Mean Platelet Volume Predicts Vascular Access Events in Hemodialysis Patients
Abstract
1. Introduction
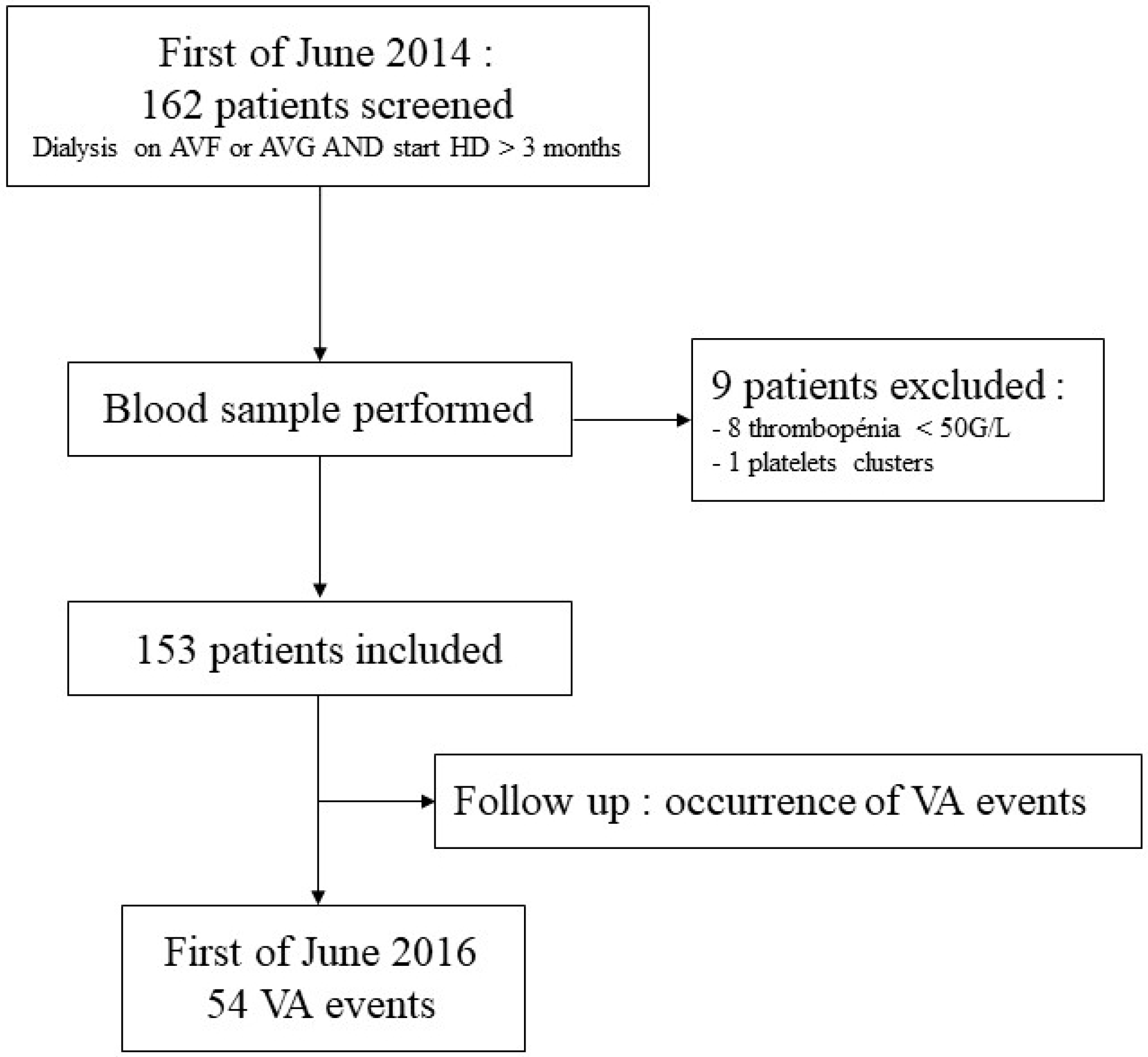
2. Methods
- Clinical parameters as extension of compression time, decreasing thrill, collateral venous circulation, edema of the arm with the VA or palpation of a stenosis;
- and/or dialysis parameters as a drop in KT/v, significant increase in venous pressure, impossibility to obtain the usual VA blood flow;
- and/or Transonic® or Doppler ultrasound parameters as a decrease in the VA blood flow higher than 20%.
