Improvement of Quality of Life with Implant-Supported Mandibular Overdentures and the Effect of Implant Type and Surgical Procedure on Bone and Soft Tissue Stability: A Three-Year Prospective Split-Mouth Trial
Abstract
1. Introduction
2. Experimental Section
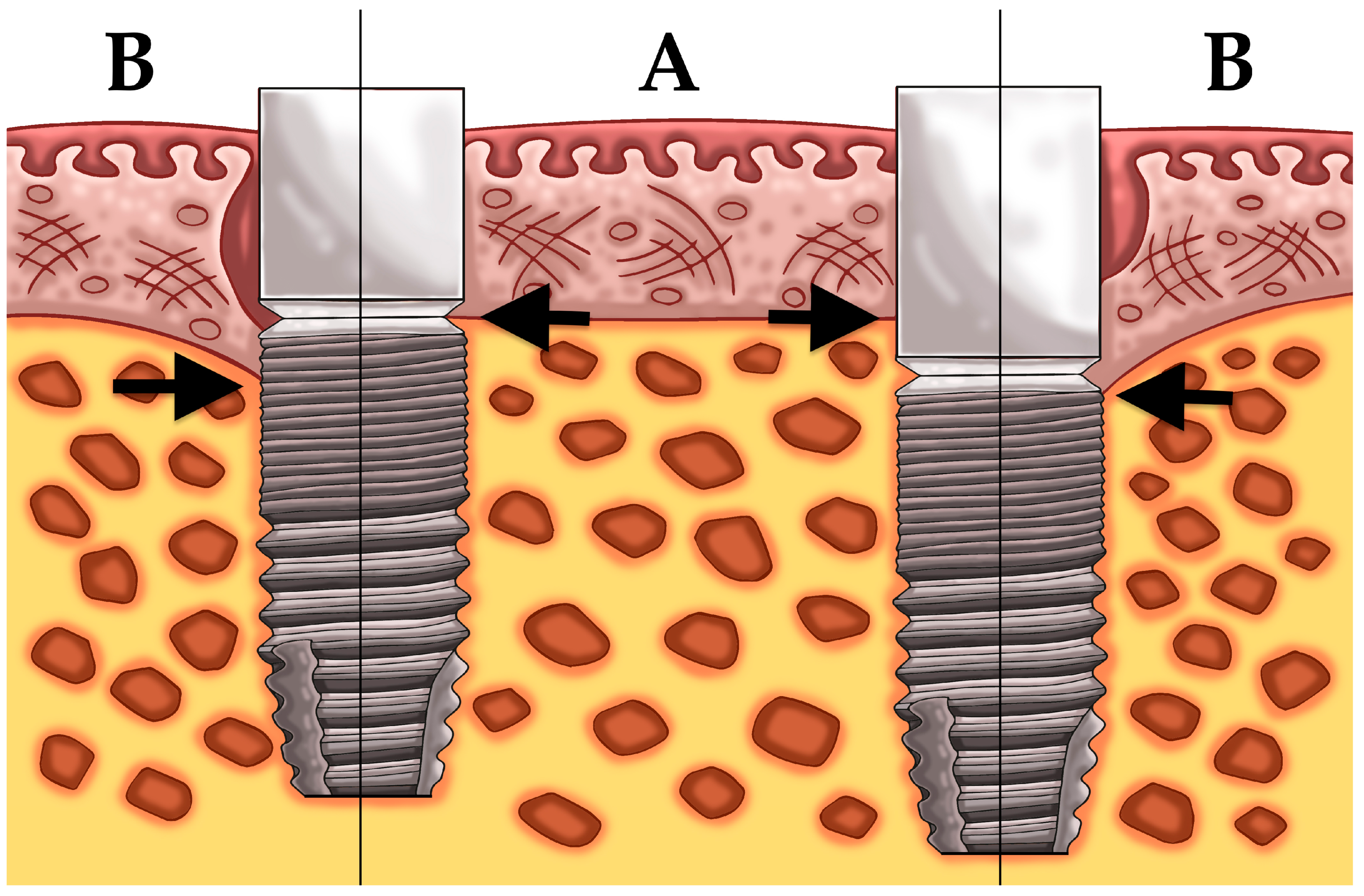
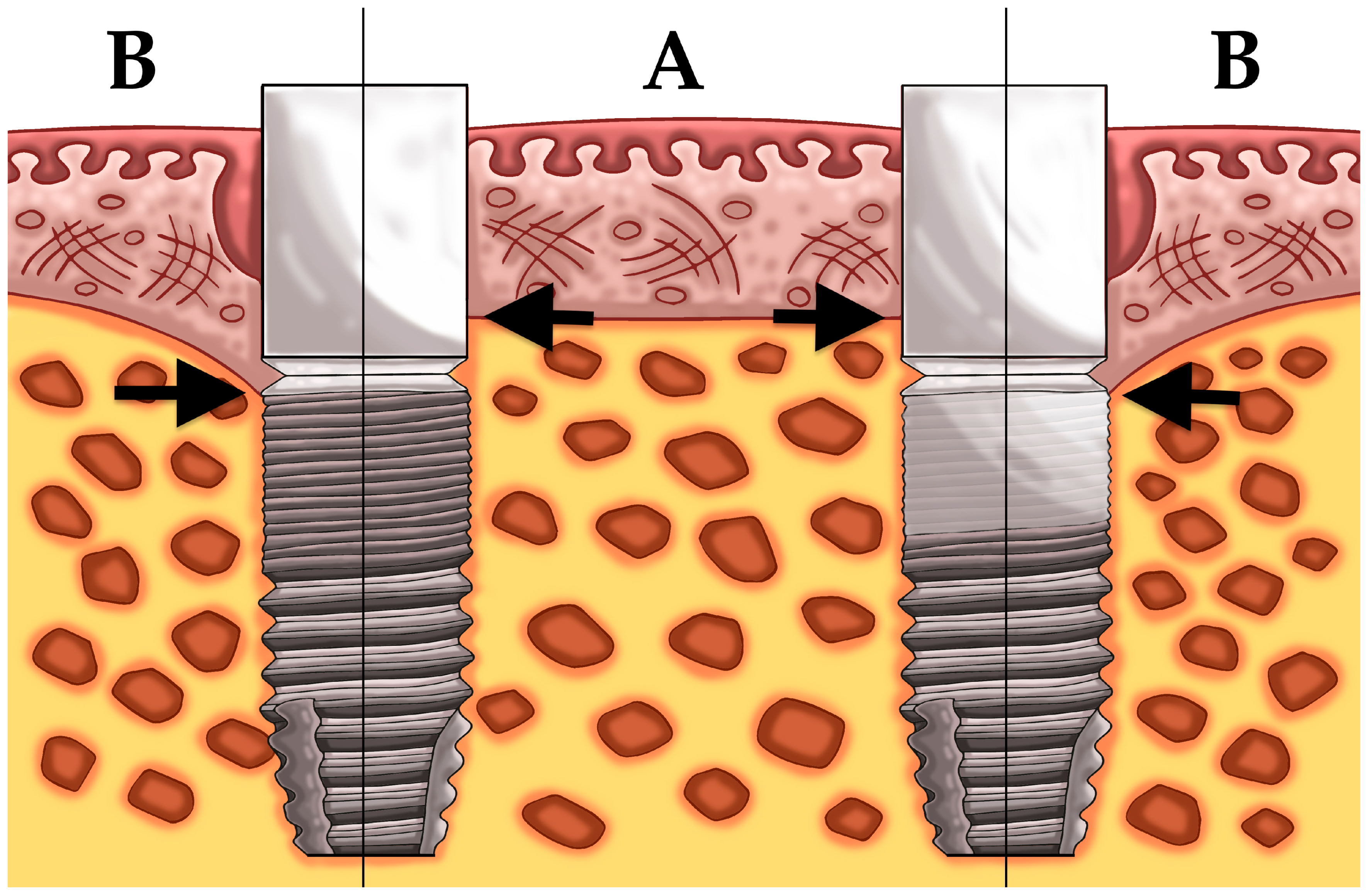
2.1. Patient Population and Surgical/Prosthetic Procedures
2.2. Clinical and Radiographic Examination
2.3. Statistics
3. Results
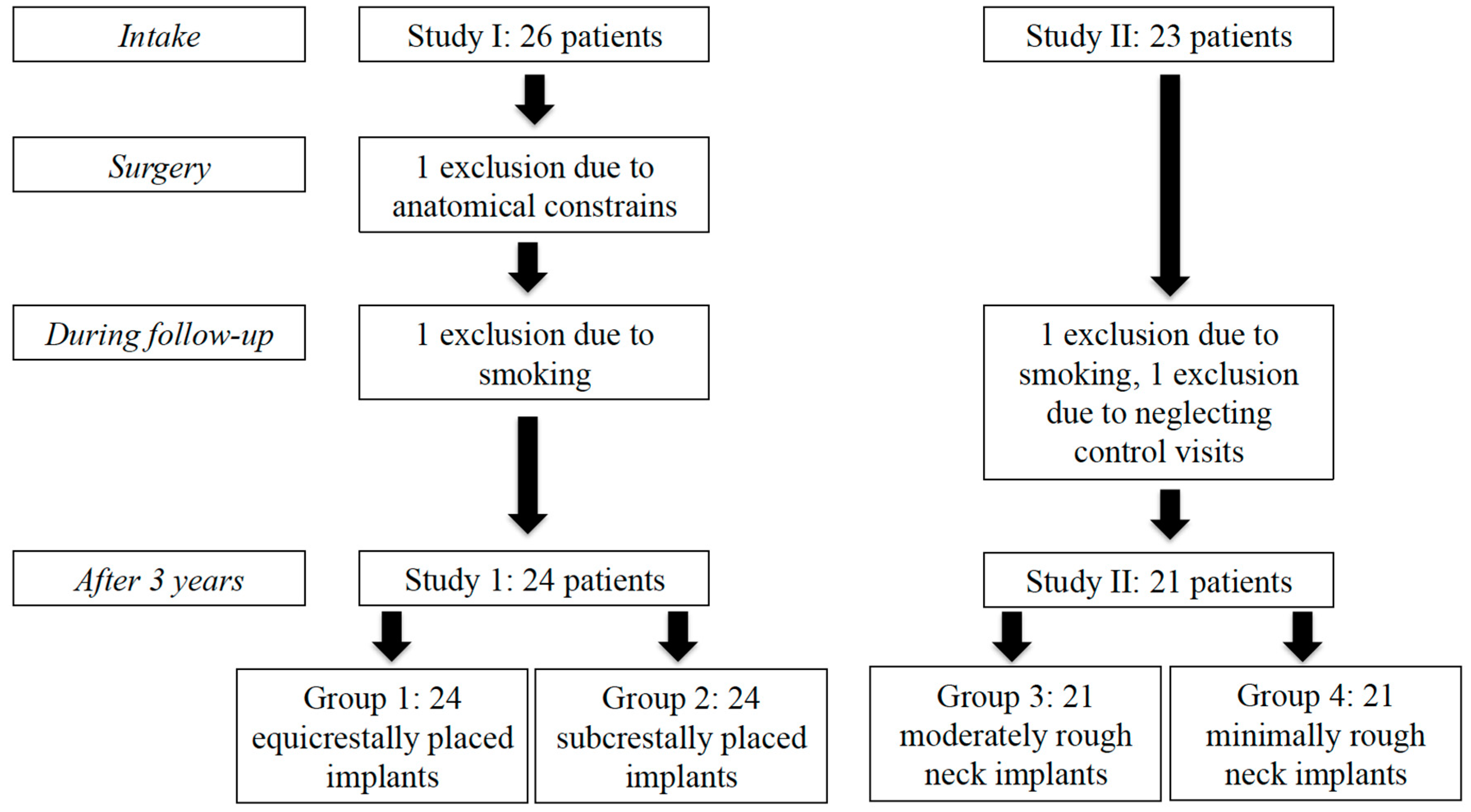
3.1. Study Population
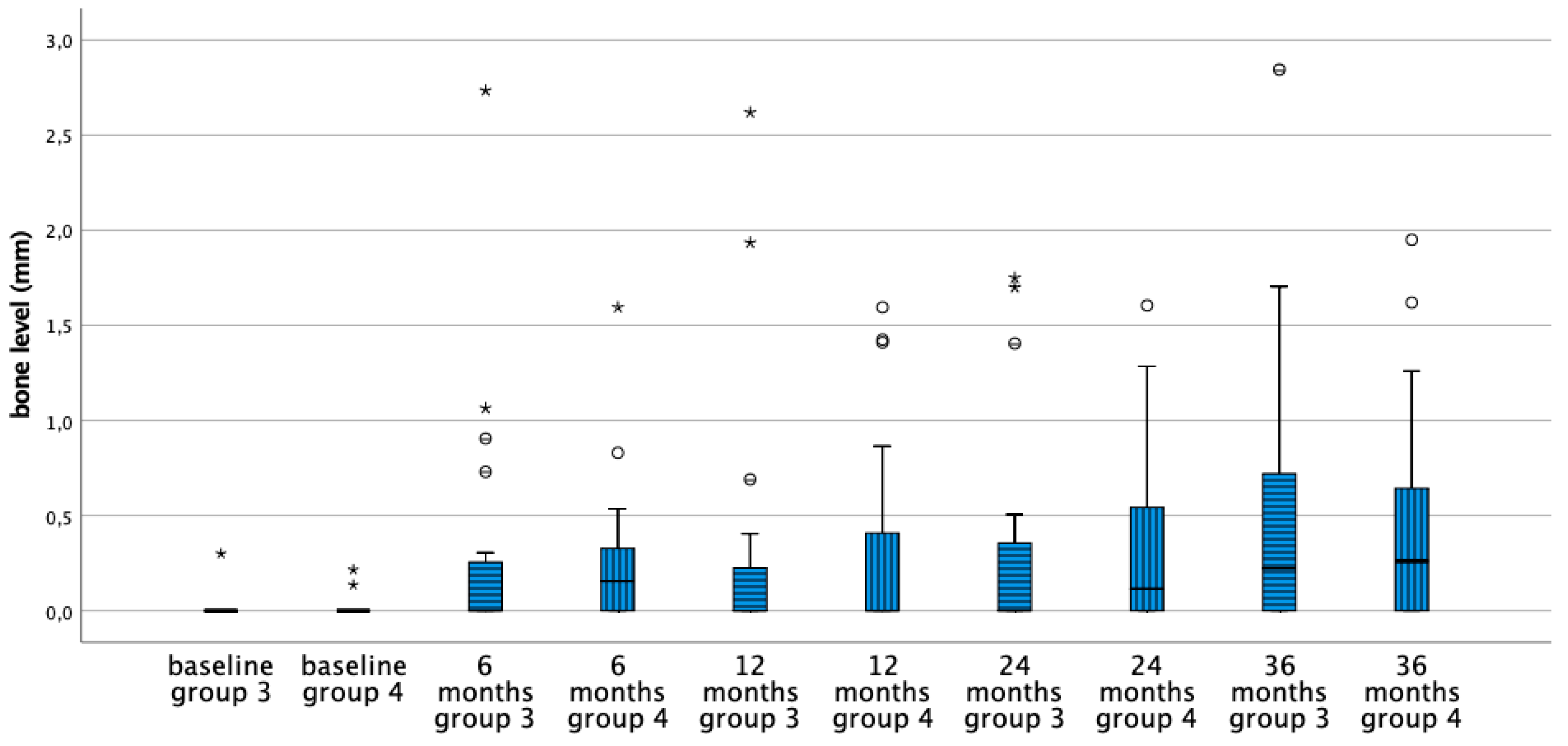
3.2. Mean Bone Level Difference
3.3. Biologic Parameters
3.4. Oral Health-Related Quality of Life
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Petersen, P.E.; Yamamoto, T. Improving the oral health of older people: the approach of the WHO Global Oral Health Programme. Community Dent. Oral Epidemiol. 2005, 33, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Sennerby, L.; De Bruyn, H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontology 2000 2017, 73, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Pelo, S.; Saponaro, G.; Patini, R.; Staderini, E.; Giordano, A.; Gasparini, G.; Garagiola, U.; Azzuni, C.; Cordaro, M.; Foresta, E.; et al. Risks in surgery-first orthognathic approach: complications of segmental osteotomies of the jaws. A systematic review. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4–12. [Google Scholar]
- Slade, G.D.; Spencer, A.J. Development and evaluation of the Oral Health Impact Profile. Community Dent. Health 1994, 11, 3–11. [Google Scholar]
- Allen, P.F.; McMillan, A.S. A longitudinal study of quality of life outcomes in older adults requesting implant prostheses and complete removable dentures. Clin. Oral Implant. Res. 2003, 14, 173–179. [Google Scholar] [CrossRef]
- Feine, J.S.; Carlsson, G.E.; Awad, M.A.; Chehade, A.; Duncan, W.J.; Gizani, S.; Head, T.; Lund, J.P.; MacEntee, M.; Mericske-Stern, R.; et al. The McGill consensus statement on overdentures. Mandibular two-implant overdentures as first choice standard of care for edentulous patients. Montreal, Quebec, 24–25 May, 2002. Int. J. Oral Maxillofac. Implant. 2002, 17, 601–602. [Google Scholar]
- De Bruyn, H.; Raes, S.; Matthys, C.; Cosyn, J. The current use of patient-centered/reported outcomes in implant dentistry: a systematic review. Clin. Oral Implant. Res. 2015, 26 (Suppl. 11), 45–56. [Google Scholar] [CrossRef]
- Zhang, L.; Lyu, C.; Shang, Z.; Niu, A.; Liang, X. Quality of Life of Implant-Supported Overdenture and Conventional Complete Denture in Restoring the Edentulous Mandible: A Systematic Review. Implant Dent. 2017, 26, 945–950. [Google Scholar] [CrossRef]
- Kutkut, A.; Bertoli, E.; Frazer, R.; Pinto-Sinai, G.; Fuentealba Hidalgo, R.; Studts, J. A systematic review of studies comparing conventional complete denture and implant retained overdenture. J. Prosthodont. Res. 2018, 62, 1–9. [Google Scholar] [CrossRef]
