Effects of Combined Remote Ischemic Pre-and Post-Conditioning on Neurologic Complications in Moyamoya Disease Patients Undergoing Superficial Temporal Artery-Middle Cerebral Artery Anastomosis
Abstract
1. Introduction
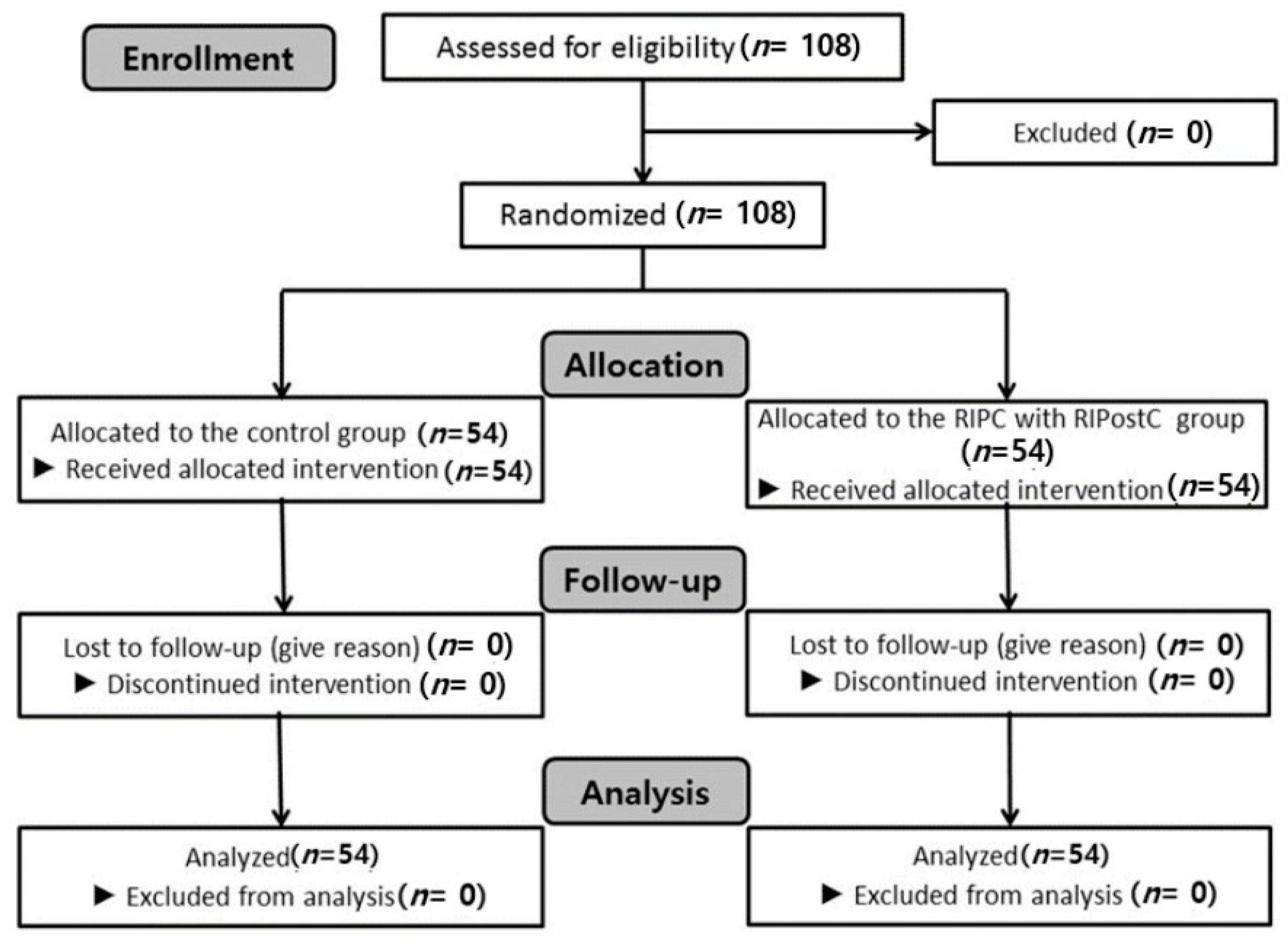
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Baaj, A.A.; Agazzi, S.; Sayed, Z.A.; Toledo, M.; Spetzler, R.F.; van Loveren, H. Surgical management of moyamoya disease: A review. Neurosurg. Focus 2009, 26, E7. [Google Scholar] [CrossRef] [PubMed]
- Burke, G.M.; Burke, A.M.; Sherma, A.K.; Hurley, M.C.; Batjer, H.H.; Bendok, B.R. Moyamoya disease: A summary. Neurosurg. Focus 2009, 26, E11. [Google Scholar] [CrossRef]
- Narisawa, A.; Fujimura, M.; Tominaga, T. Efficacy of the revascularization surgery for adult-onset moyamoya disease with the progression of cerebrovascular lesions. Clin. Neurol. Neurosurg. 2009, 111, 123–126. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Chien, C.-T.; Ma, M.-C.; Tseng, Y.-Z.; Lin, F.-Y.; Wang, S.-S.; Chen, C.-F. Protection “Outside the Box” (Skeletal Remote Preconditioning) in Rat Model is Triggered by Free Radical Pathway. J. Surg. 2005, 126, 92–101. [Google Scholar] [CrossRef]
- Przyklenk, K.; Bauer, B.; Ovize, M.; Kloner, R.A.; Whittaker, P. Regional ischemic ’preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 1993, 87, 893–899. [Google Scholar] [CrossRef]
- Yasin, N.A.H.M.; Herbison, P.; Saxena, P.; Praporski, S.; Konstantinov, I.E. The role of remote ischemic preconditioning in organ protection after cardiac surgery: A meta-analysis. J. Surg. Res. 2014, 186, 207–216. [Google Scholar] [CrossRef]
- Meng, R.; Asmaro, K.; Meng, L.; Liu, Y.; Ma, C.; Xi, C.; Li, G.; Ren, C.; Luo, Y.; Ling, F.; et al. Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology 2012, 79, 1853–1861. [Google Scholar] [CrossRef]
- Gonzalez, N.R.; Hamilton, R.; Bilgin-Freiert, A.; Dusick, J.; Vespa, P.; Hu, X.; Asgari, S. Cerebral hemodynamic and metabolic effects of remote ischemic preconditioning in patients with subarachnoid hemorrhage. Acta Neurochir. Suppl. 2013, 115, 193–198. [Google Scholar]
- Zhao, H.; Ren, C.; Chen, X.; Shen, J. From rapid to delayed and remote postconditioning: The evolving concept of ischemic postconditioning in brain ischemia. Curr. Drug Targets 2012, 13, 173–187. [Google Scholar] [CrossRef]
- Na, H.S.; Kim, Y.I.; Yoon, Y.W.; Han, H.C.; Nahm, S.H.; Hong, S.K. Ventricular premature beat—driven intermittent restoration of coronary blood flow reduces the incidence of reperfusion-induced ventricular fibrillation in a cat model of regional ischemia. Am. Heart J. 1996, 132, 78–83. [Google Scholar] [CrossRef]
- Zhao, H.; Sapolsky, R.M.; Steinberg, G.K. Interrupting reperfusion as a stroke therapy: Ischemic postconditioning reduces infarct size after focal ischemia in rats. J. Cereb. Blood Flow Metab. 2006, 26, 1114–1121. [Google Scholar] [CrossRef]
