Carbohydrate-Rich Diet Is Associated with Increased Risk of Incident Chronic Kidney Disease in Non-Diabetic Subjects
Abstract
1. Introduction
2. Materials and Methods
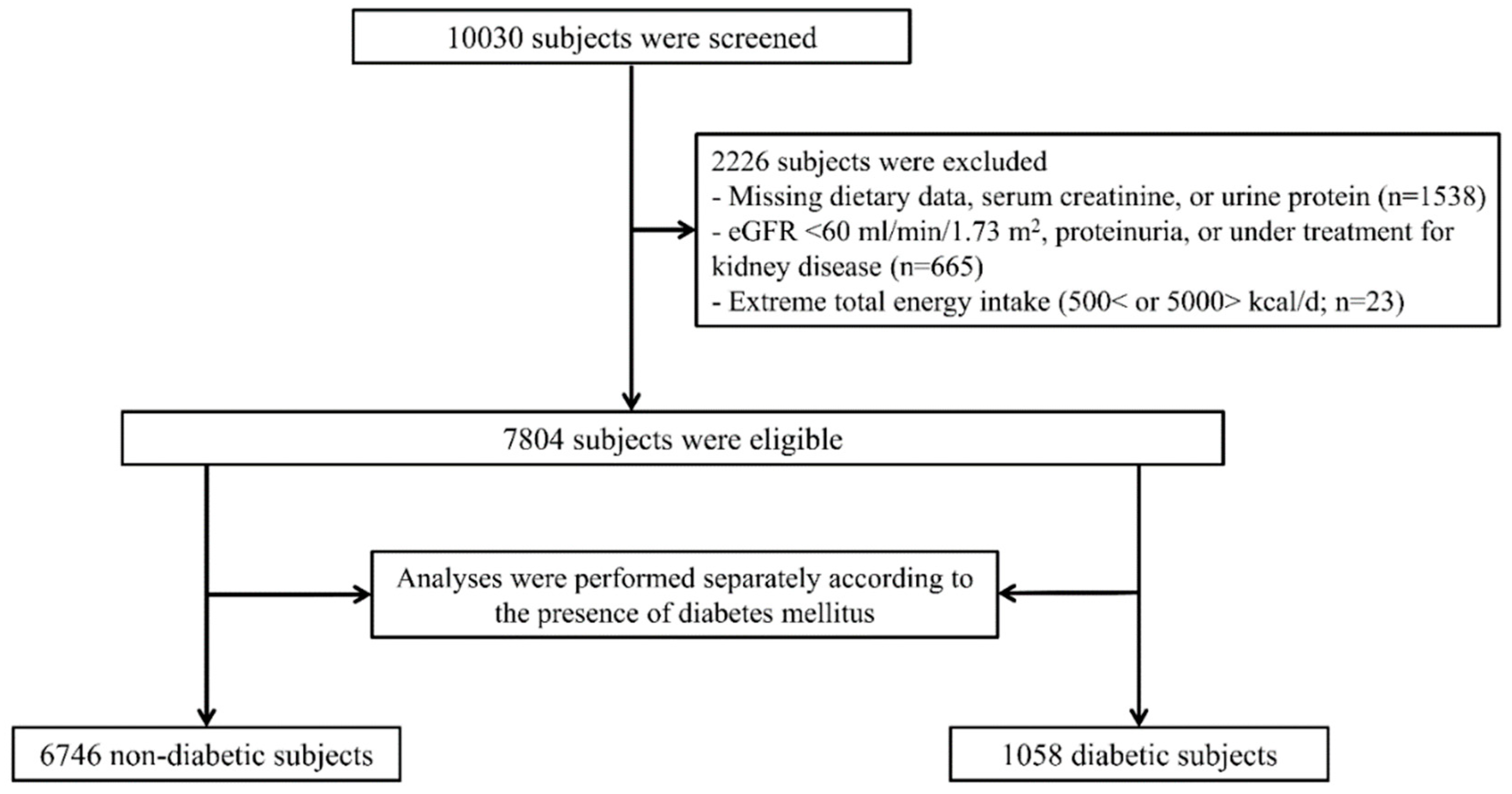
2.1. Study Design and Participants
2.2. Data Collection and Measurements
2.3. Dietary Intake Measurements
2.4. Study Endpoint
2.5. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Development of Incident CKD
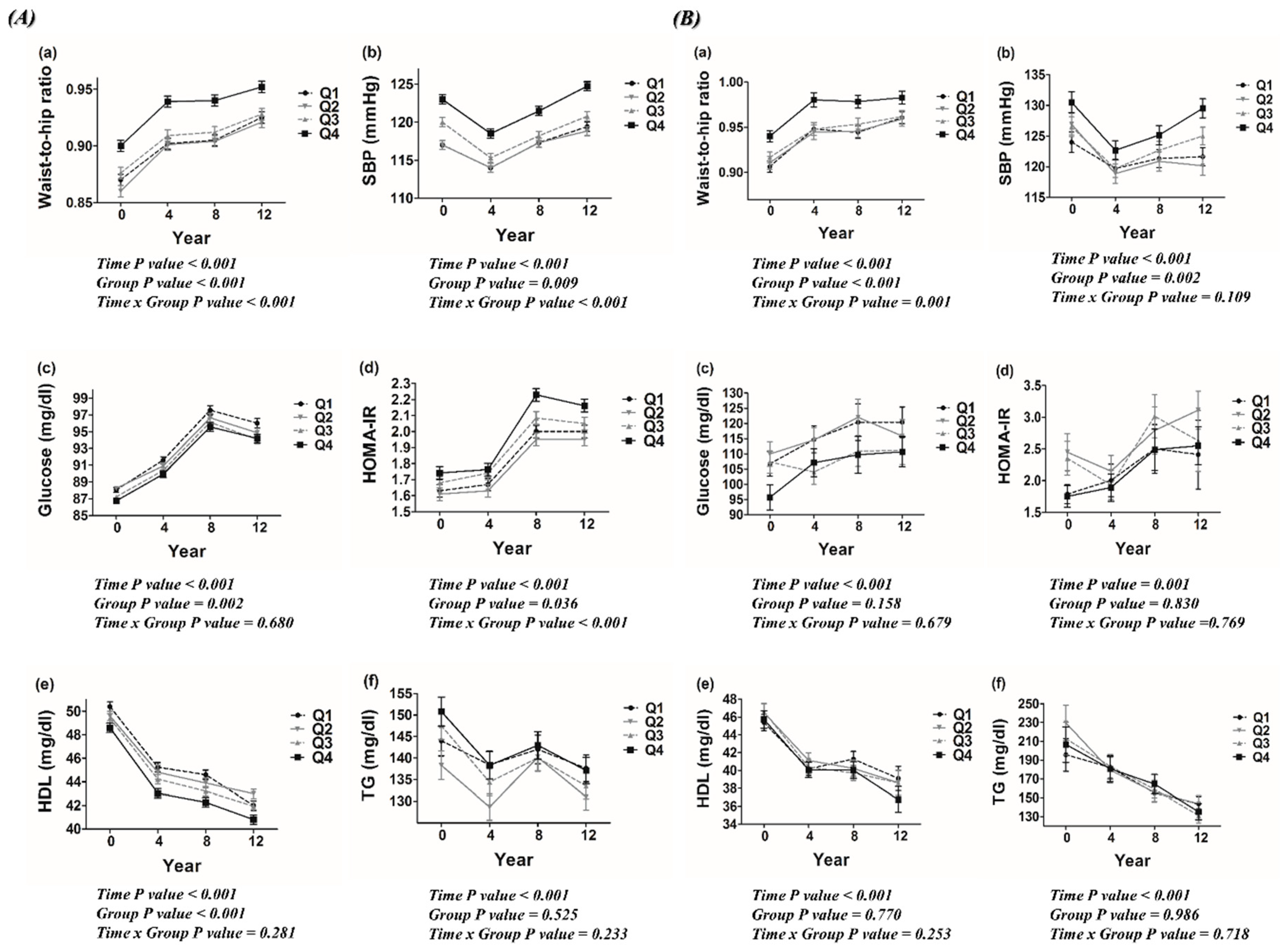
3.3. Metabolic Profiles According to Dietary Carbohydrate Density
3.4. Risk of Incident CKD According to Dietary Carbohydrate Intake
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the global burden of disease study 2013. Lancet 2015, 385, 117–171. [Google Scholar] [CrossRef]
- Sacks, F.M.; Bray, G.A.; Carey, V.J.; Smith, S.R.; Ryan, D.H.; Anton, S.D.; McManus, K.; Champagne, C.M.; Bishop, L.M.; Laranjo, N.; et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N. Engl. J. Med. 2009, 360, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, A.J.; Nordmann, A.; Briel, M.; Keller, U.; Yancy, W.S., Jr.; Brehm, B.J.; Bucher, H.C. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: A meta-analysis of randomized controlled trials. Arch. Intern. Med. 2006, 166, 285–293. [Google Scholar] [CrossRef]
- Liu, S.; Manson, J.E.; Stampfer, M.J.; Holmes, M.D.; Hu, F.B.; Hankinson, S.E.; Willett, W.C. Dietary glycemic load assessed by food-frequency questionnaire in relation to plasma high-density-lipoprotein cholesterol and fasting plasma triacylglycerols in postmenopausal women. Am. J. Clin. Nutr. 2001, 73, 560–566. [Google Scholar] [CrossRef]
- Mente, A.; Dehghan, M.; Rangarajan, S.; McQueen, M.; Dagenais, G.; Wielgosz, A.; Lear, S.; Li, W.; Chen, H.; Yi, S.; et al. Association of dietary nutrients with blood lipids and blood pressure in 18 countries: A cross-sectional analysis from the pure study. Lancet Diabetes Endocrinol. 2017, 5, 774–787. [Google Scholar] [CrossRef]
- Boden, G.; Sargrad, K.; Homko, C.; Mozzoli, M.; Stein, T.P. Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann. Intern. Med. 2005, 142, 403–411. [Google Scholar] [CrossRef]
- Jeppesen, J.; Schaaf, P.; Jones, C.; Zhou, M.Y.; Chen, Y.D.; Reaven, G.M. Effects of low-fat, high-carbohydrate diets on risk factors for ischemic heart disease in postmenopausal women. Am. J. Clin. Nutr. 1997, 65, 1027–1033. [Google Scholar] [CrossRef]
- Frost, G.; Leeds, A.A.; Dore, C.J.; Madeiros, S.; Brading, S.; Dornhorst, A. Glycaemic index as a determinant of serum HDL-cholesterol concentration. Lancet 1999, 353, 1045–1048. [Google Scholar] [CrossRef]
