CA125 as a Marker of Heart Failure in the Older Women: A Population-Based Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Biochemical Measurements
2.2. Data Analysis
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
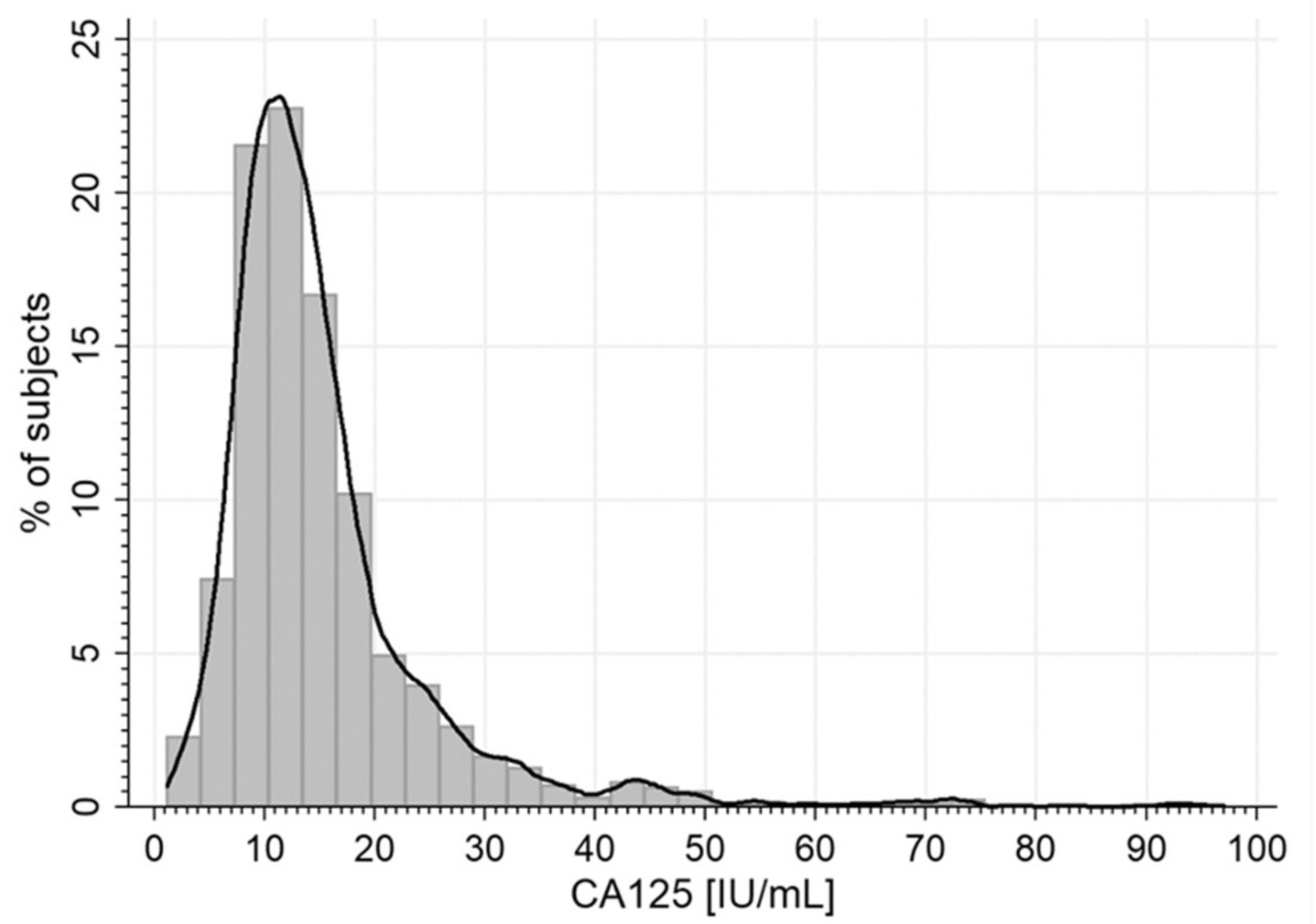
3.2. Serum CA125 Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A

References
- Weiland, F.; Fritz, K.; Oehler, M.K.; Hoffmann, P. Methods for identification of CA125 from ovarian cancer ascites by high resolution mass spectrometry. Int. J. Mol. Sci. 2012, 13, 9942–9958. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Cao, Y.; Liu, X.; Zeng, X.-T.; Li, Y. Serum CA125 is a predictive marker for breast cancer outcomes and correlates with molecular subtypes. Oncotarget 2017, 8, 63963–63970. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, Y.; Wang, Y.; Zhang, L. Role of serum CA125 and CA199 concentration in diagnosis and prognosis evaluation of lung cancer patients. Int. J. Clin. Exp. Pathol. 2016, 9, 5388–5396. [Google Scholar]
- Polat, E.; Duman, U.; Duman, M.; Derya Peker, K.; Akyuz, C.; Fatih Yasar, N.; Uzun, O.; Akbulut, S.; Birol Bostanci, E.; Yol, S. Preoperative serum tumor marker levels in gastric cancer. Pak. J. Med. Sci. 2014, 30, 145–149. [Google Scholar]
- Meden, H.; Fattahi-Meibodi, A. CA 125 in benign gynecological conditions. Int. J. Biol. Markers. 1998, 13, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Li, K.H.C.; Gong, M.; Li, G.; Baranchuk, A.; Liu, T.; Wong, M.C.S.; Jesuthasan, A.; Lai, R.W.C.; Lai, J.C.L.; Lee, A.P.W.; et al. Cancer antigen-125 and outcomes in acute heart failure: A systematic review and meta-analysis. Heart Asia 2018, 10, e011044. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.; Gong, M.; Bellanti, R.; Ali-Hasan-Al-Saegh, S.; Li, G.; Roig, E.; Núñez, J.; Stamos, T.D.; Yilmaz, M.B.; Hakki, K.; et al. Cancer antigen-125 and risk of atrial fibrillation: A systematic review and meta-analysis. Heart Asia 2018, 10, e010970. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, M.; Zhu, J.; Yao, P.; Li, X.; Yuan, J.; Min, X.; Lang, M.; Yang, H.; Hu, F.B.; et al. Higher carbohydrate antigen 125 levels are associated with increased risk of coronary heart disease in elderly chinese: a population-based case-control study. PLoS ONE 2013, 8, e81328. [Google Scholar] [CrossRef]