- Sivaramakrishnan, G.; Sridharan, K. Comparison of implant supported mandibular overdentures and conventional dentures on quality of life: A systematic review and meta-analysis of randomized controlled studies. Aust. Dent. J. 2016, 61, 482–488. [Google Scholar] [CrossRef]
- Renvert, S.; Persson, G.R.; Pirih, F.Q.; Camargo, P.M. Peri-implant health, peri-implant mucositis, and peri-implantitis: Case definitions and diagnostic considerations. J. Periodontol. 2018, 89 (Suppl. 1), S304–S312. [Google Scholar] [CrossRef]
- Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. Br. Dent. J. 2018, 225, 141. [CrossRef]
- Heitz-Mayfield, L.J.A.; Salvi, G.E. Peri-implant mucositis. J. Periodontol. 2018, 89 (Suppl. 1), S257–S266. [Google Scholar] [CrossRef]
- Pontoriero, R.; Tonelli, M.P.; Carnevale, G.; Mombelli, A.; Nyman, S.R.; Lang, N.P. Experimentally induced peri-implant mucositis. A clinical study in humans. Clin. Oral Implant. Res. 1994, 5, 254–259. [Google Scholar] [CrossRef]
- Zitzmann, N.U.; Berglundh, T.; Marinello, C.P.; Lindhe, J. Experimental peri-implant mucositis in man. J. Clin. Periodontol. 2001, 28, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Salvi, G.E.; Aglietta, M.; Eick, S.; Sculean, A.; Lang, N.P.; Ramseier, C.A. Reversibility of experimental peri-implant mucositis compared with experimental gingivitis in humans. Clin. Oral Implant. Res. 2012, 23, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.; Giannopoulou, C.; Courvoisier, D.; Schimmel, M.; Muller, F.; Mombelli, A. Experimental mucositis and experimental gingivitis in persons aged 70 or over. Clinical and biological responses. Clin. Oral Implant. Res. 2017, 28, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.G.; Lindhe, J. Peri-implant health. J. Periodontol. 2018, 89 (Suppl. 1), S249–S256. [Google Scholar] [CrossRef]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.L. Peri-implantitis. J. Periodontol. 2018, 89 (Suppl. 1), S267–S290. [Google Scholar] [CrossRef]
- Canullo, L.; Schlee, M.; Wagner, W.; Covani, U.; Montegrotto Group for the Study of Peri-implant Disease. International Brainstorming Meeting on Etiologic and Risk Factors of Peri-implantitis, Montegrotto (Padua, Italy), August 2014. Int. J. Oral Maxillofac. Implant. 2015, 30, 1093–1104. [Google Scholar] [CrossRef]
- Albrektsson, T.; Buser, D.; Chen, S.T.; Cochran, D.; DeBruyn, H.; Jemt, T.; Koka, S.; Nevins, M.; Sennerby, L.; Simion, M.; et al. Statements from the Estepona consensus meeting on peri-implantitis, 2–4 February 2012. Clin. Implant Dent. Relat. Res. 2012, 14, 781–782. [Google Scholar] [CrossRef] [PubMed]