3. Results
3.1. Baseline Characteristics of the Cohort
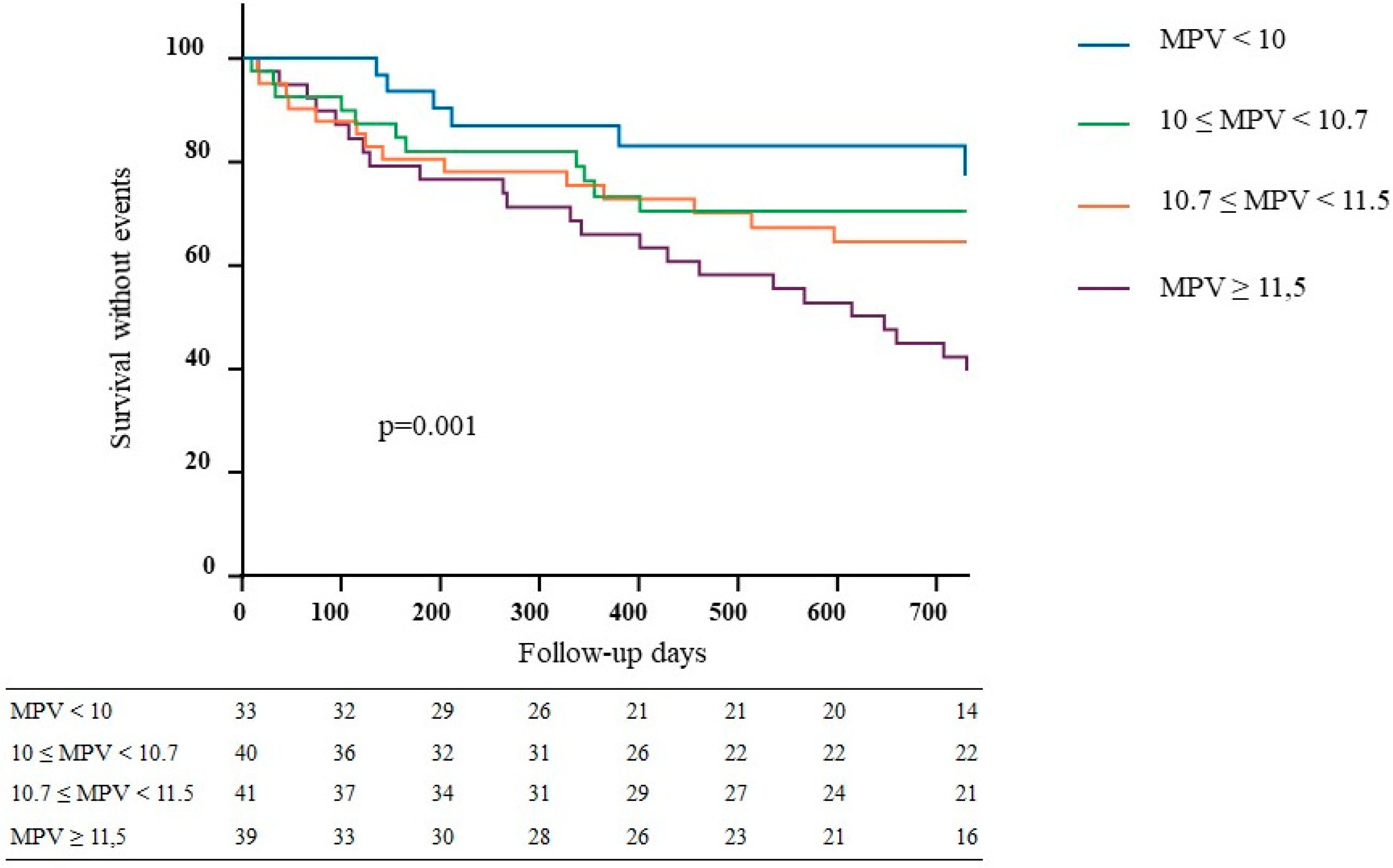
3.2. Association between MPV and VA Events
3.3. Secondary Outcomes
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- R.E.I.N RAPPORT ANNUEL 2015. Available online: https://www.agence-biomedecine.fr/IMG/pdf/rapport_rein_2015.pdf (accessed on 3 May 2019).