- Becerra-Muñoz, V.M.; Sobrino-Márquez, J.M.; Rangel-Sousa, D.; Fernández-Cisnal, A.; Lage-Gallé, E.; García-Pinilla, J.M.; Martínez-Martínez, Á.; de Teresa-Galván, E. Long-term prognostic role of CA-125 in noncongestive patients undergoing a cardiac transplantation. Biomark Med. 2017, 11, 239–243. [Google Scholar]
- Rheude, T.; Pellegrini, C.; Reinhard, W. Determinants of elevated carbohydrate antigen 125 in patients with severe symptomatic aortic valve stenosis referred for transcatheter aortic valve implantation. Biomarkers 2018, 23, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, N.M. Relationship between pro-anti-inflammatory cytokines, T-cell activation and CA 125 in obese patients with heart failure. Med. Sci. Monit. 2011, 17, CR174–CR179. [Google Scholar] [CrossRef]
- Kosar, F.; Aksoy, Y.; Ozguntekin, G.; Ozerol, I.; Varol, E. Relationship between cytokines and tumour markers in patients with chronic heart failure. Eur. J. Heart Fail 2006, 8, 270–274. [Google Scholar] [CrossRef]
- D’Aloia, A.; Faggiano, P.; Aurigemma, G.; Bontempi, L.; Ruggeri, G.; Metra, M.; Nodari, S.; Dei Cas, L. Serum levels of carbohydrate antigen 125 in patients with chronic heart failure: Relation to clinical severity, hemodynamic and Doppler echocardiographic abnormalities, and short-term prognosis. J. Am. Coll. Cardiol. 2003, 41, 1805–1811. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, Y.; Wang, Y.; Guo, Y.; Li, J. Tumor marker levels in patients aged 85 years and older chronic heart failure. Eur. J. Intern. Med. 2013, 24, 440–443. [Google Scholar] [CrossRef]
- Yucel, H.; Kaya, H.; Zorlu, A.; Yıldırımlı, K.; Sancakdar, E.; Gunes, H.; Kurt, R.; Ozgul, U.; Turgut, O.O.; Yilmaz, M.B. Cancer antigen 125 levels and increased risk of new-onset atrial fibrillation. Herz 2015, 40 (Suppl. 2), 119–124. [Google Scholar] [CrossRef]
