Long-Term Hydroxychloroquine Therapy and Risk of Coronary Artery Disease in Patients with Systemic Lupus Erythematosus
Abstract
1. Introduction
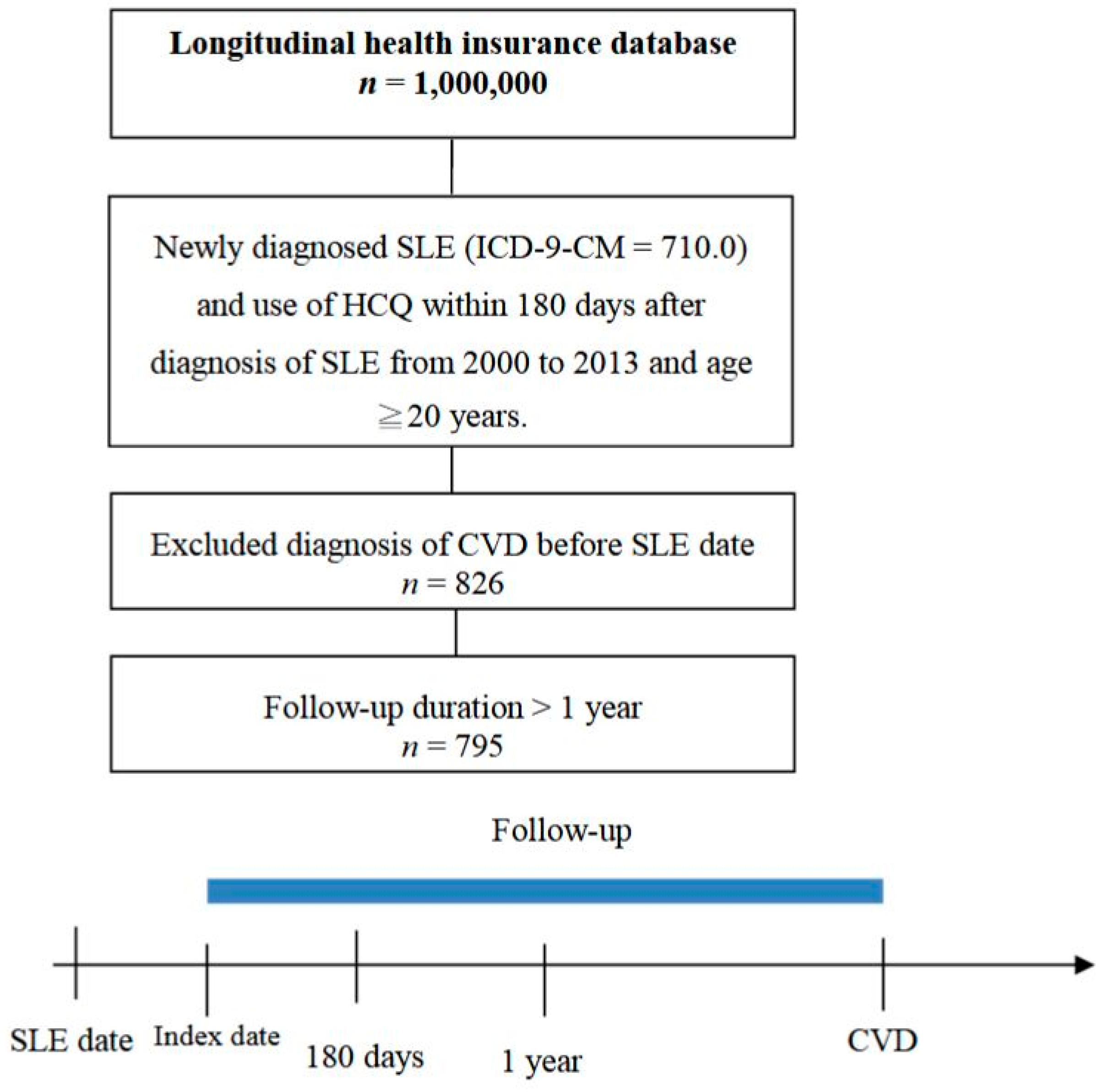
2. Experimental Section
2.1. Methodology
2.2. Statistical Analysis
3. Results
3.1. SLE Patient Characteristics
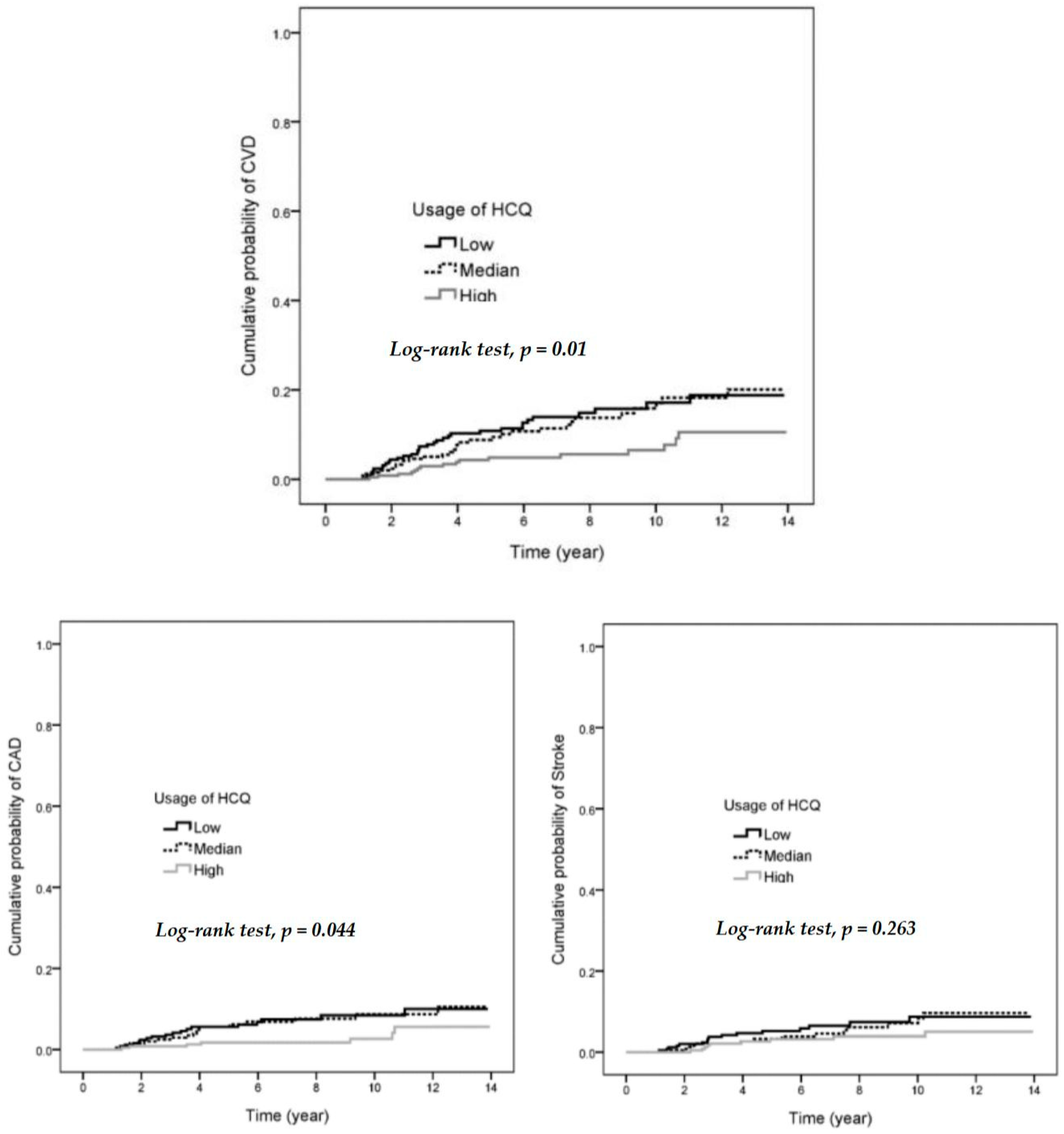
3.2. A Decreased Hazard Ratio (HR) for CVD in SLE Patients with High Usage, High Cumulative Dose of HCQ or High MPR of HCQ
3.3. Decreased HR for CAD in SLE Patients with High Usage of HCQ, High Cumulative Dose of HCQ, or High MPR of HCQ
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tsokos, G.C. Systemic lupus erythematosus. N. Engl. J. Med. 2011, 365, 2110–2121. [Google Scholar] [CrossRef] [PubMed]
- Bartels, C.M.; Buhr, K.A.; Goldberg, J.W.; Bell, C.L.; Visekruna, M.; Nekkanti, S.; Greenlee, R.T. Mortality and cardiovascular burden of systemic lupus erythematosus in a US population-based cohort. J. Rheumatol. 2014, 41, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Magder, L.S.; Petri, M. Incidence of and risk factors for adverse cardiovascular events among patients with systemic lupus erythematosus. Am. J. Epidemiol. 2012, 176, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.M.; Wang, X.P.; Cheng, Q.Y.; Zhao, Y.L.; Zhang, T.P.; Li, B.Z.; Ye, D.Q. A meta-analysis of cardiovascular events in systemic lupus erythematosus. Immunol. Investig. 2019, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.G.; Alghamdi, A.A.; ALjahlan, M.A.; Al-Homood, I.A. Echocardiographic findings in asymptomatic systemic lupus erythematosus patients. Clin. Rheumatol. 2017, 36, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Bourre-Tessier, J.; Huynh, T.; Clarke, A.E.; Bernatsky, S.; Joseph, L.; Belisle, P.; Pineau, C.A. Features associated with cardiac abnormalities in systemic lupus erythematosus. Lupus 2011, 20, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Pons-Estel, G.J.; Andreoli, L.; Scanzi, F.; Cervera, R.; Tincani, A. The antiphospholipid syndrome in patients with systemic lupus erythematosus. J. Autoimmun. 