- De Bruyn, H.; Christiaens, V.; Doornewaard, R.; Jacobsson, M.; Cosyn, J.; Jacquet, W.; Vervaeke, S. Implant surface roughness and patient factors on long-term peri-implant bone loss. Periodontology 2000 2017, 73, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, A.; Hallgren, C.; Johansson, C.; Danelli, S. A histomorphometric evaluation of screw-shaped implants each prepared with two surface roughnesses. Clin. Oral Implant. Res. 1998, 9, 11–19. [Google Scholar] [CrossRef]
- Cochran, D.L. A comparison of endosseous dental implant surfaces. J. Periodontol. 1999, 70, 1523–1539. [Google Scholar] [CrossRef]
- Lazzara, R.J.; Testori, T.; Trisi, P.; Porter, S.S.; Weinstein, R.L. A human histologic analysis of osseotite and machined surfaces using implants with 2 opposing surfaces. Int. J. Periodontics Restor. Dent. 1999, 19, 117–129. [Google Scholar]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implant. Res. 2006, 17 (Suppl. 2), 68–81. [Google Scholar] [CrossRef]
- Esposito, M.; Coulthard, P.; Thomsen, P.; Worthington, H.V. The role of implant surface modifications, shape and material on the success of osseointegrated dental implants. A Cochrane systematic review. Eur. J. Prosthodontics Restor. Dent. 2005, 13, 15–31. [Google Scholar]
- Esposito, M.; Ardebili, Y.; Worthington, H.V. Interventions for replacing missing teeth: different types of dental implants. Cochrane Database Syst. Rev. 2014, CD003815. [Google Scholar] [CrossRef]
- Glibert, M.; Matthys, C.; Maat, R.J.; De Bruyn, H.; Vervaeke, S. A randomized controlled clinical trial assessing initial crestal bone remodeling of implants with a different surface roughness. Clin. Implant Dent. Relat. Res. 2018, 20, 824–828. [Google Scholar] [CrossRef]
- Monje, A.; Catena, A.; Borgnakke, W.S. Association between diabetes mellitus/hyperglycaemia and peri-implant diseases: Systematic review and meta-analysis. J. Clin. Periodontol. 2017, 44, 636–648. [Google Scholar] [CrossRef]
- Papi, P.; Letizia, C.; Pilloni, A.; Petramala, L.; Saracino, V.; Rosella, D.; Pompa, G. Peri-implant diseases and metabolic syndrome components: a systematic review. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 866–875. [Google Scholar] [CrossRef]
- Moraschini, V.; Barboza, E.S.; Peixoto, G.A. The impact of diabetes on dental implant failure: a systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2016, 45, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- de Medeiros, F.; Kudo, G.A.H.; Leme, B.G.; Saraiva, P.P.; Verri, F.R.; Honorio, H.M.; Pellizzer, E.P.; Santiago Junior, J.F. Dental implants in patients with osteoporosis: A systematic review with meta-analysis. Int. J. Oral Maxillofac. Surg. 2018, 47, 480–491. [Google Scholar] [CrossRef]
- Jung, R.E.; Al-Nawas, B.; Araujo, M.; Avila-Ortiz, G.; Barter, S.; Brodala, N.; Chappuis, V.; Chen, B.; De Souza, A.; Almeida, R.F.; et al. Group 1 ITI Consensus Report: The influence of implant length and design and medications on clinical and patient-reported outcomes. Clin. Oral Implant. Res. 2018, 29 (Suppl. 16), 69–77. [Google Scholar] [CrossRef] [PubMed]