- Möller-Leimkühler, A.M. Gender differences in cardiovascular disease and comorbid depression. Dialogues. Clin. Neurosci. 2007, 9, 71–83. [Google Scholar] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Rev. Esp. Cardiol. 2016, 69, 1167. [Google Scholar]
- Zdrojewski, T.; Wizner, B.; Więcek, A.; Ślusarczyk, P.; Chudek, J.; Mossakowska, M.; Bandosz, P.; Bobak, M.; Kozakiewicz, K.; Broda, G.; et al. Prevalence, awareness, and control of hypertension in elderly and very elderly in Poland: Results of a cross-sectional representative survey. J. Hypertens. 2016, 34, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Pauler, D.K.; Menon, U.; McIntosh, M.; Symecko, H.L.; Skates, S.J.; Jacobs, I.J. Factors influencing serum CA125II levels in healthy postmenopausal women. Cancer Epidemiol. Biomarkers Prev. 2001, 10, 489–493. [Google Scholar] [PubMed]
- Palazzuoli, A.; Gallotta, M.; Quatrini, I.; Nuti, R. Natriuretic peptides (BNP and NT-proBNP): Measurement and relevance in heart failure. Vasc. Health Risk Manag. 2010, 6, 411–418. [Google Scholar] [CrossRef]
- Núñez, J.; Núñez, E.; Bayés-Genís, A.; Fonarow, G.C.; Miñana, G.; Bodí, V.; Pascual-Figal, D.; Santas, E.; Garcia-Blas, S.; Chorro, F.J.; et al. Long-term serial kinetics of N-terminal pro B-type natriuretic peptide and carbohydrate antigen125 for mortality risk prediction following acute heart failure. Eur. Heart J. Acute Cardiovasc. Care. 2017, 6, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Méndez, A.B.; Ordoñez-Llanos, J.; Ferrero, A. Prognostic value of increased carbohydrate antigen in patients with heart failure. World J. Cardiol. 2014, 6, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Kouris, N.T.; Kontogianni, D.D.; Papoulia, E.P.; Goranitou, G.S.; Zaharos, I.D.; Grassos, H.A.; Kalkandi, E.M.; Sifaki, M.D.; Babalis, D.K. Clinical and prognostic value of elevated CA125 levels in patients with congestive heart failure. Hellenic J. Cardiol. 2006, 47, 269–274. [Google Scholar] [PubMed]