2017, 76, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Watad, A.; Abu Much, A.; Bracco, D.; Mahroum, N.; Comaneshter, D.; Cohen, A.D.; Amital, H. Association between ischemic heart disease and systemic lupus erythematosus—A large case-control study. Immunol. Res. 2017, 65, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.C.; Liu, H.R.; Leng, R.X.; Li, X.P.; Li, X.M.; Pan, H.F.; Ye, D.Q. Subclinical atherosclerosis in patients with systemic lupus erythematosus: A systemic review and meta-analysis. Autoimmun. Rev. 2016, 15, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Arkema, E.V.; Svenungsson, E.; Von Euler, M.; Sjowall, C.; Simard, J.F. Stroke in systemic lupus erythematosus: A Swedish population-based cohort study. Ann. Rheum. Dis. 2017, 76, 1544–1549. [Google Scholar] [CrossRef]
- Roman, M.J.; Shanker, B.A.; Davis, A.; Lockshin, M.D.; Sammaritano, L.; Simantov, R.; Crow, M.K.; Schwartz, J.E.; Paget, S.A.; Devereux, R.B.; et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N. Engl. J. Med. 2003, 349, 2399–2406. [Google Scholar] [CrossRef] [PubMed]
- Leonard, D.; Svenungsson, E.; Dahlqvist, J.; Alexsson, A.; Arlestig, L.; Taylor, K.E.; Sandling, J.K.; Bengtsson, C.; Frodlund, M.; Jonsen, A.; et al. Novel gene variants associated with cardiovascular disease in systemic lupus erythematosus and rheumatoid arthritis. Ann. Rheum. Dis. 2018, 77, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Wigren, M.; Svenungsson, E.; Mattisson, I.Y.; Gustafsson, J.T.; Gunnarsson, I.; Zickert, A.; Elvin, K.; Jensen-Urstad, K.; Bengtsson, A.; Gullstrand, B.; et al. Cardiovascular disease in systemic lupus erythematosus is associated with increased levels of biomarkers reflecting receptor-activated apoptosis. Atherosclerosis 2018, 270, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Croca, S.; Rahman, A. Atherosclerosis in systemic lupus erythematosus. Best Pract. Res. Clin. Rheumatol. 2017, 31, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.K.; Kim, D.; Won, S.; Lee, J.; Park, B.; Jang, E.J.; Bae, S.C.; Sung, Y.K. Impact of anti-rheumatic treatment on cardiovascular risk in Asian patients with rheumatoid arthritis. Semin. Arthritis Rheum. 