- Saris, W.H. Sugars, energy metabolism, and body weight control. Am. J. Clin. Nutr. 2003, 78, 850S–857S. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Chiriboga, D.E.; Olendzki, B.C.; Hebert, J.R.; Li, W.; Leung, K.; Hafner, A.R.; Ockene, I.S. Association between carbohydrate intake and serum lipids. J. Am. Coll. Nutr. 2006, 25, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Shai, I.; Schwarzfuchs, D.; Henkin, Y.; Shahar, D.R.; Witkow, S.; Greenberg, I.; Golan, R.; Fraser, D.; Bolotin, A.; Vardi, H.; et al. Weight loss with a low-carbohydrate, mediterranean, or low-fat diet. N. Engl. J. Med. 2008, 359, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Kurella, M.; Lo, J.C.; Chertow, G.M. Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. J. Am. Soc. Nephrol. 2005, 16, 2134–2140. [Google Scholar] [CrossRef] [PubMed]
- Grubbs, V.; Lin, F.; Vittinghoff, E.; Shlipak, M.G.; Peralta, C.A.; Bansal, N.; Jacobs, D.R.; Siscovick, D.S.; Lewis, C.E.; Bibbins-Domingo, K. Body mass index and early kidney function decline in young adults: A longitudinal analysis of the cardia (coronary artery risk development in young adults) study. Am. J. Kidney Dis. 2014, 63, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.H.; Yun, H.R.; Joo, Y.S.; Kim, J.; Lee, S.; Lee, C.; Park, K.S.; Park, J.T.; Chang, T.I.; Kang, E.W.; et al. Changes in obese metabolic phenotypes over time and risk of incident chronic kidney disease. Diabetes Obes. Metab. 2018, 20, 2778–2791. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.H.; Yadav, D.; Kim, J.S.; Son, J.W.; Choi, E.; Kim, S.H.; Shin, C.; Sung, K.C.; Kim, J.Y. An association of metabolic syndrome and chronic kidney disease from a 10-year prospective cohort study. Metabolism 2017, 67, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Oyabu, C.; Hashimoto, Y.; Fukuda, T.; Tanaka, M.; Asano, M.; Yamazaki, M.; Fukui, M. Impact of low-carbohydrate diet on renal function: A meta-analysis of over 1000 individuals from nine randomised controlled trials. Br. J. Nutr. 2016, 116, 632–638. [Google Scholar] [CrossRef]
- Juraschek, S.P.; Chang, A.R.; Appel, L.J.; Anderson, C.A.; Crews, D.C.; Thomas, L.; Charleston, J.; Miller, E.R., III. Effect of glycemic index and carbohydrate intake on kidney function in healthy adults. BMC Nephrol. 2016, 17, 70. [Google Scholar] [CrossRef]