- Amorim, S.; Campelo, M.; Moura, B.; Martins, E.; Rodrigues, J.; Barroso, I.; Faria, M.; Guimarães, T.; Macedo, F.; Silva-Cardoso, J.; et al. The role of biomarkers in dilated cardiomyopathy: Assessment of clinical severity and reverse remodeling. Rev. Port. Cardiol. 2017, 36, 709–716. [Google Scholar] [CrossRef]
- Kouris, N.T.; Zacharos, I.D.; Kontogianni, D.D.; Goranitou, G.S.; Sifaki, M.D.; Grassos, H.E.; Kalkandi, E.M.; Babalis, D.K. Significance of CA125 levels in patients with chronic congestive heart failure. Correlation with clinical and echocardiographic parameters. Eur. J. Heart Fail. 2005, 7, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, Q.; Yang, Y.; Wang, L. Diagnostic value of copeptin and cancer antigen 125 in acute heart failure patients with atrial fibrillation and their correlations with short-term cardiovascular events. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2018, 30, 1024–1028. [Google Scholar]
- Yilmaz, M.B.; Zorlu, A.; Tandogan, I. Plasma CA-125 level is related to both sides of the heart: A retrospective analysis. Int. J. Cardiol. 2011, 149, 80–82. [Google Scholar] [CrossRef]
- Kaya, H.; Zorlu, A.; Yucel, H.; Tatlisu, M.A.; Kivrak, T.; Coskun, A.; Yilmaz, M.B. Higher cancer antigen 125 level is associated with the presence of permanent atrial fibrillation in systolic heart failure patients. Acta Cardiol. 2016, 71, 61–66. [Google Scholar] [CrossRef]
- Vizzardi, E.; Nodari, S.; D’Aloia, A.; Chiari, E.; Faggiano, P.; Metra, M.; Dei Cas, L. CA 125 tumoral marker plasma levels relate to systolic and diastolic ventricular function and to the clinical status of patients with chronic heart failure. Echocardiography 2008, 25, 955–960. [Google Scholar] [CrossRef]
- Duman, D.; Palit, F.; Simsek, E.; Bilgehan, K. Serum carbohydrate antigen 125 levels in advanced heart failure: Relation to B-type natriuretic peptide and left atrial volume. Eur. J. Heart Fail. 2008, 10, 556–559. [Google Scholar] [CrossRef]