- Zhong, H.; Gao, Z.; Chen, M.; Zhao, J.; Wang, F.; Li, L.; Dong, H.; Liu, L.; Wang, Q.; Xiong, L. Cardioprotective effect of remote ischemic postconditioning on children undergoing cardiac surgery: A randomized controlled trial. Paediatr. Anaesth. 2013, 23, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Meng, R.; Ma, C.; Hou, B.; Jiao, L.; Zhu, F.; Wu, W.; Shi, J.; Duan, Y.; Zhang, R.; et al. Safety and Efficacy of Remote Ischemic Preconditioning in Patients With Severe Carotid Artery Stenosis Before Carotid Artery Stenting: A Proof-of-Concept, Randomized Controlled Trial. Circulation 2017, 135, 1325–1335. [Google Scholar] [CrossRef]
- Sales, A.H.A.; Barz, M.; Bette, S.; Wiestler, B.; Ryang, Y.-M.; Meyer, B.; Bretschneider, M.; Ringel, F.; Gempt, J. Impact of ischemic preconditioning on surgical treatment of brain tumors: A single-center, randomized, double-blind, controlled trial. BMC Med. 2017, 15, 137. [Google Scholar] [CrossRef] [PubMed]
- Laiwalla, A.N.; Ooi, Y.C.; Liou, R.; Gonzalez, N.R. Matched Cohort Analysis of the Effects of Limb Remote Ischemic Conditioning in Patients with Aneurysmal Subarachnoid Hemorrhage. Transl. Stroke Res. 2016, 7, 42–48. [Google Scholar] [CrossRef]
- Mi, T.; Yu, F.; Ji, X.; Sun, Y.; Qu, D. The Interventional Effect of Remote Ischemic Preconditioning on Cerebral Small Vessel Disease: A Pilot Randomized Clinical Trial. Eur. Neurol. 2016, 76, 28–34. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, R.; Song, H.; Liu, G.; Hua, Y.; Cui, D.; Zheng, L.; Feng, W.; Liebeskind, D.S.; Fisher, M.; et al. Remote Ischemic Conditioning May Improve Outcomes of Patients With Cerebral Small-Vessel Disease. Stroke 2017, 48, 3064–3072. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.J.; Chen, C.; Li, X.R.; Ran, Y.Y.; Xu, T.; Zhang, Y.; Geng, X.K.; Zhang, Y.; Du, H.S.; Leak, R.K.; et al. Remote Ischemic Preconditioning-Mediated Neuroprotection against Stroke is Associated with Significant Alterations in Peripheral Immune Responses. CNS Neurosci. Ther. 2016, 22, 43–52. [Google Scholar] [CrossRef]
- Zhou, G.; Li, M.H.; Tudor, G.; Lu, H.T.; Kadirvel, R.; Kallmes, D. Remote Ischemic Conditioning in Cerebral Diseases and Neurointerventional Procedures: Recent Research Progress. Front. Neurol. 2018, 9, 339. [Google Scholar] [CrossRef] [PubMed]
- Hoda, M.N.; Siddiqui, S.; Herberg, S.; Periyasamy-Thandavan, S.; Bhatia, K.; Hafez, S.S.; Johnson, M.H.; Hill, W.D.; Ergul, A.; Fagan, S.C.; et al. Remote ischemic perconditioning is effective alone and in combination with intravenous tissue-type plasminogen activator in murine model of embolic stroke. Stroke 2012, 43, 2794–2799. [Google Scholar] [CrossRef] [PubMed]