- Kim, Y.; Han, B.G. Cohort profile: The Korean genome and epidemiology study (KoGES) consortium. Int. J. Epidemiol. 2017, 46, 1350. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Kwon, E.; Shim, J.E.; Park, M.K.; Joo, Y.; Kimm, K.; Park, C.; Kim, D.H. Validation and reproducibility of food frequency questionnaire for korean genome epidemiologic study. Eur. J. Clin. Nutr. 2007, 61, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.N.; Ogden, L.G.; Foster, G.D.; Klein, S.; Stein, R.; Miller, B.; Hill, J.O.; Brill, C.; Bailer, B.; Rosenbaum, D.R.; et al. Comparative effects of low-carbohydrate high-protein versus low-fat diets on the kidney. Clin. J. Am. Soc. Nephrol. 2012, 7, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Juraschek, S.P.; Appel, L.J.; Anderson, C.A.; Miller, E.R., 3rd. Effect of a high-protein diet on kidney function in healthy adults: Results from the omniheart trial. Am. J. Kidney Dis. 2013, 61, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, B.; Harris, D.C.; Flood, V.M.; Burlutsky, G.; Brand-Miller, J.; Mitchell, P. Carbohydrate nutrition is associated with the 5-year incidence of chronic kidney disease. J. Nutr. 2011, 141, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, M.; Mente, A.; Zhang, X.; Swaminathan, S.; Li, W.; Mohan, V.; Iqbal, R.; Kumar, R.; Wentzel-Viljoen, E.; Rosengren, A.; et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (pure): A prospective cohort study. Lancet 2017, 390, 2050–2062. [Google Scholar] [CrossRef]
- Dickinson, S.; Hancock, D.P.; Petocz, P.; Ceriello, A.; Brand-Miller, J. High-glycemic index carbohydrate increases nuclear factor-kappab activation in mononuclear cells of young, lean healthy subjects. Am. J. Clin. Nutr. 2008, 87, 1188–1193. [Google Scholar] [PubMed]
- Giugliano, D.; Ceriello, A.; Esposito, K. The effects of diet on inflammation: Emphasis on the metabolic syndrome. J. Am. Coll. Cardiol. 2006, 48, 677–685. [Google Scholar] [CrossRef]
- Williams, S.B.; Goldfine, A.B.; Timimi, F.K.; Ting, H.H.; Roddy, M.A.; Simonson, D.C.; Creager, M.A. Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation 1998, 97, 1695–1701. [Google Scholar] [CrossRef]
- Hu, Y.; Block, G.; Norkus, E.P.; Morrow, J.D.; Dietrich, M.; Hudes, M. Relations of glycemic index and glycemic load with plasma oxidative stress markers. Am. J. Clin. Nutr. 2006, 84, 70–76, quiz 266-267. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodpoor, F.; Rahbar Saadat, Y.; Barzegari, A.; Ardalan, M.; Zununi Vahed, S. The impact of gut microbiota on kidney function and pathogenesis. Biomed. Pharmacother. 2017, 93, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Rhee, E.P.; Clish, C.B.; Ghorbani, A.; Larson, M.G.; Elmariah, S.; McCabe, E.; Yang, Q.; Cheng, S.; Pierce, K.; Deik, A.; et al. A combined epidemiologic and metabolomic approach improves ckd prediction. J. Am. Soc. Nephrol. 2013, 24, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.D. Diet and gut microbiota in health and disease. Nestle Nutr. Inst. Workshop Ser. 2017, 88, 117–126. [Google Scholar]