- Huang, F.; Chen, J.; Liu, Y.; Zhang, K.; Wang, J.; Huang, H. New mechanism of elevated CA125 in heart failure: The mechanical stress and inflammatory stimuli initiate CA125 synthesis. Med. Hypotheses 2012, 79, 381–383. [Google Scholar] [CrossRef]
- Zeillemaker, A.M.; Verbrugh, H.A.; Hoynck van Papendrecht, A.A.; Leguit, P. CA-125 secretion by peritoneal mesothelial cells. J. Clin. Pathol. 1994, 47, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Duman, C.; Ercan, E.; Tengiz, I.; Bozdemir, H.; Ercan, H.E.; Nalbantgil, I. Elevated serum CA 125 levels in mitral stenotic patients with heart failure. Cardiology 2003, 100, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, A.E.; Stanciu, M.M.; Vatasescu, R.G. NT-proBNP and CA 125 levels are associated with increased pro-inflammatory cytokines in coronary sinus serum of patients with chronic heart failure. Cytokine 2018, 111, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, H.; Shimamoto, K.; Takano, M.; Kimura, M.; Takahashi, Y.; Tatsumi, F.; Watanabe, E.; Jujo, K.; Ishizuka, N.; Kawana, M.; et al. Cancer antigen-125 plasma level as a biomarker of new-onset atrial fibrillation in postmenopausalwomen. Heart 2017, 103, 1368–1373. [Google Scholar] [CrossRef]
- De Gennaro, L.; Brunetti, D.; Montrone, D.; De Rosa, F.; Cuculo, A.; Di Biase, M. Inflammatory activation and carbohydrate antigen-125 levels in subjects with atrial fibrillation. Eur. J. Clin. Invest. 2012, 42, 371–375. [Google Scholar] [CrossRef]
- Yalta, K.; Yilmaz, A.; Turgut, O.O.; Erselcan, T.; Yilmaz, M.B.; Karadas, F.; Yontar, C.; Tandogan, I. Evaluation of tumor markers CA-125 and CEA in acute myocardial infarction. Adv. Ther. 2006, 23, 1052–1059. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.L.; Yang, X.; Shan, H.; Zhang, Q.H.; Feng, X.L.; Xie, Y.Y.; Tang, J.J.; Zhang, J. Clinical significance of serum carbohydrate antigen 125 in acute exacerbation of chronic obstructive pulmonary disease. Nan Fang Yi Ke Da Xue Xue Bao 2016, 36, 1386–1389. [Google Scholar] [PubMed]