- Chazan, J.A.; London, M.R.; Pono, L.M. Long-term survival of vascular accesses in a large chronic hemodialysis population. Nephron 1995, 69, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Franzoni, M.; Cattaneo, I.; Longaretti, L.; Figliuzzi, M.; Ene-Iordache, B.; Remuzzi, A. Endothelial cell activation by hemodynamic shear stress derived from arteriovenous fistula for hemodialysis access. Am. J. Physiol. 2016, 310, H49–H59. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.; Menke, J.; Sollinger, D.; Schinzel, H.; Thürmel, K. Haemostasis in chronic kidney disease. Nephrol. Dial. Transplant. 2014, 29, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Chronic Kidney Disease Prognosis Consortium; Matsushita, K.; van der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; de Jong, P.E.; Coresh, J.; Gansevoort, R.T. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 2010, 375, 2073–2081. [Google Scholar] [PubMed]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Wattanakit, K.; Cushman, M.; Stehman-Breen, C.; Heckbert, S.R.; Folsom, A.R. Chronic kidney disease increases risk for venous thromboembolism. J. Am. Soc. Nephrol. 2008, 19, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, G.; Cohen, G.; Jankowski, J.; Vanholder, R. Platelet/Leukocyte activation, inflammation, and uremia. Semin Dial 2009, 22, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Faure, V.; Dou, L.; Sabatier, F.; Cerini, C.; Sampol, J.; Berland, Y.; Brunet, P.; Dignat-George, F. Elevation of circulating endothelial microparticles in patients with chronic renal failure. J. Thromb. Haemost. 2006, 4, 566–573. [Google Scholar] [CrossRef]
- Sallée, M.; Dou, L.; Cerini, C.; Poitevin, S.; Brunet, P.; Burtey, S. The aryl hydrocarbon receptor-activating effect of uremic toxins from tryptophan metabolism: A new concept to understand cardiovascular complications of chronic kidney disease. Toxins 2014, 6, 934–949. [Google Scholar] [CrossRef]
- Dou, L.; Sallée, M.; Cerini, C.; Poitevin, S.; Gondouin, B.; Jourde-Chiche, N.; Fallague, K.; Brunet, P.; Calaf, R.; Dussol, B.; et al. The cardiovascular effect of the uremic solute indole-3 acetic acid. J. Am. Soc. Nephrol. 2015, 26, 876–887. [Google Scholar] [CrossRef]
- Martin, J.F.; Trowbridge, E.A.; Salmon, G.; Plumb, J. The biological significance of platelet volume: Its relationship to bleeding time, platelet thromboxane B2 production and megakaryocyte nuclear DNA concentration. Thromb. Res. 1983, 32, 443–460. [Google Scholar] [CrossRef]
- Bataille, S.; Burtey, S.; Decourt, A.; Frère, C.; Henneuse, A.; Aillaud, M.-F.; Morange, P.; Bardin, N.; Duval, A.; Sallée, M.; et al. Antiphospholipids antibodies and hemodialysis: A frequent association linked to arteriovenous fistula thrombosis. Nephrol. Ther. 2015, 11, 27–33. [Google Scholar] [CrossRef]
- Salmela, B.; Hartman, J.; Peltonen, S.; Albäck, A.; Lassila, R. Thrombophilia and arteriovenous fistula survival in ESRD. Clin. J. Am. Soc. Nephrol. 2013, 8, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Klamroth, R.; Orlovic, M.; Fritsche, I.; Seibt, S.; Seibt, F.; Wegscheider, K.; Landgraf, H. The influence of thrombophilic risk factors on vascular access survival in chronic dialysis patients in a retrospective evaluation. VASA 2013, 42, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.B.; Jakubowski, J.A.; Quinn, P.G.; Deykin, D.; Valeri, C.R. Platelet size as a determinant of platelet function. J. Lab. Clin. Med. 1983, 101, 205–213. [Google Scholar] [PubMed]
- Niethammer, A.G.; Forman, E.N. Use of the platelet histogram maximum in evaluating thrombocytopenia. Am. J. Hematol. 1999, 60, 19–23. [Google Scholar] [CrossRef]
- Troussard, X.; Vol, S.; Cornet, E.; Bardet, V.; Couaillac, J.-P.; Fossat, C.; Luce, J.-C.; Maldonado, E.; Siguret, V.; Tichet, J.; et al. Full blood count normal reference values for adults in France. J. Clin. Pathol. 2014, 67, 341–344. [Google Scholar] [CrossRef]
- Anderson, J.; Abbuhl, M.; Hering, T.; Johnston, K. Immunohistochemical identification of components in thehealing response of human vascular grafts. ASAIO J. 1985, 8, 79–85. [Google Scholar]
- Malik, J.; Kudlicka, J.; Novakova, L.; Adamec, J.; Malikova, H.; Kavan, J. Surveillance of arteriovenous accesses with the use of duplex Doppler ultrasonography. J. Vasc. Access 2014, 15, S28–S32. [Google Scholar] [CrossRef] [PubMed]
- The R Project for Statistical Computing. Available online: http://www.R-project.org/ (accessed on 2 January 2019).