- Do, M.H.; Lee, E.; Oh, M.J.; Kim, Y.; Park, H.Y. High-glucose or -fructose diet cause changes of the gut microbiota and metabolic disorders in mice without body weight change. Nutrients 2018, 10, 761. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.; Thompson, C.H.; Luscombe-Marsh, N.D.; Noakes, M.; Buckley, J.D.; Wittert, G.A.; Brinkworth, G.D. Long-term effects of a very low carbohydrate compared with a high carbohydrate diet on renal function in individuals with type 2 diabetes: A randomized trial. Medicine (Baltimore) 2015, 94, e2181. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, H.J.; Davis, R.E.; Davies, J.S. A critical review of low-carbohydrate diets in people with type 2 diabetes. Diabet Med. 2016, 33, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.; Yang, C.W. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Moin, S.; Gondal, G.M.; Bano, U. Risk of development of chronic kidney disease in patients with type 2 diabetes having metabolic syndrome. J. Coll. Physicians Surg. Pak. 2008, 18, 472–476. [Google Scholar]
- Lanktree, M.B.; Theriault, S.; Walsh, M.; Pare, G. HDL cholesterol, LDL cholesterol, and triglycerides as risk factors for CKD: A mendelian randomization study. Am. J. Kidney Dis. 2018, 71, 166–172. [Google Scholar] [CrossRef]
- Yang, Y.J.; Kim, M.K.; Hwang, S.H.; Ahn, Y.; Shim, J.E.; Kim, D.H. Relative validities of 3-day food records and the food frequency questionnaire. Nutr. Res. Pract. 2010, 4, 142–148. [Google Scholar] [CrossRef] [PubMed]

| Variables | Total (n = 6746) | Quartiles of Dietary Carbohydrate Density | Pa | Pb | |||
|---|---|---|---|---|---|---|---|
| Q1 (n = 1686) <67.8 | Q2 (n = 1687) 67.8–71.9 | Q3 (n = 1687) 71.9–75.8 | Q4 (n = 1686) >75.8 | ||||
| Nutrients (per day) | |||||||
| Total energy intake (kcal) | 1883.2 ± 518.0 | 2136.4 ± 587.1 | 1943.3 ± 453.7 | 1798.0 ± 429.6 | 1655.3 ± 460.2 | <0.001 | <0.001 |
| Carbohydrate density (%) | 71.5 ± 6.0 | 63.7 ± 3.9 | 69.9 ± 1.2 | 73.8 ± 1.1 | 78.6 ± 2.2 | <0.001 | <0.001 |
| Protein density (%) | 13.3 ± 2.0 | 15.5 ± 1.7 | 13.7 ± 1.2 | 12.6 ± 1.0 | 11.2 ± 1.1 | <0.001 | <0.001 |
| Fat density (%) | 14.1 ± 4.7 | 20.1 ± 3.1 | 15.4 ± 1.5 | 12.4 ± 1.4 | 8.6 ± 1.9 | <0.001 | <0.001 |
| Demographic data | |||||||
| Age (years) | 51.3 ± 8.6 | 48.3 ± 7.4 | 50.0 ± 8.1 | 51.5 ± 8.4 | 55.5 ± 8.7 | <0.001 | <0.001 |