- Safii, S.H.; Palmer, R.M.; Wilson, R.F. Risk of implant failure and marginal bone loss in subjects with a history of periodontitis: a systematic review and meta-analysis. Clin. Implant Dent. Relat. Res. 2010, 12, 165–174. [Google Scholar] [CrossRef] [PubMed]
- De Bruyn, H.; Collaert, B. The effect of smoking on early implant failure. Clin. Oral Implant. Res. 1994, 5, 260–264. [Google Scholar] [CrossRef]
- Hinode, D.; Tanabe, S.; Yokoyama, M.; Fujisawa, K.; Yamauchi, E.; Miyamoto, Y. Influence of smoking on osseointegrated implant failure: a meta-analysis. Clin. Oral Implant. Res. 2006, 17, 473–478. [Google Scholar] [CrossRef]
- Doornewaard, R.; Christiaens, V.; De Bruyn, H.; Jacobsson, M.; Cosyn, J.; Vervaeke, S.; Jacquet, W. Long-Term Effect of Surface Roughness and Patients’ Factors on Crestal Bone Loss at Dental Implants. A Systematic Review and Meta-Analysis. Clin. Implant Dent. Relat. Res. 2017, 19, 372–399. [Google Scholar] [CrossRef]
- Vervaeke, S.; Collaert, B.; Cosyn, J.; De Bruyn, H. A 9-Year Prospective Case Series Using Multivariate Analyses to Identify Predictors of Early and Late Peri-Implant Bone Loss. Clin. Implant Dent. Relat. Res. 2016, 18, 30–39. [Google Scholar] [CrossRef]
- Berglundh, T.; Lindhe, J. Dimension of the periimplant mucosa. Biological width revisited. J. Clin. Periodontol. 1996, 23, 971–973. [Google Scholar] [CrossRef]
- Linkevicius, T.; Apse, P.; Grybauskas, S.; Puisys, A. The influence of soft tissue thickness on crestal bone changes around implants: a 1-year prospective controlled clinical trial. Int. J. Oral Maxillofac. Implant. 2009, 24, 712–719. [Google Scholar]
- Vervaeke, S.; Dierens, M.; Besseler, J.; De Bruyn, H. The influence of initial soft tissue thickness on peri-implant bone remodeling. Clin. Implant Dent. Relat. Res. 2014, 16, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Moreno, P.; Leon-Cano, A.; Ortega-Oller, I.; Monje, A.; F, O.V.; Catena, A. Marginal bone loss as success criterion in implant dentistry: Beyond 2 mm. Clin. Oral Implant. Res. 2015, 26, e28–e34. [Google Scholar] [CrossRef]
- Canullo, L.; Camacho-Alonso, F.; Tallarico, M.; Meloni, S.M.; Xhanari, E.; Penarrocha-Oltra, D. Mucosa Thickness and Peri-implant Crestal Bone Stability: A Clinical and Histologic Prospective Cohort Trial. Int. J. Oral Maxillofac. Implant. 2017, 32, 675–681. [Google Scholar] [CrossRef]
- Doornewaard, R.; Jacquet, W.; Cosyn, J.; De Bruyn, H. How do peri-implant biologic parameters correspond with implant survival and peri-implantitis? A critical review. Clin. Oral Implant. Res. 2018, 29 (Suppl. 18), 100–123. [Google Scholar] [CrossRef]