2018, 47, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Fasano, S.; Pierro, L.; Pantano, I.; Iudici, M.; Valentini, G. Longterm hydroxychloroquine therapy and low-dose aspirin may have an additive effectiveness in the primary prevention of cardiovascular events in patients with systemic lupus erythematosus. J. Rheumatol. 2017, 44, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Rempenault, C.; Combe, B.; Barnetche, T.; Gaujoux-Viala, C.; Lukas, C.; Morel, J.; Hua, C. Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: A systematic review and meta-analysis. Ann. Rheum. Dis. 2018, 77, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, X.; Zhang, Y.; Kwong, J.S.; Li, L.; Xu, C.; Li, Q.; Sun, X.; Tian, H.; Li, S.; et al. Chloroquine and hydroxychloroquine are associated with reduced cardiovascular risk: A systematic review and meta-analysis. Drug Des. Dev. Ther. 2018, 12, 1685–1695. [Google Scholar] [CrossRef]
- Andrade, S.E.; Kahler, K.H.; Frech, F.; Chan, K.A. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol. Drug Saf. 2006, 15, 565–574. [Google Scholar] [CrossRef]
- Ponticelli, C.; Moroni, G. Hydroxychloroquine in systemic lupus erythematosus (SLE). Expert Opin. Drug Saf. 2017, 16, 411–419. [Google Scholar] [CrossRef]
- Gottschalk, T.A.; Tsantikos, E.; Hibbs, M.L. Pathogenic inflammation and its therapeutic targeting in systemic lupus erythematosus. Front. Immunol. 2015, 6, 550. [Google Scholar] [CrossRef] [PubMed]
- Miranda, S.; Billoir, P.; Damian, L.; Thiebaut, P.A.; Schapman, D.; Le Besnerais, M.; Jouen, F.; Galas, L.; Levesque, H.; Le Cam-Duchez, V.; et al. Hydroxychloroquine reverses the prothrombotic state in a mouse model of antiphospholipid syndrome: Role of reduced inflammation and endothelial dysfunction. PLoS ONE 2019, 14, e0212614. [Google Scholar] [CrossRef] [PubMed]
- Urbanski, G.; Caillon, A.; Poli, C.; Kauffenstein, G.; Begorre, M.A.; Loufrani, L.; Henrion, D.; Belizna, C. Hydroxychloroquine partially prevents endothelial dysfunction induced by anti-beta-2-GPI antibodies in an in vivo mouse model of antiphospholipid syndrome. PLoS ONE 2018, 13, e0206814. [Google Scholar] [CrossRef] [PubMed]