- Roy-Chaudhury, P.; Sukhatme, V.P.; Cheung, A.K. Hemodialysis vascular access dysfunction: A cellular and molecular viewpoint. J. Am. Soc. Nephrol. 2006, 17, 1112–1127. [Google Scholar] [CrossRef] [PubMed]
- Badrnya, S.; Schrottmaier, W.C.; Kral, J.B.; Yaiw, K.-C.; Volf, I.; Schabbauer, G.; Söderberg-Nauclér, C.; Assinger, A. Platelets mediate oxidized low-density lipoprotein-induced monocyte extravasation and foam cell formation. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.H.; Kantarjian, H.M.; Cortes, J.E. Biology of platelet-derived growth factor and its involvement in disease. Mayo Clin. Proc. 2006, 81, 1241–1257. [Google Scholar] [CrossRef] [PubMed]
- Stracke, S.; Konner, K.; Köstlin, I.; Friedl, R.; Jehle, P.M.; Hombach, V.; Keller, F.; Waltenberger, J. Increased expression of TGF-beta1 and IGF-I in inflammatory stenotic lesions of hemodialysis fistulas. Kidney Int. 2002, 61, 1011–1019. [Google Scholar] [CrossRef]
- ElChoufani, S.E.; Bolin, P.; Waien, S.; Christiano, C.R.; Holbert, D.; Bode, A.P. Platelet Adhesion Testing May Predict Early Hemodialysis Arteriovenous Graft and Fistula Failure in End-Stage Renal Disease Patients. Clin. Appl. Thromb./Hemos. 2008, 14, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Suzuki, Y.; Nakayama, T.; Ohmori, M.; Masai, S.; Sasagawa, N.; Ohyama, K. Duplex ultrasound for the prediction of vascular events associated with arteriovenous fistulas in hemodialysis patients. J. Vasc. Access 2016, 17, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Ravani, P.; Quinn, R.R.; Oliver, M.J.; Karsanji, D.J.; James, M.T.; MacRae, J.M.; Palmer, S.C.; Strippoli, G.F.M. Preemptive Correction of Arteriovenous Access Stenosis: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Am. J. Kidney Dis. 2016, 67, 446–460. [Google Scholar] [CrossRef] [PubMed]
- Tordoir, J.; Canaud, B.; Haage, P.; Konner, K.; Basci, A.; Fouque, D.; Kooman, J.; Martin-Malo, A.; Pedrini, L.; Pizzarelli, F.; et al. EBPG on Vascular Access. Nephrol. Dial. Transplant. 2007, 22, ii88–ii117. [Google Scholar] [CrossRef]
- Tessitore, N.; Bedogna, V.; Poli, A.; Mantovani, W.; Lipari, G.; Baggio, E.; Mansueto, G.; Lupo, A. Adding access blood flow surveillance to clinical monitoring reduces thrombosis rates and costs, and improves fistula patency in the short term: A controlled cohort study. Nephrol. Dial. Transplant. 2008, 23, 3578–3584. [Google Scholar] [CrossRef]
- Parisotto, M.T.; Schoder, V.U.; Miriunis, C.; Grassmann, A.H.; Scatizzi, L.P.; Kaufmann, P.; Stopper, A.; Marcelli, D. Cannulation technique influences arteriovenous fistula and graft survival. Kidney Int. 2014, 86, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.G.; Becker, R.C.; Berger, P.B.; Bhatt, D.L.; Eikelboom, J.W.; Konkle, B.; Mohler, E.R.; Reilly, M.P.; Berger, J.S. Mean platelet volume as a predictor of cardiovascular risk: A systematic review and meta-analysis. J. Thromb. Haemost. 2010, 8, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Noris, P.; Melazzini, F.; Balduini, C.L. New roles for mean platelet volume measurement in the clinical practice? Platelets 2016, 27, 607–612. [Google Scholar] [CrossRef]