- Loukogeorgakis, S.P.; Williams, R.; Panagiotidou, A.T.; Kolvekar, S.K.; Donald, A.; Cole, T.J.; Yellon, D.M.; Deanfield, J.E.; MacAllister, R.J. Transient limb ischemia induces remote preconditioning and remote postconditioning in humans by a K(ATP)-channel dependent mechanism. Circulation 2007, 116, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, M.; Anderson, M.F.; Mallard, C.; Hagberg, H. Hypoxic preconditioning confers long-term reduction of brain injury and improvement of neurological ability in immature rats. Pediatr. Res. 2005, 57, 305–309. [Google Scholar] [CrossRef]
- Pignataro, G.; Esposito, E.; Sirabella, R.; Vinciguerra, A.; Cuomo, O.; Di Renzo, G.; Annunziato, L. nNOS and p-ERK involvement in the neuroprotection exerted by remote postconditioning in rats subjected to transient middle cerebral artery occlusion. Neurobiol. Dis. 2013, 54, 105–114. [Google Scholar] [CrossRef]
- Hess, D.C.; Hoda, M.N.; Bhatia, K. Remote limb perconditioning [corrected] and postconditioning: Will it translate into a promising treatment for acute stroke? Stroke 2013, 44, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Bastide, M.; Gele, P.; Petrault, O.; Pu, Q.; Caliez, A.; Robin, E.; Deplanque, D.; Duriez, P.; Bordet, R. Delayed cerebrovascular protective effect of lipopolysaccharide in parallel to brain ischemic tolerance. J. Cereb. Blood Flow Metab. 2003, 23, 399–405. [Google Scholar] [CrossRef]
- Lecour, S. Activation of the protective Survivor Activating Factor Enhancement (SAFE) pathway against reperfusion injury: Does it go beyond the RISK pathway? J. Mol. Cell Cardiol. 2009, 47, 32–40. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Iliodromitis, E.K.; Andreadou, I.; Papalois, A.; Gritsopoulos, G.; Anastasiou-Nana, M.; Kremastinos, D.T.; Yellon, D.M. Investigating the signal transduction pathways underlying remote ischemic conditioning in the porcine heart. Cardiovasc. Drugs Ther. 2012, 26, 87–93. [Google Scholar] [CrossRef]
- Hegelmaier, T.; Kumowski, N.; Mainka, T.; Vollert, J.; Goertz, O.; Lehnhardt, M.; Zahn, P.; Kolbenschlag, J.; Maier, C. Remote ischaemic conditioning decreases blood flow and improves oxygen extraction in patients with early complex regional pain syndrome. Eur. J. Pain. 2017, 21, 1346–1354. [Google Scholar] [CrossRef]
- Xiao, Y.; Hafeez, A.; Zhang, Y.; Liu, S.; Kong, Q.; Duan, Y.; Luo, Y.; Ding, Y.; Shi, H.; Ji, X. Neuroprotection by peripheral nerve electrical stimulation and remote postconditioning against acute experimental ischaemic stroke. Neurol. Res. 2015, 37, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Huo, K.; Liu, R.; Yang, J.; Cheng, Y.; Chang, S.; Ren, D.; Luo, G. The Design and Rationale of a Clinical Trial Evaluating Limb Postconditioning in Young Patients with Intracranial Arterial Stenosis. J. Stroke Cerebrovasc. Dis. 2016, 25, 2506–2512. [Google Scholar] [CrossRef]
- Cho, Y.J.; Lee, E.-H.; Lee, K.; Kim, T.K.; Hong, D.M.; Chin, J.-H.; Choi, D.-K.; Bahk, J.-H.; Sim, J.-Y.; Choi, I.-C.; et al. Long-term clinical outcomes of Remote Ischemic Preconditioning and Postconditioning Outcome (RISPO) trial in patients undergoing cardiac surgery. Int. J. Cardiol. 2017, 231, 84–89. [Google Scholar] [CrossRef]