| Male (%) | 3182 (47.2) | 973 (57.7) | 895 (53.1) | 767 (45.5) | 547 (32.4) | <0.001 | <0.001 |
| SBP (mmHg) | 120.0 ± 17.8 | 117.4 ± 16.4 | 118.1 ± 16.9 | 120.7 ± 18.3 | 123.9 ± 18.6 | <0.001 | <0.001 |
| DBP (mmHg) | 79.8 ± 11.3 | 79.2 ± 11.3 | 78.9 ± 11.4 | 80.0 ± 11.2 | 81.1 ± 11.3 | <0.001 | <0.001 |
| Body mass index (kg/m2) | 24.6 ± 2.9 | 24.5 ± 2.8 | 24.5 ± 2.9 | 24.6 ± 3.0 | 24.6 ± 3.0 | 0.471 | 0.175 |
| Waist-to-hip ratio | 0.88 ± 0.07 | 0.86 ± 0.07 | 0.86 ± 0.07 | 0.87 ± 0.07 | 0.90 ± 0.07 | <0.001 | <0.001 |
| Education (%) | <0.001 | <0.001 | |||||
| Low | 2074 (30.9) | 268 (16.0) | 344 (20.4) | 544 (32.4) | 918 (55.0) | ||
| Intermediate | 3698 (55.1) | 1069 (63.7) | 1032 (61.3) | 952 (56.7) | 645 (38.7) | ||
| High | 935 (13.9) | 340 (20.3) | 307 (18.2) | 183 (10.9) | 105 (6.3) | ||
| Income (%) | <0.001 | <0.001 | |||||
| Low | 2152 (32.4) | 293 (17.5) | 395 (23.7) | 519 (31.3) | 945 (57.1) | ||
| Intermediate | 1993 (30.0) | 515 (30.8) | 519 (31.1) | 543 (32.8) | 416 (20.9) | ||
| High | 2505 (37.6) | 862 (51.6) | 756 (45.3) | 594 (35.9) | 293 (17.7) | ||
| Marriage (yes) | 6130 (91.2) | 1586 (94.3) | 1569 (93.3) | 1533 (91.1) | 1442 (85.8) | <0.001 | <0.001 |
| Alcohol (%) | 3647 (54.2) | 1130 (67.1) | 990 (59.0) | 892 (52.9) | 635 (37.9) | <0.001 | <0.001 |
| Smoking (%) | 2691 (40.2) | 842 (50.2) | 749 (44.6) | 637 (38.0) | 463 (27.8) | <0.001 | <0.001 |
| Exercise (MET, k) | 9.8 ± 6.3 | 9.3 ± 6.0 | 9.2 ± 5.8 | 9.8 ± 6.4 | 10.9 ± 6.9 | <0.001 | <0.001 |
| Comorbidities | |||||||
| Hypertension | 2470 (36.6) | 541 (32.2) | 552 (32.7) | 611 (36.2) | 766 (45.4) | <0.001 | <0.001 |
| CVD | 163 (2.4) | 31 (1.8) | 32 (1.9) | 35 (2.1) | 65 (3.9) | <0.001 | <0.001 |
| Dyslipidemia | 148 (2.2) | 39 (2.3) | 42 (2.5) | 40 (2.4) | 27 (1.6) | 0.253 | 0.149 |
| Variables | Total (n = 1058) | Quartiles of Dietary Carbohydrate Density | Pa | Pb | |||
|---|---|---|---|---|---|---|---|
| Q1 (n = 264) <68.0 | Q2 (n = 265) 68.0–72.2 | Q3 (n = 265) 72.2–76.3 | Q4 (n = 264) >76.3 | ||||
| Nutrients (per day) | |||||||
| Total energy intake (kcal) | 1859.9 ± 517.5 | 2152.0 ± 528.4 | 1888.0 ± 466.4 | 1767.2 ± 440.8 | 1632.5 ± 485.5 | <0.001 | <0.001 |
| Carbohydrate density (%) | 71.9 ± 6.1 | 63.8 ± 3.7 | 70.2 ± 1.2 | 74.2 ± 1.2 | 79.3 ± 2.2 | <0.001 | <0.001 |
| Protein density (%) | 13.3 ± 2.1 | 15.8 ± 1.8 | 13.8 ± 1.1 | 12.8 ± 1.04 | 11.0 ± 1.0 | <0.001 | <0.001 |
| Fat density (%) | 13.6 ± 4.7 | 19.6 ± 3.1 | 14.9 ± 1.5 | 11.7 ± 1.4 | 8.0 ± 1.9 | <0.001 | <0.001 |
| Demographic data | |||||||
| Age (years) | 55.4 ± 8.8 | 52.3 ± 8.7 | 54.2 ± 8.3 | 55.9 ± 8.7 | 59.3 ± 7.9 | <0.001 | <0.001 |