- Henning, B.F.; Zidek, W.; Linder, B.; Tepel, M. Mean platelet volume and coronary heart disease in hemodialysis patients. Kidney Blood Press. Res. 2002, 25, 103–108. [Google Scholar] [CrossRef]
- Bal, Z.; Bal, U.; Okyay, K.; Yilmaz, M.; Balcioglu, S.; Turgay, O.; Hasirci, S.; Aydinalp, A.; Yildirir, A.; Sezer, S.; et al. Hematological parameters can predict the extent of coronary artery disease in patients with end-stage renal disease. Int. Urol. Nephrol. 2015, 47, 1719–1725. [Google Scholar] [CrossRef]
- Kim, S.; Molnar, M.Z.; Fonarow, G.C.; Streja, E.; Wang, J.; Gillen, D.L.; Mehrotra, R.; Brunelli, S.M.; Kovesdy, C.P.; Kalantar-Zadeh, K.; et al. Mean platelet volume and mortality risk in a national incident hemodialysis cohort. Int. J. Cardiol. 2016, 220, 862–870. [Google Scholar] [CrossRef]
- Cho, A.; Choi, M.J.; Lee, Y.-K.; Hoon, H.C.; Koo, J.-R.; Yoon, J.-W.; Noh, J.-W. Effects of aspirin resistance and mean platelet volume on vascular access failure in hemodialysis patients. Korean J. Intern. Med. 2018. [Google Scholar] [CrossRef]
- Shin, D.H.; Rhee, S.Y.; Jeon, H.J.; Park, J.-Y.; Kang, S.-W.; Oh, J. An Increase in Mean Platelet Volume/Platelet Count Ratio Is Associated with Vascular Access Failure in Hemodialysis Patients. PLoS ONE 2017, 12, e0170357. [Google Scholar] [CrossRef]
- Coleman, C.I.; Tuttle, L.A.; Teevan, C.; Baker, W.L.; White, C.M.; Reinhart, K.M. Antiplatelet agents for the prevention of arteriovenous fistula and graft thrombosis: A meta analysis: Antiplatelet agents and access thrombosis. Int. J. Clin. Pract. 2010, 64, 1239–1244. [Google Scholar] [CrossRef]
- Tanner, N.C.; Da Silva, A. Medical adjuvant treatment to increase patency of arteriovenous fistulae and grafts. In Cochrane Database of Systematic Reviews; The Cochrane Collaboration, Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2015. [Google Scholar]
- De Marchi, S.; Falleti, E.; Giacomello, R.; Stel, G.; Cecchin, E.; Sepiacci, G.; Bortolotti, N.; Zanello, F.; Gonano, F.; Bartoli, E. Risk factors for vascular disease and arteriovenous fistula dysfunction in hemodialysis patients. J. Am. Soc. Nephrol. 1996, 7, 1169–1177. [Google Scholar]

| Patients Characteristics | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | All Population | p |
|---|---|---|---|---|---|---|
| N = 33 | N = 40 | N = 41 | N = 39 | N = 153 | ||
| Age, years, mean (SD) | 68.1 (16.9) | 64.8 (15.9) | 65.8 (14.5) | 63.7 (19) | 65.5 (16.5) | 0.66 |
| Female (%) | 16 (48) | 18 (45) | 13 (32) | 15 (38) | 62 (41) | 0.46 |
| BMI (kg/m2), mean (SD) | 23.6 (4.4) | 24.8 (4.4) | 25.2 (5.2)) | 24.4 (4.7) | 24.6 (4.6) | 0.56 |
| ESRD vintage, years. mean (SD) | 8.1 (8.4) | 6.9 (10.5) | 8.5 (8.1) | 7.9 (8.7) | 7.8 (8.8) | 0.44 |
| Transplantation n (%) | 5 (15) | 3 (7) | 6 (15) | 8 (20) | 22 (14) | 0.43 |
| Vascular access n (%) | ||||||
| AVF | 24 (73) | 31 (78) | 34 (82) | 31 (79) | 120 (78) | 0.76 |