- Chiu, D.; Shedden, P.; Bratina, P.; Grotta, J.C. Clinical features of moyamoya disease in the United States. Stroke 1998, 29, 1347–1351. [Google Scholar] [CrossRef]
- Iwama, T.; Hashimoto, N.; Yonekawa, Y. The relevance of hemodynamic factors to perioperative ischemic complications in childhood moyamoya disease. Neurosurgery 1996, 38, 1120–1126. [Google Scholar]
- Kurehara, K.; Ohnishi, H.; Touho, H.; Furuya, H.; Okuda, T. Cortical blood flow response to hypercapnia during anaesthesia in Moyamoya disease. Can. J. Anaesth. 1993, 40, 709–713. [Google Scholar] [CrossRef]
- Lee, S.H.; Heros, R.C.; Mullan, J.C.; Korosue, K. Optimum degree of hemodilution for brain protection in a canine model of focal cerebral ischemia. J. Neurosurg. 1994, 80, 469–475. [Google Scholar] [CrossRef]
- Kansha, M.; Irita, K.; Takahashi, S.; Matsushima, T. Anesthetic management of children with moyamoya disease. Clin. Neurol. Neurosurg. 1997, 99 (Suppl. 2), S110–S113. [Google Scholar] [CrossRef]
- Healy, D.; Khan, W.; Wong, C.; Moloney, M.C.; Grace, P.; Coffey, J.; Dunne, C.; Walsh, S.; Sadat, U.; Gaunt, M.; et al. Remote preconditioning and major clinical complications following adult cardiovascular surgery: Systematic review and meta-analysis. Int. J. Cardiol. 2014, 176, 20–31. [Google Scholar] [CrossRef]
- Przyklenk, K.; Whittaker, P. Remote ischemic preconditioning: Current knowledge, unresolved questions, and future priorities. J. Cardiovasc. Pharmacol. Ther. 2011, 16, 255–259. [Google Scholar] [CrossRef]
- Murphy, P.G.; Myer, D.S.; Davies, M.J.; Webster, N.R.; Jones, J.G. The antioxidant potential of profopol(2,6-diisopropylphenol). Br. J. Anaesth. 1992, 68, 613–618. [Google Scholar] [CrossRef]
- Hong, D.M.; Jeon, Y.; Lee, C.-S.; Kim, H.J.; Lee, J.-M.; Bahk, J.-H.; Kim, K.-B.; Hwang, H.Y. Effects of remote ischemic preconditioning with postconditioning in patients undergoing off-pump coronary bypass surgery. Circ. J. 2012, 76, 884–890. [Google Scholar] [CrossRef]
- Hong, D.M.; Min, J.J.; Kim, J.H.; Sohn, I.S.; Lim, T.W.; Lim, Y.J.; Bahk, J.H.; Jeon, Y. The effects of remote ischemic preconditioning on myocardial injury in patients undergoing off-pump coronary bypass graft surgery. Anaesth. Intensive Care 2010, 38, 924–929. [Google Scholar] [CrossRef]
- Lucchinetti, E.; Bestmann, L.; Feng, J.; Freidank, H.; Clanachan, A.S.; Finegan, B.A.; Zaugg, M. Remote ischaemic preconditioning applied during isoflurane inhalation provides no benefit to the myocarduim of patients undergoing on-pump coronary bypass graft surgery. Anesthesiology 2012, 116, 296–310. [Google Scholar] [CrossRef]
- Kottenberg, E.; Thielmann, M.; Bergmann, L.; Heine, T.; Jakob, H.; Heusch, G.; Peters, J. Protection by remote ischemic preconditioning during coronary artery bypass graft surgerywith isoflurane but not profopol-a clinical trail. Acta Anaesth. Scand. 2012, 56, 30–38. [Google Scholar] [CrossRef]