- Vervaeke, S.; Matthys, C.; Nassar, R.; Christiaens, V.; Cosyn, J.; De Bruyn, H. Adapting the vertical position of implants with a conical connection in relation to soft tissue thickness prevents early implant surface exposure: A 2-year prospective intra-subject comparison. J. Clin. Periodontol. 2018, 45, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Meyer, S.; Mombelli, A.; Muller, F. Dental implants in the elderly population: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2017, 28, 920–930. [Google Scholar] [CrossRef]
- Lesaffre, E.; Philstrom, B.; Needleman, I.; Worthington, H. The design and analysis of split-mouth studies: what statisticians and clinicians should know. Stat. Med. 2009, 28, 3470–3482. [Google Scholar] [CrossRef]
- Suarez-Lopez Del Amo, F.; Lin, G.H.; Monje, A.; Galindo-Moreno, P.; Wang, H.L. Influence of Soft Tissue Thickness on Peri-Implant Marginal Bone Loss: A Systematic Review and Meta-Analysis. J. Periodontol. 2016, 87, 690–699. [Google Scholar] [CrossRef]
- Quirynen, M.; Abarca, M.; Van Assche, N.; Nevins, M.; van Steenberghe, D. Impact of supportive periodontal therapy and implant surface roughness on implant outcome in patients with a history of periodontitis. J. Clin. Periodontol. 2007, 34, 805–815. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T.; Chrcanovic, B. Long-term clinical outcome of implants with different surface modifications. Eur. J. Oral Implantol. 2018, 11 (Suppl. 1), S123–S136. [Google Scholar]
- Koodaryan, R.; Hafezeqoran, A. Evaluation of Implant Collar Surfaces for Marginal Bone Loss: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2016, 2016, 4987526. [Google Scholar] [CrossRef] [PubMed]
- Donati, M.; Ekestubbe, A.; Lindhe, J.; Wennstrom, J.L. Marginal bone loss at implants with different surface characteristics - A 20-year follow-up of a randomized controlled clinical trial. Clin. Oral Implant. Res. 2018, 29, 480–487. [Google Scholar] [CrossRef]
- Raes, M.; D’Hondt, R.; Teughels, W.; Coucke, W.; Quirynen, M. A 5-year randomized clinical trial comparing minimally with moderately rough implants in patients with severe periodontitis. J. Clin. Periodontol. 2018, 45, 711–720. [Google Scholar] [CrossRef] [PubMed]
- De Waal, Y.C.; van Winkelhoff, A.J.; Meijer, H.J.; Raghoebar, G.M.; Winkel, E.G. Differences in peri-implant conditions between fully and partially edentulous subjects: A systematic review. J. Clin. Periodontol. 2013, 40, 266–286. [Google Scholar] [CrossRef] [PubMed]
- De Waal, Y.C.; Winkel, E.G.; Meijer, H.J.; Raghoebar, G.M.; van Winkelhoff, A.J. Differences in peri-implant microflora between fully and partially edentulous patients: A systematic review. J. Clin. Periodontol. 2014, 85, 68–82. [Google Scholar] [CrossRef] [PubMed]



| Domain 1: Functional Limitation | |
| 1 | Have you had trouble pronouncing any words because of problems with your teeth, mouth, or denture? |