- Muller-Calleja, N.; Manukyan, D.; Canisius, A.; Strand, D.; Lackner, K.J. Hydroxychloroquine inhibits proinflammatory signalling pathways by targeting endosomal NADPH oxidase. Ann. Rheum. Dis. 2017, 76, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, K.; Breen, K.; Parmar, K.; Rand, J.H.; Wu, X.X.; Hunt, B.J. The effect of hydroxychloroquine on haemostasis, complement, inflammation and angiogenesis in patients with antiphospholipid antibodies. Rheumatology (Oxford) 2018, 57, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, Y.; Oeser, A.; Shintani, A.K.; Turner, E.; Olsen, N.; Fazio, S.; Linton, M.F.; Raggi, P.; Stein, C.M. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N. Engl. J. Med. 2003, 349, 2407–2415. [Google Scholar] [CrossRef]
- Floris, A.; Piga, M.; Mangoni, A.A.; Bortoluzzi, A.; Erre, G.L.; Cauli, A. Protective effects of hydroxychloroquine against accelerated atherosclerosis in systemic lupus erythematosus. Mediat. Inflamm. 2018, 2018, 3424136. [Google Scholar] [CrossRef]
- Morris, S.J.; Wasko, M.C.; Antohe, J.L.; Sartorius, J.A.; Kirchner, H.L.; Dancea, S.; Bili, A. Hydroxychloroquine use associated with improvement in lipid profiles in rheumatoid arthritis patients. Arthritis Care Res. (Hoboken) 2011, 63, 530–534. [Google Scholar] [CrossRef]
- Kerr, G.; Aujero, M.; Richards, J.; Sayles, H.; Davis, L.; Cannon, G.; Caplan, L.; Michaud, K.; Mikuls, T. Associations of hydroxychloroquine use with lipid profiles in rheumatoid arthritis: Pharmacologic implications. Arthritis Care Res. (Hoboken) 2014, 66, 1619–1626. [Google Scholar] [CrossRef]
- Ammirati, E.; Bozzolo, E.P.; Contri, R.; Baragetti, A.; Palini, A.G.; Cianflone, D.; Banfi, M.; Uboldi, P.; Bottoni, G.; Scotti, I.; et al. Cardiometabolic and immune factors associated with increased common carotid artery intima-media thickness and cardiovascular disease in patients with systemic lupus erythematosus. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 751–759. [Google Scholar] [CrossRef]
- Sazliyana, S.; Mohd Shahrir, M.S.; Kong, C.T.; Tan, H.J.; Hamidon, B.B.; Azmi, M.T. Implications of immunosuppressive agents in cardiovascular risks and carotid intima media thickness among lupus nephritis patients. Lupus 2011, 20, 1260–1266. [Google Scholar] [CrossRef]