| AVG | 9 (27) | 9 (22) | 7 (18) | 8 (21) | 33 (22) | 0.76 |
| Comorbidities (%) | ||||||
| Congestive heart failure | 10 (30) | 10 (25) | 8 (20) | 10 (26) | 38 (25) | 0.45 |
| Atrial fibrillation | 9 (27) | 12 (30) | 11 (27) | 14 (36) | 46 (30) | 0.81 |
| Ischemic heart disease | 9 (27) | 12 (30) | 17 (41) | 16 (41) | 54 (35) | 0.45 |
| Stroke | 4 (12) | 6 (15) | 2 (5) | 7 (18) | 19 (12) | 0.32 |
| Venous thrombosis | 8 (24) | 4 (10) | 4 (10) | 3 (8) | 21 (14) | 0.14 |
| Dyslipidemia | 13 (36) | 12 (30) | 17 (41) | 20 (51) | 61 (40) | 0.26 |
| Tobacco past/present | 11 (35) | 17 (49) | 18 (50) | 20 (54) | 66 (43) | 0.47 |
| Diabetes | 10 (30) | 15 (37) | 15 (37) | 15 (38) | 55 (40) | 0.91 |
| Hypertension | 28 (85) | 37 (92) | 35 (85) | 35 (90) | 135 (88) | 0.69 |
| Peripheral vascular disease | 7 (21) | 10 (25) | 11 (27) | 12 (31) | 40 (55) | 0.83 |
| Cancer | 6 (19) | 10 (25) | 8 (20) | 6 (15) | 30 (20) | 0.76 |
| Laboratory variable mean (± SD) | ||||||
| Hemoglobin (g/dL) | 10.8 (1.9) | 10.6 (1.4) | 10.6 (1.1) | 10.7 (1.0) | 10.7 (1.2) | 0.9 |
| Platelet count (G/L) | 219 (76) | 225 (87) | 205 (60) | 153 (47) | 200 (74) | <0.001 |
| MPV (fL) | 9.5 (0.5) | 10.3 (0.1) | 11.0 (0.2) | 12.8 (0.5) | 10.8 (1.1) | <0.001 |
| Leukocytes (G/L) | 6.8 (1.9) | 7.4 (2.9) | 6.6 (2.7) | 6.4 (2.1) | 6.7 (2.5) | 0.36 |
| Beta2 microglobulin (mg/L) | 28.4 (7.8) | 26.7 (6.5) | 29.4 (5.3) | 26.9 (8) | 27.9 (6.9) | 0.09 |
| Urea (mg/dL) | 56.3 (12.9) | 61.9 (19.3) | 59.1 (21.6) | 59.7 (14.0) | 59.7 (17.6) | 0.81 |
| Creatinine (mg/dL) | 7.96 (2.58) | 8.87 (2.62) | 9.60 (3.00) | 8.50 (2.15) | 8.78 (2.65) | 0.05 |
| Potassium (mmol/L) | 4.8 (0.6) | 5.1 (0.8) | 5.0 (0.7) | 4.9 (0.6) | 5.0 (0.7) | 0.32 |
| Bicarbonate (mmol/L) | 20.3 (2.1) | 20.6 (2.4) | 21.1 (2.5) | 21.3 (2.4) | 21.0 (2.3) | 0.53 |
| Calcium (mg/dL) | 9.38 (2.04) | 9.58 (0.48) | 9.54 (0.68) | 9.30 (0.68) | 9.46 (0.68) | 0.27 |
| Phosphorus (mg/dL) | 4.77 (1.58) | 5.17 (2.26) | 5.20 (2.20) | 4.49 (1.52) | 4.96 (1.86) | 0.34 |
| Albumin (g/L) | 36.6 (5.3) | 38.2 (4.8) | 39.4 (5.0) | 38.7 (4.9) | 38.3 (5.0) | 0.06 |
| Parathyroid hormone (pg/mL) | 23.8 (26.9) | 29.8 (31.2) | 30.1 (39.5) | 29.3 (41.2) | 28.6 (35.6) | 0.9 |
| C reactive protein (mg/L) | 19.1 (25.5) | 10.8 (15.7) | 17.5 (41.7) | 10.1 (16.6) | 14.2 (27.3) | 0.28 |
| Iron saturation (%) | 26 (10) | 27 (18) | 23 (9) | 31 (14) | 27 (13) | 0.06 |
| Ferritin (µg/L) | 580 (319) | 465 (278) | 494 (345) | 530 (291) | 515 (310) | 0.38 |
| Dialysis modality | ||||||
| Hemodialysis n (%) | 13 (40) | 13 (32) | 14 (35) | 21 (54) | 61 (40) | 0.20 |
| Hemofiltration/Hemodiafiltration n (%) | 20 (60) | 27 (67) | 27 (65) | 18 (46) | 92 (60) | 0.20 |
| Kt/V. mean (SD) | 1.41 (0.25) | 1.50 (0.28) | 1.53 (0.30) | 1.53 (0.21 | 1.50 (0.26) | 0.31 |
| Time per week. hours. mean (SD) | 12.3 (1.7) | 13.5 (2.6) | 13.5 (2.63) | 12.8 (2.1) | 13.1 (2.2) | 0.08 |
| Medication | ||||||
| ACEI/ARB n (%) | 5 (17) | 11 (27) | 9 (22) | 13 (33) | 38 (25) | 0.32 |