| 2 | Have you felt that your sense of taste has worsened because of problems with your teeth, mouth, or denture? |
| Domain 2: Physical Pain | |
| 3 | Have you had painful aching in your mouth? |
| 4 | Have you found it uncomfortable to eat any foods because of problems with your teeth, mouth, or denture? |
| Domain 3: Psychological Discomfort | |
| 5 | Have you been self-conscious because of your teeth, mouth, or denture? |
| 6 | Have you felt tense because of problems with your teeth, mouth, or denture? |
| Domain 4: Physical Disability | |
| 7 | Has been your diet been unsatisfactory because of problems with your teeth, mouth, or denture? |
| 8 | Have you interrupt meals because of problems with your teeth, mouth, or denture? |
| Domain 5: Psychological Disability | |
| 9 | Have you found it difficult to relax because of problems with your teeth, mouth or denture? |
| 10 | Have you been a bit embarrassed because of problems with your teeth, mouth, or denture? |
| Domain 6: Social Disability | |
| 11 | Have you been a bit irritable with other people because of problems with your teeth, mouth, or denture? |
| 12 | Have you had difficulty doing your usual jobs because of problems with your teeth, mouth, or denture? |
| Domain 7: Handicap | |
| 13 | Have you felt that life, in general, was less satisfying because of problems with your teeth, mouth, or denture? |
| 14 | Have you been totally unable to function because of problems with your teeth, mouth, or denture? |
| Bone Level | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1: Equicrestal | Group 2: Subcrestal | Paired Difference | |||||||||
| Mean (SD) | Median | Min | Max | Mean (SD) | Median | Min | Max | Mean dif | 95% CI | p | |
| Baseline | 0.03 (0.09) | 0.00 | 0.00 | 0.40 | 0.00 (0.00) | 0.00 | 0.00 | 0.00 | 0.030 | (−0.009,0.070) | 0.123 |
| 6 months | 0.72 (0.74) | 0.59 | 0.00 | 2.45 | 0.04 (0.11) | 0.00 | 0.00 | 0.45 | 0.678 | (0.360,0.996) | <0.001 |
| 12 months | 0.78 (0.81) | 0.54 | 0.00 | 2.92 | 0.03 (0.10) | 0.00 | 0.00 | 0.36 | 0.746 | (0.397,1.096) | <0.001 |
| 24 months | 0.69 (0.70) | 0.51 | 0.00 | 2.61 | 0.04 (0.10) | 0.00 | 0.00 | 0.30 | 0.644 | (0.337,0.951) | <0.001 |
| 36 months | 0.59 (0.59) | 0.44 | 0.00 | 2.25 | 0.04 (0.10) | 0.00 | 0.00 | 0.36 | 0.549 | (0.297,0.802) | <0.001 |
| Group 3: Moderately Rough Neck | Group 4: Minimally Rough Neck | Paired Difference | |||||||||
| Mean (SD) | Median | Min | Max | Mean (SD) | Median | Min | Max | Mean dif | 95% CI | p | |
| Baseline | 0.01 (0.07) | 0.00 | 0.00 | 0.30 | 0.02 (0.05) | 0.00 | 0.00 | 0.22 | −0.002 | (−0.424,0.037) | 0.902 |
| 6 months | 0.33 (0.64) | 0.00 | 0.00 | 2.74 | 0.27 (0.38) | 0.18 | 0.00 | 1.60 | 0.064 | (−0.118,0.245) | 0.474 |
| 12 months | 0.34 (0.68) | 0.00 | 0.00 | 2.62 | 0.34 (0.53) | 0.00 | 0.00 | 1.61 | 0.009 | (−0.191,0.209) | 0.926 |