- Li, S.; Ma, H.; Gan, L.; Ma, X.; Wu, S.; Li, M.; Tang, C.H.; Tsai, H.C. Cancer antigen-125 levels correlate with pleural effusions and COPD-related complications in people living at high altitude. Medicine 2018, 97, e12993. [Google Scholar] [CrossRef]
- Barouchos, N.; Papazafiropoulou, A.; Iacovidou, N.; Vrachnis, N.; Barouchos, N.; Armeniakou, E.; Dionyssopoulou, V.; Mathioudakis, A.G.; Christopoulou, E.; Koltsida, S.; et al. Comparison of tumor markers and inflammatory biomarkers in chronic obstructive pulmonary disease (COPD) exacerbations. Scand. J. Clin. Lab Invest. 2015, 75, 126–132. [Google Scholar] [CrossRef]
- Yilmaz, M.B.; Zorlu, A.; Dogan, O.T.; Karahan, O.; Tandogan, I.; Akkurt, I. Role of CA-125 in identification of right ventricular failure in chronic obstructive pulmonary disease. Clin. Cardiol. 2011, 34, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.C.; Kessel, B.; Riley, T.L.; Ragard, L.R.; Williams, C.R.; Xu, J.L.; Buys, S.S.; ProsTate, Lung, Colorectal and Ovarian Cancer Project Team. The epidemiology of CA-125 in women without evidence of ovarian cancer in the prostate, lung, colorectal and ovarian cancer (PLCO). Screening Trial. Gynecol. Oncol. 2008, 110, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Sikaris, K.A. CA125-A test with a change of heart. Heart Lung Circ. 2011, 20, 634–640. [Google Scholar] [CrossRef] [PubMed]
| All (n = 1565) | |
|---|---|
| Age (years) | 78 ± 9 |
| 65–69 years, n (%) | 293 (18.7) |
| 70–74 years, n (%) | 321 (20.5) |
| 75–79 years, n (%) | 259 (16.5) |
| 80–84 years, n (%) | 229 (14.6) |
| 85–90 years, n (%) | 233 (14.9) |
| >90 years, n (%) | 230 (14.7) |
| BMI (kg/m2) | 29.1 ± 5.5 |
| <18.5 kg/m2, n (%) | 16 (1.1) |
| 18.5–24.9 kg/m2, n (%) | 324 (2.2) |
| 25–29.9 kg/m2, n (%) | 527 (36.2) |
| ≥30 kg/m2, n (%) | 590 (40.5) |
| Current smoker, n (%) | 67 (4.3) |
| Hypertension, n (%) | 1218 (78.2) |
| Coronary artery disease, n (%) | 291 (18.6) |
| Hospitalization for MI, n (%) | 105 (6.7) |
| Hospitalization for HF, n (%) | 160 (10.2) |
| AF, n (%) | 297 (19.0) |
| Diabetes, n (%) | 384 (24.5) |
| COPD, n (%) | 249 (15.9) |
