 2008, 11, S105–S120. [Google Scholar]
- Portenoy, R.K.; Ahmed, E. Principles of opioid use in cancer pain. J. Clin. Oncol. 2014, 32, 1662–1670. [Google Scholar] [CrossRef]
- Choo, E.K.; Ewing, S.W.F.; Lovejoy, T.I. Opioids out, cannabis in: Negotiating the unknowns in patient care for chronic pain. JAMA 2016, 316, 1763–1764. [Google Scholar] [CrossRef] [PubMed]
- Ryan-Ibarra, S.; Induni, M.; Ewing, D. Prevalence of medical marijuana use in California, 2012. Drug Alcohol. Rev. 2015, 34, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Nunberg, H.; Kilmer, B.; Pacula, R.L.; Burgdorf, J. An Analysis of Applicants Presenting to a Medical Marijuana Specialty Practice in California. J. Drug Policy Anal. 2011, 4. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.T.; Atzeni, F.; Beasley, M.; Fluss, E.; Sarzi-Puttini, P.; Macfarlane, G.J. The prevalence of fibromyalgia in the general population: A comparison of the American College of Rheumatology 1990, 2010, and modified 2010 classification criteria. Arthritis Rheumatol. 2015, 67, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Walitt, B.; Nahin, R.L.; Katz, R.S.; Bergman, M.J.; Wolfe, F. The Prevalence and Characteristics of Fibromyalgia in the 2012 National Health Interview Survey. PLoS ONE 2015, 10, e0138024. [Google Scholar] [CrossRef] [PubMed]
- Hazekamp, A.; Heerdink, E.R. The prevalence and incidence of medicinal cannabis on prescription in The Netherlands. Eur. J. Clin. Pharmacol. 2013, 69, 1575–1580. [Google Scholar] [CrossRef] [PubMed]
- Kaskie, B.; Ayyagari, P.; Milavetz, G.; Shane, D.; Arora, K. The Increasing Use of Cannabis Among Older Americans: A Public Health Crisis or Viable Policy Alternative? Gerontologist 2017, 57, 1166–1172. [Google Scholar] [CrossRef]
- Goldenberg, D.; Mayskiy, M.; Mossey, C.; Ruthazer, R.; Schmid, C. A randomized, double-blind crossover trial of fluoxetine and amitriptyline in the treatment of fibromyalgia. Arthritis Rheum. 1996, 39, 1852–1859. [Google Scholar] [CrossRef]
- Reiman, A. Medical cannabis patients: Patient profiles and health care utilization patterns. Complement. Health Pract. Rev. 2007, 12, 31–50. [Google Scholar] [CrossRef]
- Harris, D.; Jones, R.T.; Shank, R.; Nath, R.; Fernandez, E.; Goldstein, K.; Mendelson, J. Self-reported marijuana effects and characteristics of 100 San Francisco medical marijuana club members. J. Addict. Dis. 2000, 19, 89–103. [Google Scholar] [CrossRef]
- Wang, T.; Collet, J.P.; Shapiro, S.; Ware, M.A. Adverse effects of medical cannabinoids: A systematic review. CMAJ 2008, 178, 1669–1678. [Google Scholar] [CrossRef] [PubMed]
- Ware, M.A.; Wang, T.; Shapiro, S.; Collet, J.P.; Boulanger, A.; Esdaile, J.M.; Gordon, A.; Lynch, M.; Moulin, D.E.; O’Connell, C. Cannabis for the management of pain: Assessment of safety study (COMPASS). J. Pain 2015, 16, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Casarett, D. The Achilles Heel of Medical Cannabis Research—Inadequate Blinding of Placebo-Controlled Trials. JAMA Intern. Med. 2018, 178, 9–10. [Google Scholar] [CrossRef] [PubMed]
| Variable | Number of Patients (N = 367) |
|---|---|
| Age (years), mean ± SD | 52.9 (15.1) |
| Age groups, n (%) | |
| 40 years and below | 75 (20.4) |
| 40–60 years | 181 (49.3) |
| 60 years and above | 111 (30.2) |
| Females, n (%) | 301 (82.0) |
| BMI (kg/m2), mean ± SD | 28.6 (18.2) |
| Work status: works regularly | 59 (16.1) |
| Part-time work | 53 (14.4) |
| Unemployed/retired | 233 (63.4) |
| Other | 22 (5.9) |
| Driving a car, n (%) | 231 (62.9) |
| Approved monthly dosage of cannabis ≤ 20 g, n (%) | 328 (89.4%) |
| Approved route of administration, n (%) | Oil 74 (20.2) Inflorescence 247 (67.3) Oil + Inflorescence 44 (12.0) |
| Previous experience with cannabis, n (%) | 166 (45.2) |
| Cigarette smokers, n (%) | 137 (37.3) |
| Number of regularly used medications, median (i.q range) | 5.0 (3.0–8.0) |
| Number of regularly used medications for fibromyalgia, median (i.q range) | 1.0 (1.0–2.0) |
| Treatment indication: primary fibromyalgia, n (%) | 283 (77.1) |
| Cancer, n (%) | 35 (9.5) |
| PTSD, n (%) | 22 (6.0)) |
| Other, n (%) | 27 (7.4) |
| Years of chronic pain, median (i.q. range) | 7.0 (3.0–13.0) |
| Type of pain: Daily, n (%) | 320 (87.2) |
| Episodic, n (%) | 47 (12.8) |
| Variable | p Value | Odds Ratio | 95% Confidence Interval |
|---|---|---|---|
| Age > 60 years | 0.01 | 0.34 | 0.16–0.72 |
| Concerns about cannabis-prior to treatment initiation | 0.01 | 0.36 | 0.16–0.80 |
| Spasticity | 0.03 | 2.26 | 1.08–4.72 |
| Previous experience with cannabis | 0.04 | 2.46 | 1.06–5.74 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagy, I.; Bar-Lev Schleider, L.; Abu-Shakra, M.; Novack, V. Safety and Efficacy of Medical Cannabis in Fibromyalgia. J. Clin. Med. 2019, 8, 807. https://doi.org/10.3390/jcm8060807
Sagy I, Bar-Lev Schleider L, Abu-Shakra M, Novack V. Safety and Efficacy of Medical Cannabis in Fibromyalgia. Journal of Clinical Medicine. 2019; 8(6):807. https://doi.org/10.3390/jcm8060807
Chicago/Turabian StyleSagy, Iftach, Lihi Bar-Lev Schleider, Mahmoud Abu-Shakra, and Victor Novack. 2019. "Safety and Efficacy of Medical Cannabis in Fibromyalgia" Journal of Clinical Medicine 8, no. 6: 807. https://doi.org/10.3390/jcm8060807
APA StyleSagy, I., Bar-Lev Schleider, L., Abu-Shakra, M., & Novack, V. (2019). Safety and Efficacy of Medical Cannabis in Fibromyalgia. Journal of Clinical Medicine, 8(6), 807. https://doi.org/10.3390/jcm8060807