- Tam, H.W.; Chen, C.M.; Leong, P.Y.; Chen, C.H.; Li, Y.C.; Wang, Y.H.; Lin, L.C.; Chiou, J.Y.; Wei, J.C. Methotrexate might reduce ischemic stroke in patients with rheumatoid arthritis: A population-based retrospective cohort study. Int. J. Rheum. Dis. 2018, 21, 1591–1599. [Google Scholar] [CrossRef] [PubMed]
- Bessant, R.; Duncan, R.; Ambler, G.; Swanton, J.; Isenberg, D.A.; Gordon, C.; Rahman, A. Prevalence of conventional and lupus-specific risk factors for cardiovascular disease in patients with systemic lupus erythematosus: A case-control study. Arthritis Rheum. 2006, 55, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Hanaoka, H.; Iida, H.; Kiyokawa, T.; Takakuwa, Y.; Kawahata, K. Hydroxychloroquine improves the disease activity and allows the reduction of the corticosteroid dose regardless of background treatment in japanese patients with systemic lupus erythematosus. Intern. Med. 2019, 58, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Shippey, E.A.; Wagler, V.D.; Collamer, A.N. Hydroxychloroquine: An old drug with new relevance. Clevel. Clin. J. Med. 2018, 85, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Kim, Y.Y.; Lee, H.; Park, S.H.; Kim, S.K.; Choe, J.Y. Risk of retinal toxicity in longterm users of hydroxychloroquine. J. Rheumatol. 2017, 44, 1674–1679. [Google Scholar] [CrossRef] [PubMed]
- Hartman, O.; Kovanen, P.T.; Lehtonen, J.; Eklund, K.K.; Sinisalo, J. Hydroxychloroquine for the prevention of recurrent cardiovascular events in myocardial infarction patients: Rationale and design of the OXI trial. Eur. Heart J. Cardiovasc. Pharmacother. 2017, 3, 92–97. [Google Scholar] [CrossRef]
| n | % | |
|---|---|---|
| Age | ||
| <30 | 208 | 26.2 |
| 30–45 | 318 | 40.0 |
| ≥45 | 269 | 33.8 |
| Mean ± SD | 40 ± 13.3 | |
| Sex | ||
| Female | 715 | 89.9 |
| Male | 80 | 10.1 |
| Hypertension | 54 | 6.8 |
| Hyperlipidemia | 22 | 2.8 |
| Chronic liver disease | 42 | 5.3 |
| Chronic kidney disease | 4 | 0.5 |
| COPD | 26 | 3.3 |
| Diabetes | 20 | 2.5 |
| Usage of HCQ (days) | ||
| Low (<105) | 264 | 33.2 |
| Median (105–318) | 265 | 33.3 |
| High (≥318) | 266 | 33.5 |