| Glucose, mg/dL | 94.5 (85.7–107.1) |
| Impaired fasting glucose, n (%) | 558 (35.6) |
| HOMA-IR | 1.44 (0.74–2.76) |
| Total cholesterol, mg/dL | 211.1 ± 47.2 |
| LDL-cholesterol, mg/dL | 124.9 ± 41.3 |
| HDL-cholesterol, mg/dL | 52.9 ± 13.8 |
| Triglycerydes, mg/dL | 121.5 (93.1–158.9) |
| eGFR-MDRD short, mL/min/1.73 m2 | 73.5 ± 21.1 |
| eGFR < 60 mL/min/1.73 m2, n (%) | 387 (24.7) |
| ACR | 5.56 (2.36–15.75) |
| ACR ≥ 300, n (%) | 39 (2.7) |
| hs-CRP, mg/dL | 2.47 (1.21–4.88) |
| Il-6, pg/mL | 2.2 (1.5–3.7) |
| NT-proBNP, pg/mL | 225 (114–504) |
| Ca125, U/mL | 13.0 (9.7–17.6) |
| Ca125 > 35 U/mL, n (%) | 79 (5.1) |
| β-blockers, n (%) | 603; 38.5% |
| ACE-I or ARB, n (%) | 764 (48.8) |
| Diuretics, n (%) | 513 (32.8) |
| Spironolactone, n (%) | 193 (12.3) |
| Statins, n (%) | 382 (24.4) |
| Fibrates, n (%) | 19 (1.2) |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| log10 (CA125 U/mL) * 100 | β | 95% CI: β | p | β | 95% CI: β | p |
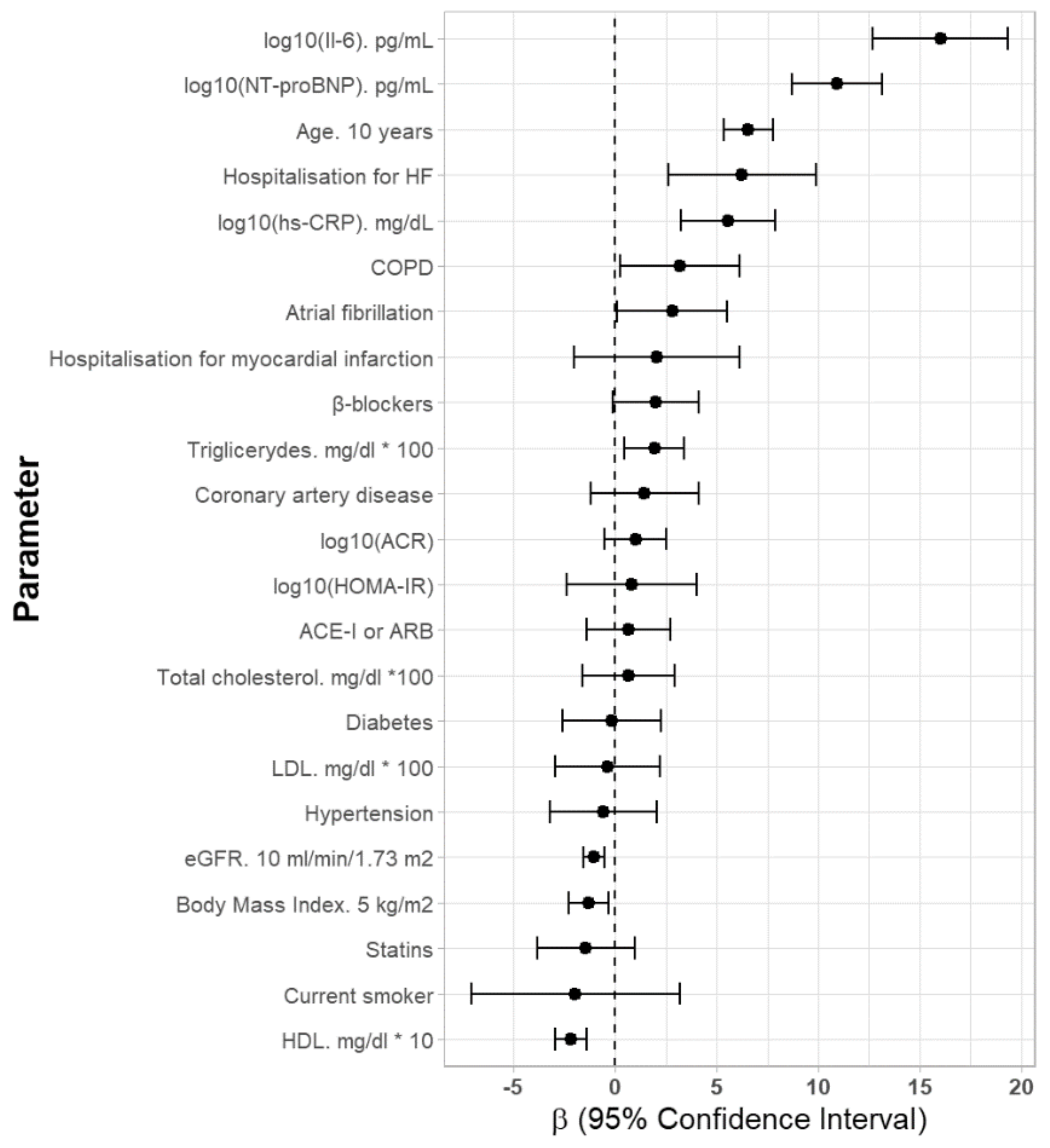
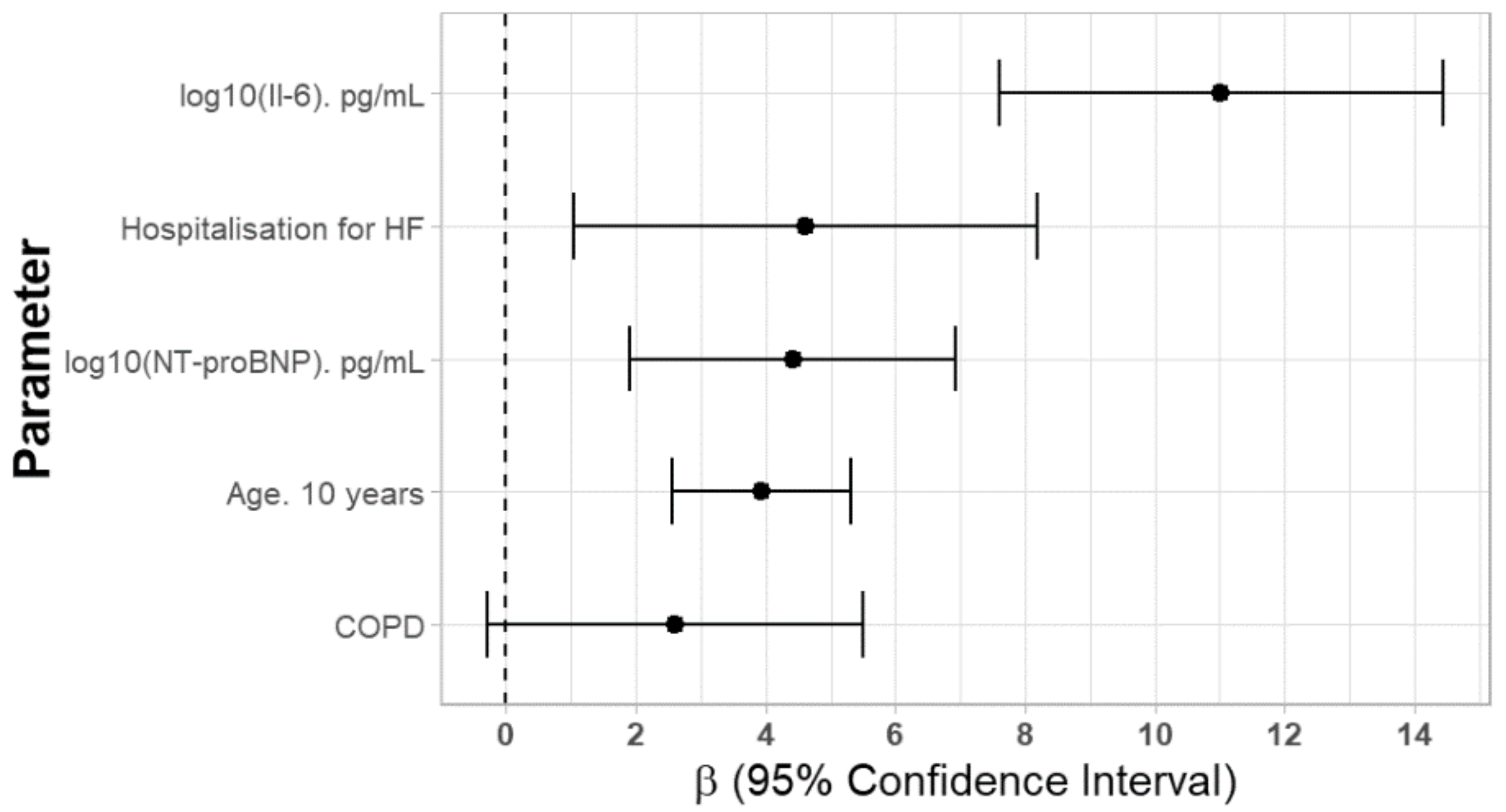
| Age (years) | 0.656 | 0.534–0.777 | <0.001 | 0.393 | 0.255–0.532 | <0.001 |
| BMI (kg/m2) | −0.260 | −0.454–−0.066 | <0.01 | - | - | - |
| Current smoker (Yes) | −1.950 | −7.107–3.206 | 0.46 | - | - | - |
| AF (Yes) | 2.805 | 0.081–5.528 | <0.05 | - | - | - |
| Coronary artery disease (Yes) | 1.438 | −1.222–4.098 | 0.29 | - | - | - |
| Hospitalization for MI (Yes) | 2.040 | −2.043–6.124 | 0.33 | - | - | - |
| Hospitalization for HF (Yes) | 6.249 | 2.615–9.882 | <0.01 | 4.619 | 1.042–8.196 | <0.05 |
| Hypertension (Yes) | −0.577 | −3.218–2.065 | 0.67 | - | - | - |
| Diabetes (Yes) | −0.183 | −2.603–2.237 | 0.88 | - | - | - |
| COPD (Yes) | 3.173 | 0.234–6.111 | <0.05 | 2.607 | −0.281–5.495 | 0.08 |
| Total cholesterol (mg/dL) *100 | 0.636 | −1.635–0.291 | 0.58 | - | - | - |
| LDL-cholesterol (mg/dL) * 100 | −0.366 | −2.931–2.198 | 0.78 | - | - | - |
| HDL-cholesterol (mg/dL) | −0.218 | −0.945–−0.142 | <0.001 | - | - | - |
| Triglycerides (mg/dL) * 100 | 1.937 | 0.461–3.413 | <0.05 | - | - | - |