| Demographic Data | Control Group (n = 54) | RIPC with RIPostC Group (n = 54) | p Value |
|---|---|---|---|
| Age (year) | 39.0 ± 10.7 | 37.2 ± 10.8 | 0.378 |
| Sex (male/female) | 20/34 (37.0%/63.0%) | 19/35 (35.2%/64.8%) | 1.000 |
| Height (cm) | 164.7 ± 6.7 | 164.6 ± 8.0 | 0.955 |
| Weight (kg) | 68.3 ± 14.7 | 68.5 ± 13.7 | 0.917 |
| BMI | 24.6 ± 4.6 | 24.7 ± 4.2 | 0.862 |
| HTN | 15 (27.8%) | 15 (27.8%) | 1.000 |
| DM | 1 (1.9%) | 4 (7.4%) | 0.243 |
| Operation site (right/left) | 31/23 (57.4%/42.6%) | 26/28 (48.1%/51.9%) | 0.441 |
| Operation time (min) | 367.7 ± 94.1 | 360.0 ± 65.6 | 0.623 |
| Anesthetic time (min) | 429.7 ± 106.3 | 436.0 ± 71.7 | 0.719 |
| Postoperative Course | Control Group (n = 54) | RIPC with RIPostC Group (n = 54) | p Value |
|---|---|---|---|
| ICU stay duration (day) | 2.4 ± 1.0 | 2.1 ± 0.7 | 0.092 |
| Hospital stay duration (day) | 17.8 ± 11.3 | 13.8 ± 5.9 | 0.023 |
| MCA velocity (cc/min) | 33.9 ± 22.6 | 34.6 ± 19.0 | 0.872 |
| Neurologic Outcome | Control Group (n = 54) | RIPC with RIPostC Group (n = 54) | p Value |
|---|---|---|---|
| Hypoperfusion complication | |||
| TIA | 28 (51.9%) | 25 (46.3%) | 0.700 |
| Acute infarction | 8 (14.8%) | 2 (3.7%) | 0.093 |
| Hyperperfusion complication | |||
| Seizure | 3 (5.6%) | 0 (0%) | 0.243 |
| Hyperperfusion syndrome | 2 (3.7%) | 1 (1.9%) | 1.00 |
| Overall neurologic complication | 13 (24%) | 3 (5.6%) | 0.013 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, E.-S.; Lee, Y.-S.; Park, B.-S.; Kim, B.-G.; Sohn, H.-M.; Jeon, Y.-T. Effects of Combined Remote Ischemic Pre-and Post-Conditioning on Neurologic Complications in Moyamoya Disease Patients Undergoing Superficial Temporal Artery-Middle Cerebral Artery Anastomosis. J. Clin. Med. 2019, 8, 638. https://doi.org/10.3390/jcm8050638
Choi E-S, Lee Y-S, Park B-S, Kim B-G, Sohn H-M, Jeon Y-T. Effects of Combined Remote Ischemic Pre-and Post-Conditioning on Neurologic Complications in Moyamoya Disease Patients Undergoing Superficial Temporal Artery-Middle Cerebral Artery Anastomosis. Journal of Clinical Medicine. 2019; 8(5):638. https://doi.org/10.3390/jcm8050638
Chicago/Turabian StyleChoi, Eun-Su, Yoon-Sook Lee, Byeong-Seon Park, Byung-Gun Kim, Hye-Min Sohn, and Young-Tae Jeon. 2019. "Effects of Combined Remote Ischemic Pre-and Post-Conditioning on Neurologic Complications in Moyamoya Disease Patients Undergoing Superficial Temporal Artery-Middle Cerebral Artery Anastomosis" Journal of Clinical Medicine 8, no. 5: 638. https://doi.org/10.3390/jcm8050638
APA StyleChoi, E.-S., Lee, Y.-S., Park, B.-S., Kim, B.-G., Sohn, H.-M., & Jeon, Y.-T. (2019). Effects of Combined Remote Ischemic Pre-and Post-Conditioning on Neurologic Complications in Moyamoya Disease Patients Undergoing Superficial Temporal Artery-Middle Cerebral Artery Anastomosis. Journal of Clinical Medicine, 8(5), 638. https://doi.org/10.3390/jcm8050638