| Male (%) | 556 (52.5) | 187 (70.8) | 173 (65.3) | 121 (45.7) | 75 (28.3) | <0.001 | <0.001 |
| SBP (mmHg) | 127.7 ± 18.7 | 124.9 ± 16.9 | 127.2 ± 19.6 | 127.4 ± 19.3 | 131.3 ± 18.3 | 0.001 | <0.001 |
| DBP (mmHg) | 82.9 ± 10.8 | 82.6 ± 10.8 | 82.9 ± 11.8 | 82.2 ± 10.5 | 83.8 ± 10.2 | 0.417 | 0.472 |
| Body mass index (kg/m2) | 25.7 ± 3.1 | 25.9 ± 2.9 | 25.5 ± 3.1 | 25.7 ± 3.2 | 25.6 ± 3.1 | 0.502 | <0.001 |
| Waist-to-hip ratio | 0.92 ± 0.07 | 0.91 ± 0.06 | 0.90 ± 0.07 | 0.91 ± 0.06 | 0.94 ± 0.07 | <0.001 | <0.001 |
| Education (%) | <0.001 | <0.001 | |||||
| Low | 435 (41.3) | 54 (20.5) | 83 (31.4) | 123 (46.6) | 175 (67.0) | ||
| Intermediate | 480 (45.6) | 143 (54.4) | 151 (57.2) | 109 (41.3) | 77 (29.5) | ||
| High | 137 (13.0) | 66 (25.1) | 30 (11.4) | 32 (12.1) | 9 (3.4) | ||
| Income (%) | <0.001 | <0.001 | |||||
| Low | 448 (42.8) | 63 (24.0) | 88 (33.6) | 114 (43.5) | 183 (70.7) | ||
| Intermediate | 279 (26.7) | 79 (30.0) | 78 (29.8) | 73 (27.9) | 49 (18.9) | ||
| High | 319 (30.5) | 1221(46.0) | 96 (36.6) | 75 (28.6) | 27 (10.4) | ||
| Marriage (yes) | 921 (87.5) | 248 (94.3) | 244 (92.4) | 232 (87.9) | 197 (75.2) | <0.001 | <0.001 |
| Alcohol (%) | 568 (53.9) | 178 (67.4) | 176 (66.7) | 129 (48.9) | 85 (32.6) | <0.001 | <0.001 |
| Smoking (%) | 484 (46.3) | 164 (63.3) | 146 (55.3) | 100 (38.2) | 74 (28.4) | <0.001 | <0.001 |
| Exercise (MET, k) | 9.7 ± 6.4 | 9.1 ± 5.9 | 9.0 ± 5.7 | 9.5 ± 6.4 | 11.2 ± 7.2 | <0.001 | <0.001 |
| Comorbidities | |||||||
| Hypertension | 590 (55.8) | 135 (51.1) | 153 (57.7) | 139 (52.5) | 163 (61.5) | 0.083 | 0.062 |
| CVD | 50 (4.7) | 11 (4.2) | 12 (4.5) | 11 (4.2) | 16 (6.1) | 0.703 | 0.370 |
| Dyslipidemia | 41 (3.9) | 10 (3.8) | 11 (4.2) | 9 (3.4) | 11 (4.2) | 0.964 | 0.950 |
| Variables | Total (n = 6746) | Quartiles of Dietary Carbohydrate Density | Pa | Pb | |||
|---|---|---|---|---|---|---|---|
| Q1 (n = 1686) <67.8 | Q2 (n = 1687) 67.8–71.9 | Q3 (n = 1687) 71.9–75.8 | Q4 (n = 1686) >75.8 | ||||
| Laboratory parameters | |||||||
| BUN (mg/dL) | 14.2 ± 3.5 | 14.2 ± 3.4 | 14.3 ± 3.5 | 14.1 ± 3.6 | 14.0 ± 3.5 | 0.066 | 0.031 |
| Creatinine (mg/dL) | 0.83 ± 0.17 | 0.87 ± 0.17 | 0.85 ± 0.17 | 0.82 ± 0.16 | 0.78 ± 0.15 | <0.001 | <0.001 |
| eGFR (mL/min/1.73 m2) | 93.2 ± 13.0 | 93.6 ± 13.1 | 93.3 ± 13.4 | 93.8 ± 13.0 | 92.4 ± 12.3 | 0.100 | 0.032 |
| WBC (×1000 cells/μL) | 6.46 ± 1.75 | 6.47 ± 1.68 | 6.54 ± 1.75 | 6.41 ± 1.78 | 6.40 ± 1.78 | 0.079 | 0.082 |
| Hemoglobin (g/dL) | 13.6 ± 1.6 | 13.9 ± 1.6 | 13.7 ± 1.6 | 13.5 ± 1.6 | 13.2 ± 1.5 | <0.001 | <0.001 |