| Beta-blocker n (%) | 10 (30) | 19 (47) | 18 (44) | 20 (51) | 67 (44) | 0.31 |
| Calcium channel blocker n (%) | 3 (9) | 9 (22) | 9 (22) | 11 (28) | 32 (21) | 0.25 |
| Diuretic n (%) | 10 (30) | 14 (35) | 18 (43) | 16 (41) | 58 (38) | 0.63 |
| Antiplatelet agent n (%) | 15 (45) | 19 (47) | 22 (54) | 23 (60) | 79 (52) | 0.64 |
| Aspirin n (%) | 11 (35) | 20 (56) | 20 (53) | 14 (42) | 65 (42) | 0.33 |
| Clopidogrel n (%) | 4 (12) | 8 (20) | 9 (22) | 11 (28) | 32 (21) | 0.42 |
| Vitamin K antagonist n (%) | 6 (18) | 7 (17) | 6 (15) | 12 (31) | 31 (20) | 0.29 |
| Statin n (%) | 7 (21) | 12 (30) | 17 (41) | 16 (41) | 52 (34) | 0.21 |
| Intravenous iron n (%) | 20 (61) | 24 (60) | 22 (54) | 24 (62) | 90 (59) | 0.9 |
| Erythropoietin. µg/s (SD) | 57 (52) | 52 (60) | 62 (70) | 33 (30) | 51 (55) | 0.10 |
| Patients Characteristics | With VA Events | Without VA Events | p |
|---|---|---|---|
| n = 54 | n = 99 | ||
| Age, years, mean (SD) | 66.0 (18.1) | 65.3 (15.7) | 0.62 |
| Female (%) | 21 (39) | 41 (41) | 0.86 |
| BMI (kg/m2), mean (SD) | 24.5 (4.6) | 24.6 (4.6) | 0.88 |
| ESRD vintage, years, mean (SD) | 8.4 (9.0) | 7.5 (8.5) | 0.27 |
| Transplantation n (%) | 8 (15) | 14 (14) | 0.43 |
| Vascular access n (%) | |||
| AVF | 38 (70) | 82 (83) | 0.10 |
| AVG | 16 (30) | 17 (17) | 0.10 |
| Comorbidities (%) | |||
| Congestive heart failure | 16 (30) | 22 (22) | 0.33 |
| Atrial fibrillation | 17 (31) | 29 (29) | 0.86 |
| Ischemic heart disease | 19 (35) | 35 (35) | 0.90 |
| Stroke | 10 (19) | 9 (9) | 0.12 |
| Venous thrombosis | 8 (15) | 13 (13) | 0.52 |
| Dyslipidemia | 19 (35) | 42 (42) | 0.40 |
| Tobacco past/present | 22 (41) | 44 (44) | 0.60 |
| Diabetes | 18 (33) | 37 (37) | 0.72 |
| Hypertension | 48 (89) | 87 (88) | 0.90 |
| Peripheral vascular disease | 16 (30) | 24 (24) | 0.56 |
| Cancer | 9 (17) | 21 (21) | 0.52 |
| Laboratory variable mean (± SD) | |||
| Hemoglobin (g/dL) | 10.7 (1.4) | 10.6 (1.2) | 0.68 |
| Platelet count (G/L) | 161 (56) | 222 (74) | <0.001 |
| MPV (fL) | 11.3 (1.2) | 10.6 (0.9) | <0.001 |
| Leukocytes (G/L) | 6.3 (0.3) | 6.9 (2.7) | 0.23 |
| Beta2 microglobulin (mg/L) | 27.7 (7.2) | 27.9 (6.7) | 0.9 |
| Urea (mg/dL) | 58.0 (22.4) | 60.8 (14.0) | 0.08 |
| Creatinine (mg/dL) | 8.76 (2.93) | 8.78 (2.51) | 0.84 |
| Potassium (mmol/L) | 5.0 (0.8) | 5.0 (0.6) | 0.87 |
| Bicarbonate(mmol/L) | 21.2 (2.6) | 20.9 (2.2) | 0.29 |
| Calcium (mg/dL) | 9.46 (0.64) | 9.48 (1.08) | 0.73 |
| Phosphorus (mg/dL) | 4.86 (1.24) | 4.96 (1.86) | 0.36 |
| Albumin (g/L) | 38.4 (5.5) | 38.2 (4.8) | 0.57 |
| Parathyroid hormone (pg/mL) | 31 (40) | 27 (33) | 0.62 |
| C reactive protein (mg/L) | 11.5 (17.6) | 15.7 (31.3) | 0.61 |
| Iron saturation (%) | 28 (14) | 27 (13) | 0.69 |
| Ferritin (µg/L) | 488 (285) | 530 (323) | 0.69 |
| Dialysis modality | |||
| Hemodialysis n (%) | 25 (46) | 36 (36) | 0.30 |
| Hemofiltration/Hemodiafiltration n (%) | 29 (54) | 63 (64) | 0.30 |
| Kt/V, mean (SD) | 1.51 (0.31) | 1.50 (0.23) | 0.9 |
| Time per week, hours, mean (SD) | 13.2 (2.2) | 13 (2.3) | 0.50 |