| 24 months | 0.36 (0.58) | 0.00 | 0.00 | 1.75 | 0.37 (0.49) | 0.23 | 0.00 | 1.60 | −0.014 | (−0.170,0.142) | 0.853 |
| 36 months | 0.51 (0.74) | 0.22 | 0.00 | 2.84 | 0.45 (0.58) | 0.26 | 0.00 | 1.95 | 0.066 | (−0.114,0.246) | 0.453 |
| Plaque | |||||
| Group 1: Equicrestal | Group 2: Subcrestal | Paired Difference | |||
| Mean (SD) | Mean (SD) | Mean dif | 95% CI | p | |
| 6 months | 0.44 (0.47) | 0.52 (0.45) | −0.083 | (−0.221,0.055) | 0.224 |
| 12 months | 0.45 (0.39) | 0.56 (0.44) | −0.115 | (−0.285,0.056) | 0.178 |
| 24 months | 0.42 (0.40) | 0.51 (0.40) | −0.091 | (−0.178,−0.003) | 0.042 |
| 36 months | 0.39 (0.43) | 0.41 (0.42) | −0.022 | (−0.148,0.104) | 0.724 |
| Group 3: Moderately Rough Neck | Group 4: Minimally Rough Neck | Paired Difference | |||
| Mean (SD) | Mean (SD) | Mean dif | 95% CI | p | |
| 6 months | 0.38 (0.33) | 0.40 (0.31) | −0.025 | (−0.144,0.094) | 0.666 |
| 12 months | 0.37 (0.31) | 0.35 (0.31) | 0.017 | (−0.136,0.169) | 0.818 |
| 24 months | 0.57 (0.36) | 0.52 (0.36) | 0.054 | (−0.030,0.137) | 0.189 |
| 36 months | 0.39 (0.41) | 0.43 (0.38) | −0.038 | (−0.147,0.072) | 0.481 |
| Bleeding on Probing | |||||
| Group 1: Equicrestal | Group 2: Subcrestal | Paired Difference | |||
| Mean (SD) | Mean (SD) | Mean dif | 95% CI | p | |
| 6 months | 0.15 (0.22) | 0.15 (0.22) | 0.000 | (−0.093,0.0933) | 1.000 |
| 12 months | 0.19 (0.18) | 0.19 (0.18) | 0.000 | (−0.125,0.125) | 1.000 |
| 24 months | 0.23 (0.30) | 0.20 (0.28) | 0.023 | (−0.090,0.136) | 0.680 |
| 36 months | 0.30 (0.33) | 0.23 (0.25) | 0.076 | (−0.048,0.200) | 0.216 |
| Group 3: Moderately Rough Neck | Group 4: Minimally Rough Neck | Paired Difference | |||
| Mean (SD) | Mean (SD) | Mean dif | 95% CI | p | |
| 6 months | 0.24 (0.31) | 0.23 (0.24) | 0.013 | (−0.110,0.135) | 0.834 |
| 12 months | 0.20 (0.32) | 0.23 (0.24) | −0.033 | (−0.189,0.122) | 0.653 |
| 24 months | 0.25 (0.29) | 0.30 (0.37) | −0.054 | (−0.243,0.136) | 0.551 |
| 36 months | 0.08 (0.14) | 0.07 (0.12) | 0.013 | (−0.084,0.109) | 0.789 |
| Probing Pocket Depth | |||||||||
| Group 1: Equicrestal | Group 2: Subcrestal | Paired Difference | |||||||
| Mean (SD) | Min | Max | Mean (SD) | Min | Max | Mean dif | 95% CI | p | |
| 6 months | 1.88 (0.53) | 1.00 | 3.25 | 2.01 (0.66) | 1.00 | 3.75 | −0.135 | (−0.311,0.041) | 0.125 |
| 12 months | 1.70 (0.44) | 1.00 | 2.50 | 1.83 (0.53) | 1.00 | 2.75 | −0.130 | (−0.312,0.051) | 0.149 |
| 24 months | 2.30 (0.66) | 1.50 | 4.50 | 2.57 (0.84) | 1.25 | 4.50 | −0.261 | (−0.473,−0.048) | 0.018 |
| 36 months | 2.42 (0.69) | 1.00 | 4.00 | 2.59 (0.71) | 1.00 | 3.75 | −0.163 | (−0.0378,0.052) | 0.130 |
| Group 3: Moderately Rough Neck | Group 4: Minimally Rough Neck | Paired Difference | |||||||
| Mean (SD) | Min | Max | Mean (SD) | Min | Max | Mean dif | 95% CI | p | |
