Maintenance Negative Pressure Ventilation Improves Survival in COPD Patients with Exercise Desaturation
Abstract
1. Introduction
2. Materials and Methods
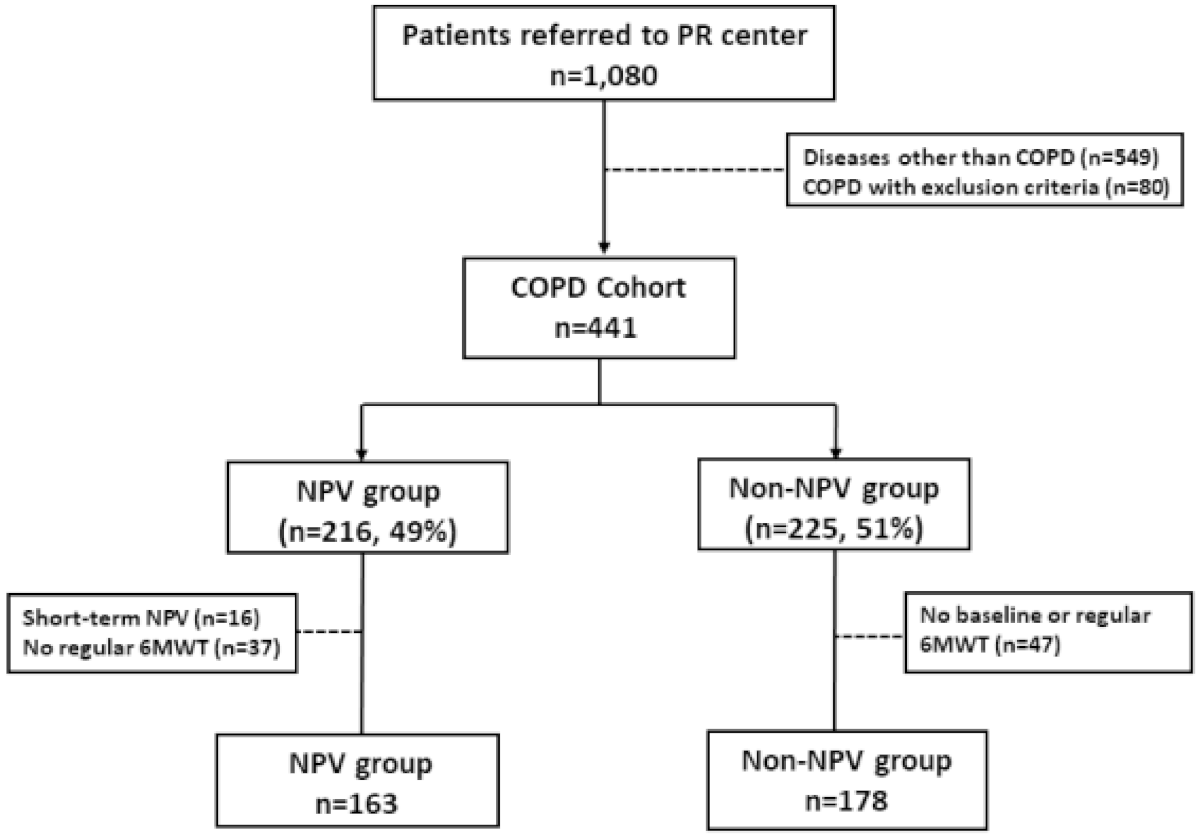
2.1. Study Population
2.2. Maintenance Negative Pressure Ventilation (NPV) Program
2.3. Clinical Assessment
2.4. Sample Size Calculation
2.5. Statistical Analysis
3. Results