| Glucose (mg/dL) | 82.8 ± 8.5 | 83.6 ± 8.4 | 83.3 ± 8.7 | 82.3 ± 8.7 | 81.8 ± 8.2 | <0.001 | <0.001 |
| HbA1c (%) | 5.5 ± 0.3 | 5.5 ± 0.3 | 5.5 ± 0.4 | 5.6 ± 0.3 | 5.6 ± 0.3 | <0.001 | <0.001 |
| HOMA-IR | 1.6 ± 1.0 | 1.6 ± 0.9 | 1.6 ± 1.1 | 1.6 ± 1.0 | 1.7 ± 1.1 | 0.004 | 0.001 |
| Albumin (g/dL) | 4.3 ± 0.3 | 4.3 ± 0.3 | 4.3 ± 0.3 | 4.2 ± 0.3 | 4.2 ± 0.3 | <0.001 | <0.001 |
| Cholesterol (mg/dL) | 190 ± 33 | 192 ± 34 | 191 ± 35 | 188 ± 34 | 189 ± 33 | <0.001 | <0.001 |
| Triglyceride (mg/dL) | 155 ± 95 | 154 ± 98 | 150 ± 88 | 156 ± 100 | 158 ± 92 | 0.105 | <0.001 |
| HDL-C (mg/dL) | 44.8 ± 9.9 | 45.7 ± 10.0 | 45.2 ± 9.9 | 44.4 ± 9.8 | 44.0 ± 9.6 | <0.001 | <0.001 |
| LDL-C (mg/dL) | 115 ± 32 | 116 ± 33 | 116 ± 32 | 112 ± 31 | 113 ± 31 | <0.001 | <0.001 |
| CRP (mg/L) | 0.22 (0.09, 0.31) | 0.21 (0.09, 0.30) | 0.21 (0.10, 0.29) | 0.22 (0.09, 0.33) | 0.23 (0.09, 0.31) | 0.462 c | 0.124 |
| Variables | Total (n = 1058) | Quartiles of Dietary Carbohydrate Density | Pa | Pb | |||
|---|---|---|---|---|---|---|---|
| Q1 (n = 264) <68.0 | Q2 (n = 265) 68.0–72.2 | Q3 (n = 265) 72.2–76.3 | Q4 (n = 264) >76.3 | ||||
| Laboratory parameters | |||||||
| BUN (mg/dL) | 14.6 ± 3.6 | 14.8 ± 3.8 | 15.1 ± 3.5 | 14.4 ± 3.5 | 14.2 ± 3.8 | 0.042 | 0.018 |
| Creatinine (mg/dL) | 0.84 ± 0.17 | 0.89 ± 0.18 | 0.88 ± 0.17 | 0.81 ± 0.16 | 0.77 ± 0.14 | <0.001 | <0.001 |
| eGFR (mL/min/1.73 m2) | 90.3 ± 13.1 | 91.2 ± 13.7 | 89.2 ± 13.5 | 91.2 ± 13.0 | 89.6 ± 11.9 | 0.155 | 0.423 |
| WBC (×1000 cells/μL) | 6.85 ± 1.90 | 6.84 ± 1.85 | 6.81 ± 1.79 | 7.10 ± 2.08 | 6.66 ± 1.83 | 0.058 | 0.585 |
| Hemoglobin (g/dL) | 13.9 ± 1.5 | 14.3 ± 1.4 | 14.2 ± 1.5 | 13.8 ± 1.5 | 13.1 ± 1.4 | <0.001 | <0.001 |
| Glucose (mg/dL) | 120.3 ± 42.3 | 127.2 ± 48.6 | 124.0 ± 43.0 | 118.1 ± 35.5 | 110.0 ± 35.2 | <0.001 | <0.001 |
| HbA1c (%) | 7.3 ± 1.6 | 7.3 ± 1.7 | 7.3 ± 1.6 | 7.4 ± 1.5 | 7.1 ± 1.4 | 0.409 | 0.266 |
| HOMA-IR | 2.7 ± 2.6 | 2.9 ± 2.2 | 2.6 ± 1.7 | 2.8 ± 4.2 | 2.7 ± 2.2 | 0.750 | 0.825 |
| Albumin (g/dL) | 4.3 ± 0.3 | 4.3 ± 0.4 | 4.3 ± 0.4 | 4.2 ± 0.3 | 4.2 ± 0.3 | <0.001 | <0.001 |
| Cholesterol (mg/dL) | 200 ± 40 | 205 ± 39 | 206 ± 41 | 197 ± 37 | 196 ± 40 | <0.001 | 0.002 |
| Triglyceride (mg/dL) | 209 ± 143 | 213 ± 138 | 217 ± 162 | 208 ± 129 | 200 ± 140 | 0.597 | 0.248 |
| HDL-C (mg/dL) | 42.5 ± 9.4 | 42.2 ± 9.0 | 43.2 ± 9.3 | 41.9 ± 9.0 | 42.6 ± 10.2 | 0.460 | 0.931 |
| LDL-C (mg/dL) | 117 ± 36 | 120 ± 36 | 120 ± 36 | 115 ± 36 | 114 ± 36 | 0.073 | 0.018 |
| CRP (mg/L) | 0.27 (0.09, 0.31) | 0.25 (0.09, 0.30) | 0.28 (0.10, 0.29) | 0.29 (0.09, 0.33) | 0.29 (0.09, 0.31) | 0.614c | 0.210 |