| Medication | |||
| ACEI/ARB n (%) | 16 (30) | 23 (23) | 0.43 |
| Beta-blocker n (%) | 27 (50) | 41 (41) | 0.39 |
| Calcium channel blocker n (%) | 13 (24) | 18 (18) | 0.40 |
| Diuretic n (%) | 14 (26) | 43 (43) | 0.39 |
| Ant platelet agent n (%) | 26 (48) | 53 (52) | 0.61 |
| Aspirin n (%) | 21 (39) | 45 (45) | 0.50 |
| Clopidogrel n (%) | 14 (26) | 15 (15) | 0.13 |
| Vitamin K antagonist (%) | 10 (19) | 21 (21) | 0.67 |
| Statin n (%) | 22 (41) | 30 (30) | 0.28 |
| Intra venous iron n (%) | 30 (56) | 60 (61) | 0.49 |
| Erythropoietin, µg/s (SD) | 50 (56) | 51 (58) | 0.45 |
| Odds Ratios (95% CI) of VA Events | p | |
|---|---|---|
| Age/years | 0,99 (0.97–1) | 0.09 |
| Sex (female) | 1.42 (0.77–2.60) | 0.26 |
| MPV | 1.58 (1.17–2.14) | 0.003 |
| platelets | 1 (0.99–1) | 0.7 |
| AVF vs. AVG | 0.48 (0.22–1.08) | 0.08 |
| No APA | 0.72 (0.38–1.36) | 0.31 |
| No AVK | 1.07 (0.52–2.18) | 0.86 |
| CRP | 1 (0.99–1.01) | 0.20 |
| Hemoglobin | 0.83 (0.59–1.17) | 0.29 |
| ESA | 0.99 (0.99–1) | 0.40 |
| Odds Ratios (95% CI) of VA Stenosis | p | |
|---|---|---|
| Age/years | 0.99 (0.97–1) | 0.13 |
| Sex (female) | 1.78 (0.89–3.53) | 0.10 |
| MPV | 1.55 (1.10–2.18) | 0.001 |
| platelets | 1 (0.99–1) | 0.95 |
| AVF vs. AVG | 0.37 (0.13–0,96) | 0.04 |
| No APA | 0.70 (0.33–1.38) | 0.28 |
| No AVK | 1.20 (0.55–2.65) | 0.66 |
| CRP | 1 (1–1.02) | 0.03 |
| Hemoglobin | 0.84 (0.57–1.25) | 0.39 |
| ESA | 0.99 (0.98–1.00) | 0.33 |
| Odds Ratios (95% CI) of VA Thrombosis | p | |
|---|---|---|
| Age/years | 0.99 (0.97–1.02) | 0.70 |
| Sex (female) | 0.75 (0.32–1.77) | 0.51 |
| MPV | 1.32 (0.90–1.92) | 0.16 |
| platelets | 1 (0.99–1) | 0.80 |
| AVF vs. AVG | 0.83 (0.31–2.21) | 0.71 |
| No APA | 0.50 (0.21–1.19) | 0.12 |
| No AVK | 1.07 (0.40–2.83) | 0.89 |
| CRP | 1 (0.99–1.02) | 0.26 |
| Hemoglobin | 0.83 (0.53–1.29) | 0.41 |
| ESA | 1 (0.99–1.00) | 0.70 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lano, G.; Sallée, M.; Pelletier, M.; Bataille, S.; Fraisse, M.; Berda-Haddad, Y.; Brunet, P.; Burtey, S. Mean Platelet Volume Predicts Vascular Access Events in Hemodialysis Patients. J. Clin. Med. 2019, 8, 608. https://doi.org/10.3390/jcm8050608
Lano G, Sallée M, Pelletier M, Bataille S, Fraisse M, Berda-Haddad Y, Brunet P, Burtey S. Mean Platelet Volume Predicts Vascular Access Events in Hemodialysis Patients. Journal of Clinical Medicine. 2019; 8(5):608. https://doi.org/10.3390/jcm8050608
Chicago/Turabian StyleLano, Guillaume, Marion Sallée, Marion Pelletier, Stanislas Bataille, Megan Fraisse, Yaël Berda-Haddad, Philippe Brunet, and Stéphane Burtey. 2019. "Mean Platelet Volume Predicts Vascular Access Events in Hemodialysis Patients" Journal of Clinical Medicine 8, no. 5: 608. https://doi.org/10.3390/jcm8050608
APA StyleLano, G., Sallée, M., Pelletier, M., Bataille, S., Fraisse, M., Berda-Haddad, Y., Brunet, P., & Burtey, S. (2019). Mean Platelet Volume Predicts Vascular Access Events in Hemodialysis Patients. Journal of Clinical Medicine, 8(5), 608. https://doi.org/10.3390/jcm8050608