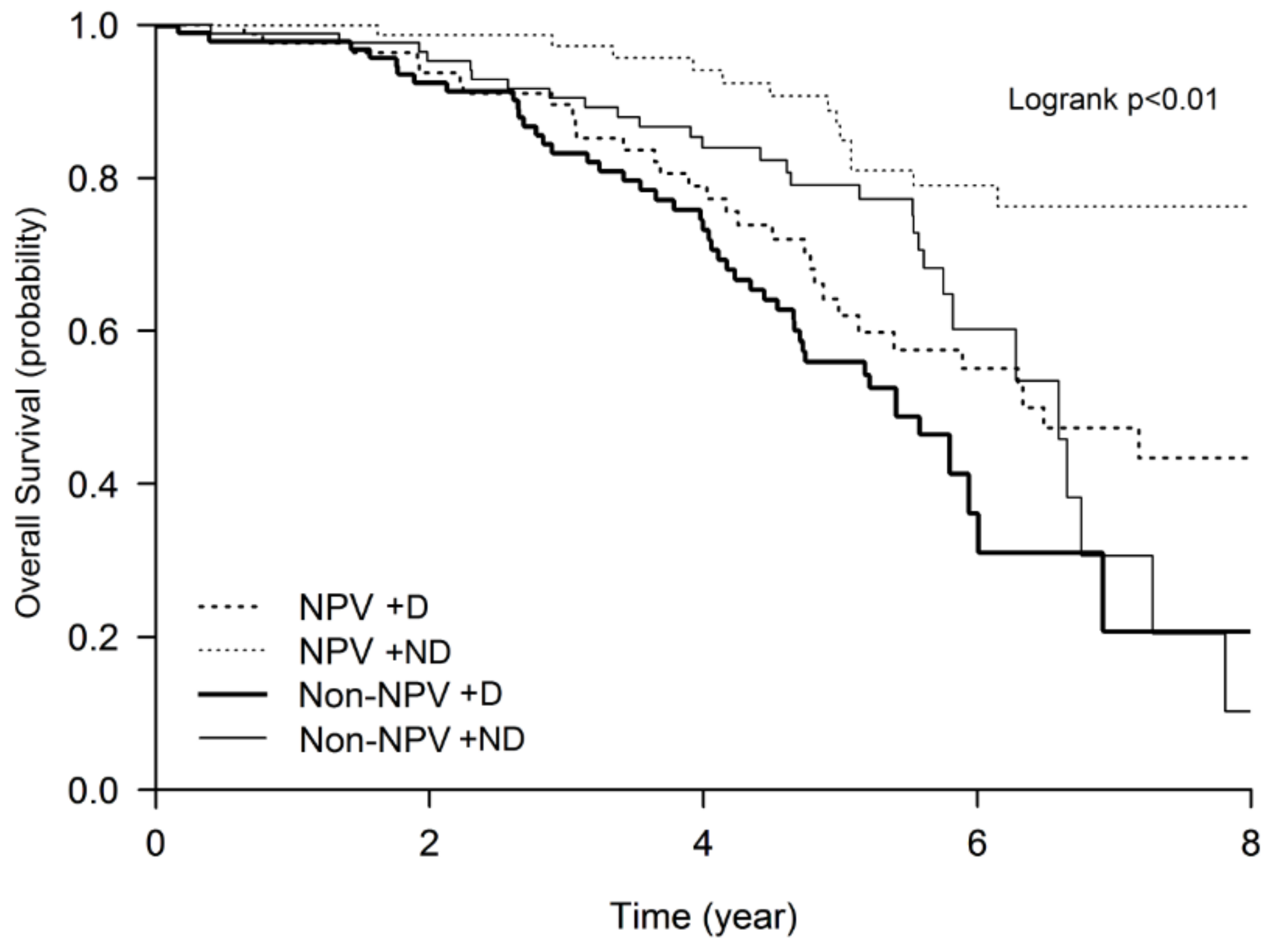
3.1. NPV Program Improved Survival in the COPD Patients
3.2. Desaturation Associated with Lower Lung Function and Higher Mortality
3.3. Higher Mortality Rate of the Desaturators Improved by the Maintenance NPV Program
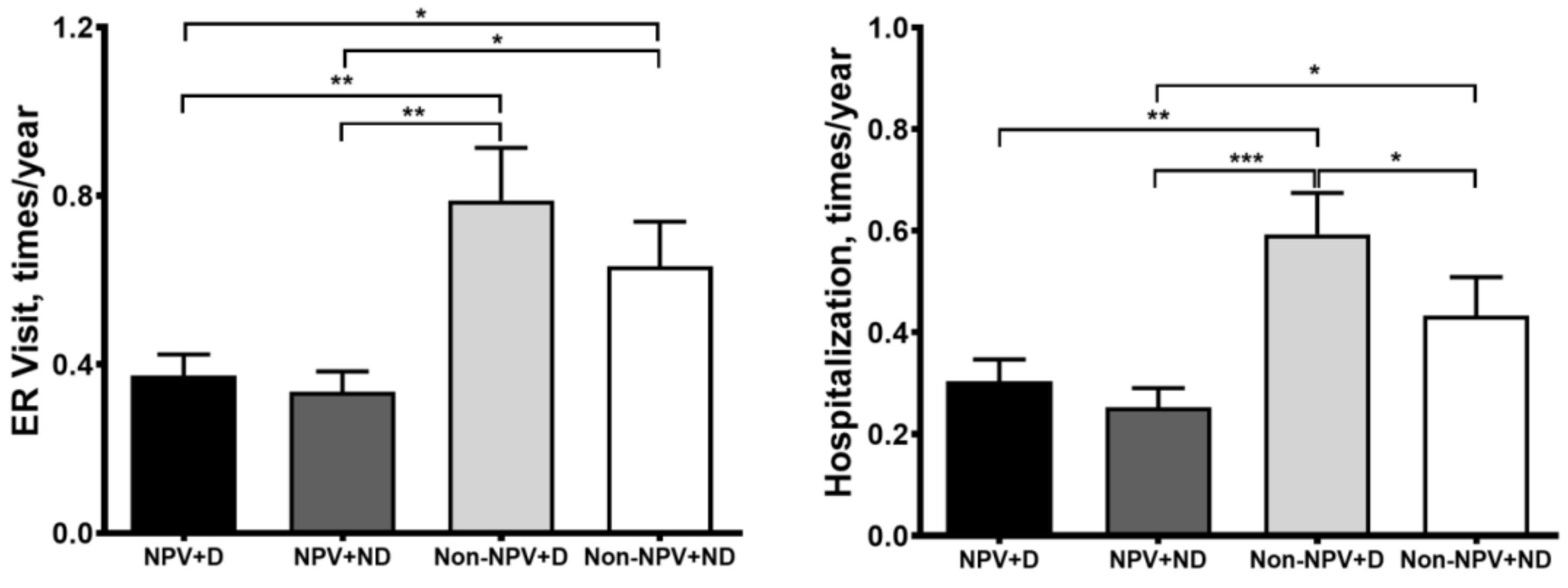
3.4. Exacerbations and Hospitalizations
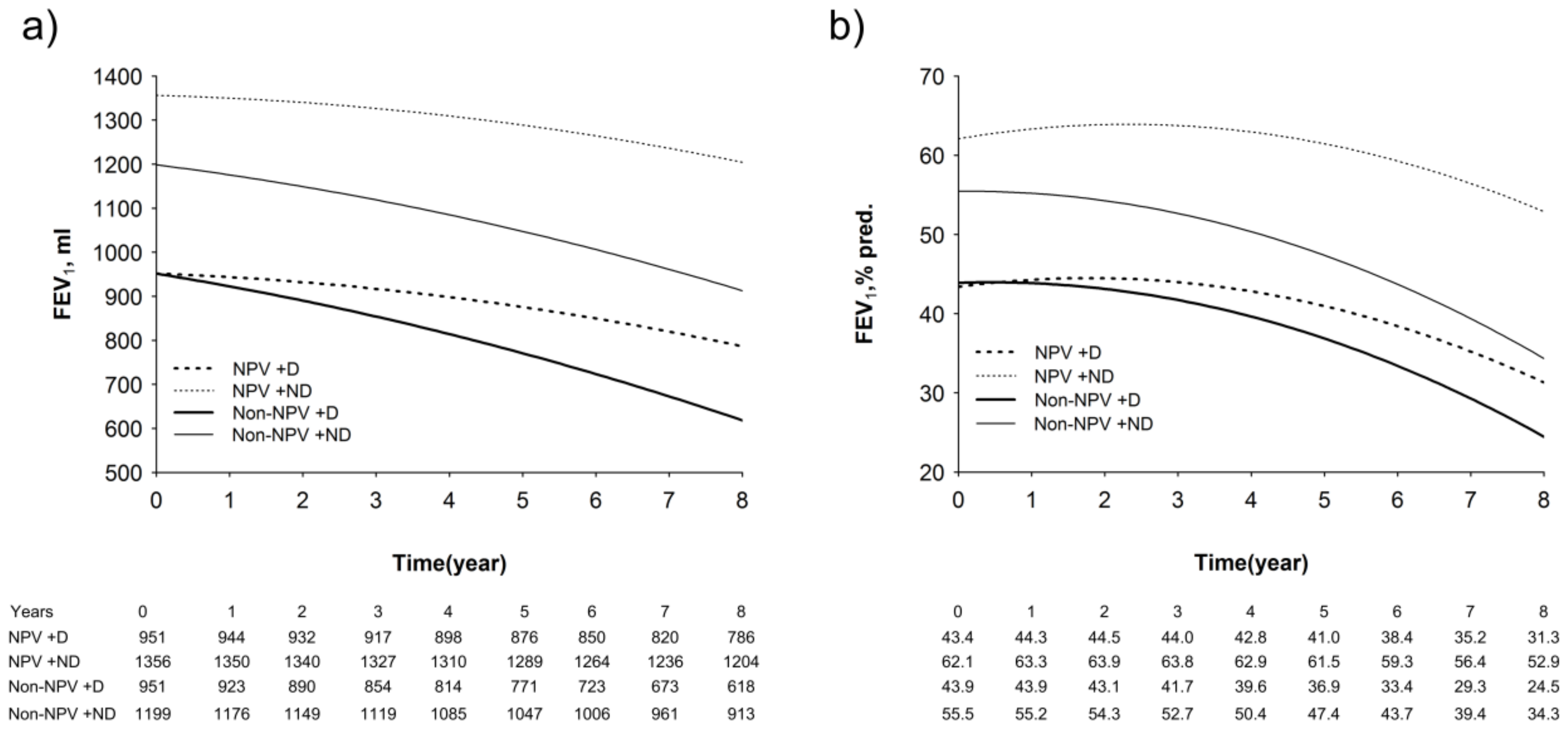