| Carbohydrate Density (%) | Continuous a | P | Dietary Carbohydrate Density (vs. Q1) | |||||
|---|---|---|---|---|---|---|---|---|
| Q2 | P | Q3 | P | Q4 | P | |||
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||||
| Non-DM | ||||||||
| Model 1 | 1.71 (1.55, 1.88) | <0.001 | 1.30 (1.09, 1.54) | 0.003 | 1.46 (1.23, 1.72) | <0.001 | 2.32 (1.98, 2.71) | <0.001 |
| Model 2 | 1.12 (1.01, 1.25) | 0.030 | 1.14 (0.96, 1.36) | 0.134 | 1.19 (1.00, 1.41) | 0.047 | 1.35 (1.14, 1.60) | 0.001 |
| Model 3 | 1.18 (1.06, 1.31) | 0.002 | 1.16 (0.97, 1.38) | 0.103 | 1.22 (1.03, 1.45) | 0.024 | 1.40 (1.17, 1.66) | 0.024 |
| Model 4 | 1.15 (1.04, 1.28) | 0.008 | 1.14 (0.06, 1.36) | 0.136 | 1.20 (1.01, 1.43) | 0.037 | 1.35 (1.13, 1.60) | 0.001 |
| DM | ||||||||
| Model 1 | 1.45 (1.22, 1.73) | <0.001 | 0.87 (0.64, 1.17) | 0.357 | 1.36 (1.03, 1.80) | 0.032 | 1.62 (1.23, 2.12) | 0.001 |
| Model 2 | 1.02 (0.82, 1.27) | 0.839 | 0.76 (0.53, 1.08) | 0.121 | 1.07 (0.77, 1.51) | 0.683 | 0.86 (0.60, 1.24) | 0.419 |
| Model 3 | 1.00 (0.80, 1.26) | 0.999 | 0.74 (0.52, 1.06) | 0.096 | 1.12 (0.79, 1.58) | 0.525 | 0.83 (0.57, 1.21) | 0.336 |
| Model 4 | 0.96 (0.76, 1.20) | 0.712 | 0.74 (0.52, 1.05) | 0.091 | 1.04 (0.73, 1.47) | 0.830 | 0.78 (0.53, 1.15) | 0.208 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, K.H.; An, S.Y.; Joo, Y.S.; Lee, S.; Yun, H.-R.; Jhee, J.H.; Han, S.H.; Yoo, T.-H.; Kang, S.-W.; Park, J.T. Carbohydrate-Rich Diet Is Associated with Increased Risk of Incident Chronic Kidney Disease in Non-Diabetic Subjects. J. Clin. Med. 2019, 8, 793. https://doi.org/10.3390/jcm8060793
Nam KH, An SY, Joo YS, Lee S, Yun H-R, Jhee JH, Han SH, Yoo T-H, Kang S-W, Park JT. Carbohydrate-Rich Diet Is Associated with Increased Risk of Incident Chronic Kidney Disease in Non-Diabetic Subjects. Journal of Clinical Medicine. 2019; 8(6):793. https://doi.org/10.3390/jcm8060793
Chicago/Turabian StyleNam, Ki Heon, Seong Yeong An, Young Su Joo, Sangmi Lee, Hae-Ryong Yun, Jong Hyun Jhee, Seung Hyeok Han, Tae-Hyun Yoo, Shin-Wook Kang, and Jung Tak Park. 2019. "Carbohydrate-Rich Diet Is Associated with Increased Risk of Incident Chronic Kidney Disease in Non-Diabetic Subjects" Journal of Clinical Medicine 8, no. 6: 793. https://doi.org/10.3390/jcm8060793
APA StyleNam, K. H., An, S. Y., Joo, Y. S., Lee, S., Yun, H.-R., Jhee, J. H., Han, S. H., Yoo, T.-H., Kang, S.-W., & Park, J. T. (2019). Carbohydrate-Rich Diet Is Associated with Increased Risk of Incident Chronic Kidney Disease in Non-Diabetic Subjects. Journal of Clinical Medicine, 8(6), 793. https://doi.org/10.3390/jcm8060793