| Cumulative HCQ dose (mg) | ||
| Low (<30,800) | 263 | 33.1 |
| Median (30,800–100,267) | 267 | 33.6 |
| High (≥100,267) | 265 | 33.3 |
| MPR of HCQ | ||
| Low (<0.29) | 264 | 33.2 |
| Median (0.29–0.87) | 265 | 33.3 |
| High (≥0.87) | 266 | 33.5 |
| CVD Event | Observed Person–Years | Incidence (/1000 Person–Years) | HR | 95% CI | Adjusted HR † | 95% CI | |
|---|---|---|---|---|---|---|---|
| Age (years) | |||||||
| <30 | 7 | 1623 | 4.3 | 1 | 1 | ||
| 30–45 | 18 | 2146 | 8.4 | 1.95 | 0.81–4.66 | 1.85 | 0.77–4.44 |
| ≥45 | 56 | 1726 | 32.4 | 7.53 | 3.43–16.53 | 6.29 | 2.83–14.02 |
| Sex | |||||||
| Female | 70 | 5023 | 13.9 | 1 | 1 | ||
| Male | 11 | 472 | 23.3 | 1.68 | 0.89–3.18 | 1.24 | 0.65–2.38 |
| Hypertension | 16 | 279 | 57.3 | 4.55 | 2.62–7.88 | 3.08 | 1.65–5.74 |
| Hyperlipidemia | 4 | 132 | 30.4 | 2.11 | 0.77–5.75 | 0.68 | 0.23–2.01 |
| Chronic liver disease | 8 | 280 | 28.6 | 2.03 | 0.98–4.21 | 1.48 | 0.68–3.19 |
| COPD | 4 | 163 | 24.5 | 1.72 | 0.63–4.69 | 0.68 | 0.23–2.02 |
| Diabetes | 3 | 125 | 24.1 | 1.65 | 0.52–5.22 | 0.67 | 0.20–2.26 |
| Usage of HCQ (days) | |||||||
| Low (<105) | 34 | 1745 | 19.5 | 1 | 1 | ||
| Median (105–318) | 31 | 1787 | 17.3 | 0.90 | 0.55–1.47 | 0.76 | 0.46–1.25 |
| High (≥318) | 16 | 1962 | 8.2 | 0.42 | 0.23–0.76 | 0.38 | 0.21–0.70 |
| Cumulative HCQ dose (mg) | |||||||
| Low (<308,00) | 33 | 1750 | 18.9 | 1 | 1 | ||
| Median (308,00–100,267) | 30 | 1754 | 17.1 | 0.92 | 0.56–1.51 | 0.92 | 0.56–1.52 |
| High (≥100,267) | 18 | 1991 | 9.0 | 0.48 | 0.27–0.86 | 0.42 | 0.23–0.77 |
| N | CVD Event | HR | 95% CI | Adjusted HR † | 95% CI | |
|---|---|---|---|---|---|---|
| MPR of HCQ | ||||||
| Low (<0.29) | 264 | 34 | 1 | 1 | ||
| Median (0.29–0.87) | 265 | 31 | 0.90 | 0.55–1.47 | 0.77 | 0.47–1.27 |
| High (≥0.87) | 266 | 16 | 0.42 | 0.23–0.76 | 0.38 | 0.21–0.70 |
| n | Event | HR | 95% CI | Adjusted HR † | 95% CI | |
|---|---|---|---|---|---|---|
| CAD | ||||||
| Usage of HCQ (days) | ||||||
| Low (<105) | 264 | 18 | 1 | 1 | ||
| Median (105–318) | 265 | 17 | 0.95 | 0.49–1.84 | 0.71 | 0.36–1.40 |
| High (≥318) | 266 | 7 | 0.36 | 0.15–0.87 | 0.31 | 0.12–0.76 |
| Cumulative HCQ dose (mg) | ||||||