| 6 months | 2.93 (0.71) | 1.75 | 5.25 | 2.88 (0.65) | 1.75 | 4.75 | 0.050 | (−0.142,0.242) | 0.592 |
| 12 months | 2.65 (0.72) | 1.75 | 4.75 | 2.68 (0.68) | 1.75 | 4.50 | −0.033 | (−0.221,0.154) | 0.709 |
| 24 months | 2.48 (0.58) | 1.25 | 3.50 | 2.34 (0.60) | 1.00 | 3,25 | 0.143 | (−0.114,0.401) | 0.252 |
| 36 months | 2.10 (0.68) | 1.25 | 4.25 | 2.01 (0.58) | 1.00 | 3.00 | 0.088 | (−0.259,0.434) | 0.603 |
| Domain | Mean-OHIP (SD) | Paired Difference | Effect-Size | |||
|---|---|---|---|---|---|---|
| Before Surgery | Three Months after Connection | Mean Dif | 95% CI | p | ||
| functional limitation | 2.30 (1.85) | 1.14 (1.42) | 1.16 | (0.540,1.785) | 0.001 | 0.63 |
| physical pain | 3.37 (2.06) | 1.21 (1.55) | 2.16 | (1.440,2.886) | <0.001 | 1.04 |
| psychological discomfort | 2.52 (2.35) | 0.65 (1.43) | 1.87 | (1.034,2.687) | <0.001 | 0.80 |
| physical disability | 2.12 (2.16) | 0.44 (0.85) | 1.68 | (0.971,2.378) | <0.001 | 0.78 |
| psychological disability | 2.21 (1.91) | 0.58 (0.93) | 1.63 | (0.930,2.326) | <0.001 | 0.85 |
| social disability | 1.67 (1.49) | 0.16 (0.49) | 1.51 | (1.007,2.016) | <0.001 | 1.01 |
| handicap | 1.42 (1.48) | 0.26 (0.66) | 1.16 | (0.683,1.642) | <0.001 | 0.78 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doornewaard, R.; Glibert, M.; Matthys, C.; Vervaeke, S.; Bronkhorst, E.; de Bruyn, H. Improvement of Quality of Life with Implant-Supported Mandibular Overdentures and the Effect of Implant Type and Surgical Procedure on Bone and Soft Tissue Stability: A Three-Year Prospective Split-Mouth Trial. J. Clin. Med. 2019, 8, 773. https://doi.org/10.3390/jcm8060773
Doornewaard R, Glibert M, Matthys C, Vervaeke S, Bronkhorst E, de Bruyn H. Improvement of Quality of Life with Implant-Supported Mandibular Overdentures and the Effect of Implant Type and Surgical Procedure on Bone and Soft Tissue Stability: A Three-Year Prospective Split-Mouth Trial. Journal of Clinical Medicine. 2019; 8(6):773. https://doi.org/10.3390/jcm8060773
Chicago/Turabian StyleDoornewaard, Ron, Maarten Glibert, Carine Matthys, Stijn Vervaeke, Ewald Bronkhorst, and Hugo de Bruyn. 2019. "Improvement of Quality of Life with Implant-Supported Mandibular Overdentures and the Effect of Implant Type and Surgical Procedure on Bone and Soft Tissue Stability: A Three-Year Prospective Split-Mouth Trial" Journal of Clinical Medicine 8, no. 6: 773. https://doi.org/10.3390/jcm8060773
APA StyleDoornewaard, R., Glibert, M., Matthys, C., Vervaeke, S., Bronkhorst, E., & de Bruyn, H. (2019). Improvement of Quality of Life with Implant-Supported Mandibular Overdentures and the Effect of Implant Type and Surgical Procedure on Bone and Soft Tissue Stability: A Three-Year Prospective Split-Mouth Trial. Journal of Clinical Medicine, 8(6), 773. https://doi.org/10.3390/jcm8060773