| log10 (NT-proBNP (pg/mL)) | 10.928 | 8.701–13.156 | <0.001 | 4.416 | 1.904–6.927 | <0.001 |
| eGFR (mL/min/1.73 m2) | −0.107 | −0.158–−0.055 | <0.001 | - | - | - |
| log10 (IL-6 (pg/mL)) | 16.028 | 12.691–19.365 | <0.001 | 11.022 | 7.608–14.436 | <0.001 |
| log10 (hs-CRP (mg/dL)) | 5.570 | 3.255–7.884 | <0.001 | - | - | - |
| log10 (HOMA-IR) | 0.809 | −2.404–4.021 | 0.62 | - | - | - |
| log10 (ACR) | 1.002 | −0.533–2.537 | 0.20 | - | - | - |
| Medication | ||||||
| β-blockers, n (%) | 1.980 | −0.138–4.099 | 0.07 | - | - | - |
| ACE-I or ARB, n (%) | 0.654 | −1.419–2.727 | 0.54 | - | - | - |
| Statins, n (%) | −1.436 | −3.837–0.966 | 0.24 | - | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulska-Będkowska, W.; Chełmecka, E.; Owczarek, A.J.; Mizia-Stec, K.; Witek, A.; Szybalska, A.; Grodzicki, T.; Olszanecka-Glinianowicz, M.; Chudek, J. CA125 as a Marker of Heart Failure in the Older Women: A Population-Based Analysis. J. Clin. Med. 2019, 8, 607. https://doi.org/10.3390/jcm8050607
Bulska-Będkowska W, Chełmecka E, Owczarek AJ, Mizia-Stec K, Witek A, Szybalska A, Grodzicki T, Olszanecka-Glinianowicz M, Chudek J. CA125 as a Marker of Heart Failure in the Older Women: A Population-Based Analysis. Journal of Clinical Medicine. 2019; 8(5):607. https://doi.org/10.3390/jcm8050607
Chicago/Turabian StyleBulska-Będkowska, Weronika, Elżbieta Chełmecka, Aleksander J. Owczarek, Katarzyna Mizia-Stec, Andrzej Witek, Aleksandra Szybalska, Tomasz Grodzicki, Magdalena Olszanecka-Glinianowicz, and Jerzy Chudek. 2019. "CA125 as a Marker of Heart Failure in the Older Women: A Population-Based Analysis" Journal of Clinical Medicine 8, no. 5: 607. https://doi.org/10.3390/jcm8050607
APA StyleBulska-Będkowska, W., Chełmecka, E., Owczarek, A. J., Mizia-Stec, K., Witek, A., Szybalska, A., Grodzicki, T., Olszanecka-Glinianowicz, M., & Chudek, J. (2019). CA125 as a Marker of Heart Failure in the Older Women: A Population-Based Analysis. Journal of Clinical Medicine, 8(5), 607. https://doi.org/10.3390/jcm8050607