| Low (<30,800) | 263 | 17 | 1 | 1 | ||
| Median (30,800–100,267) | 267 | 19 | 1.16 | 0.60–2.23 | 1.14 | 0.58–2.24 |
| High (≥100,267) | 265 | 6 | 0.32 | 0.13–0.81 | 0.25 | 0.09–0.66 |
| MPR of HCQ | ||||||
| Low (<0.29) | 264 | 18 | 1 | 1 | ||
| Median (0.29–0.87) | 265 | 17 | 0.95 | 0.49–1.84 | 0.71 | 0.36–1.40 |
| High (≥0.87) | 266 | 7 | 0.36 | 0.15–0.87 | 0.31 | 0.12–0.76 |
| Stroke | ||||||
| Usage of HCQ (days) | ||||||
| Low (<105) | 264 | 16 | 1 | 1 | ||
| Median (105–318) | 265 | 14 | 0.88 | 0.43–1.80 | 0.82 | 0.39–1.70 |
| High ≥318) | 266 | 9 | 0.52 | 0.23–1.17 | 0.51 | 0.22–1.16 |
| Cumulative HCQ dose (mg) | ||||||
| Low (<30,800) | 263 | 16 | 1 | 1 | ||
| Median (30,800–100,267) | 267 | 11 | 0.68 | 0.32–1.47 | 0.68 | 0.31–1.46 |
| High (≥100,267) | 265 | 12 | 0.69 | 0.33–1.46 | 0.67 | 0.31–1.42 |
| MPR of HCQ | ||||||
| Low (<0.29) | 264 | 16 | 1 | 1 | ||
| Median (0.29–0.87) | 265 | 14 | 0.88 | 0.43–1.80 | 0.82 | 0.39–1.70 |
| High (≥0.87) | 266 | 9 | 0.52 | 0.23–1.17 | 0.51 | 0.22–1.16 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.-H.; Leong, P.-Y.; Sia, S.-K.; Wang, Y.-H.; Wei, J.C.-C. Long-Term Hydroxychloroquine Therapy and Risk of Coronary Artery Disease in Patients with Systemic Lupus Erythematosus. J. Clin. Med. 2019, 8, 796. https://doi.org/10.3390/jcm8060796
Yang D-H, Leong P-Y, Sia S-K, Wang Y-H, Wei JC-C. Long-Term Hydroxychloroquine Therapy and Risk of Coronary Artery Disease in Patients with Systemic Lupus Erythematosus. Journal of Clinical Medicine. 2019; 8(6):796. https://doi.org/10.3390/jcm8060796
Chicago/Turabian StyleYang, Deng-Ho, Pui-Ying Leong, Sung-Kien Sia, Yu-Hsun Wang, and James Cheng-Chung Wei. 2019. "Long-Term Hydroxychloroquine Therapy and Risk of Coronary Artery Disease in Patients with Systemic Lupus Erythematosus" Journal of Clinical Medicine 8, no. 6: 796. https://doi.org/10.3390/jcm8060796
APA StyleYang, D.-H., Leong, P.-Y., Sia, S.-K., Wang, Y.-H., & Wei, J. C.-C. (2019). Long-Term Hydroxychloroquine Therapy and Risk of Coronary Artery Disease in Patients with Systemic Lupus Erythematosus. Journal of Clinical Medicine, 8(6), 796. https://doi.org/10.3390/jcm8060796