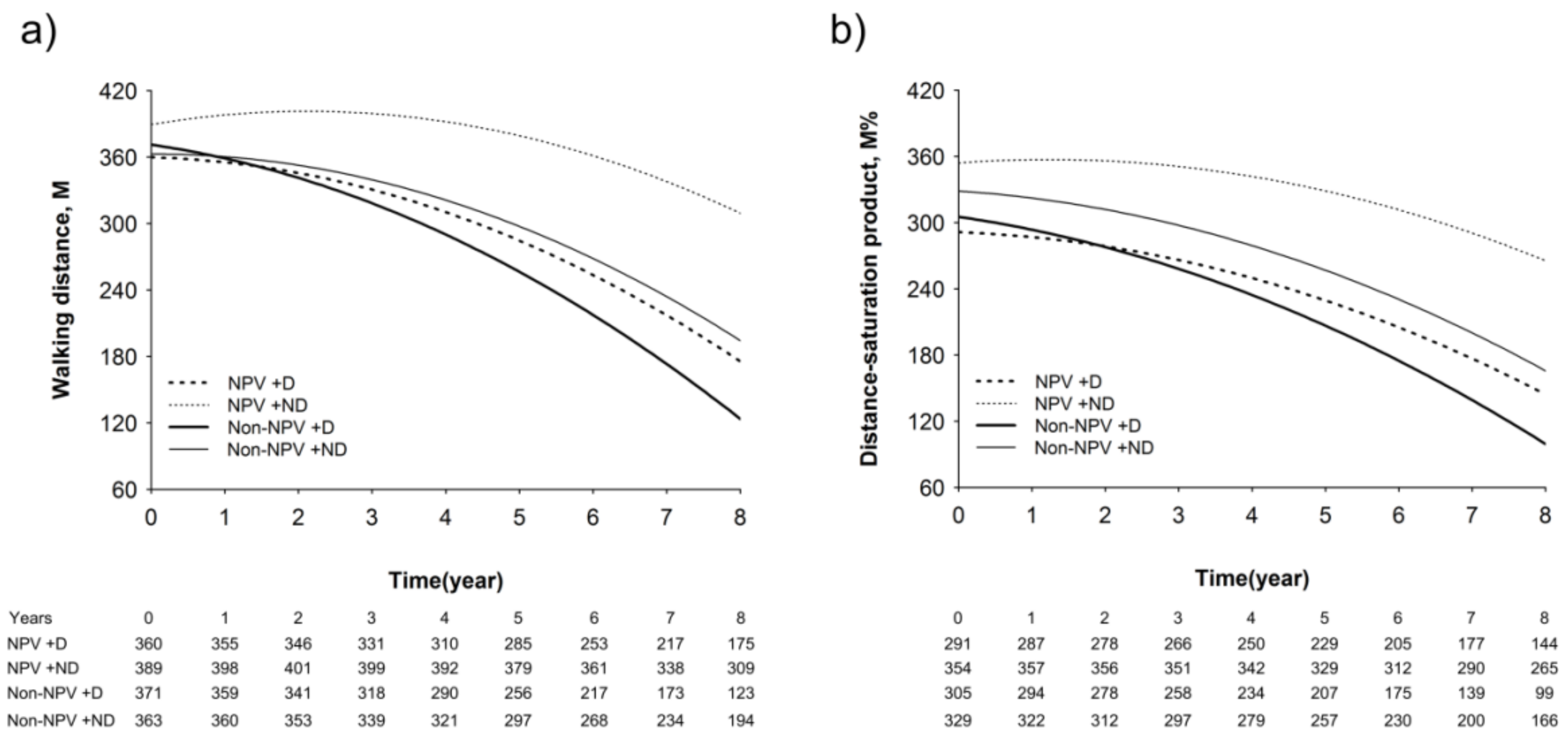
3.5. Longitudinal Changes in Lung Function, Walking Distance, and Hypoxia Index
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beckett, E.L.; Stevens, R.L.; Jarnicki, A.G.; Kim, R.Y.; Hanish, I.; Hansbro, N.G.; Deane, A.; Keely, S.; Horvat, J.C.; Yang, M.; et al. A new short-term mouse model of chronic obstructive pulmonary disease identifies a role for mast cell tryptase in pathogenesis. J. Allergy Clin. Immunol. 2013, 131, 752–762. [Google Scholar] [CrossRef] [PubMed]
- ATS committee on proficiency standards for clinical pulmonary function laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Takigawa, N.; Tada, A.; Soda, R.; Date, H.; Yamashita, M.; Endo, S.; Takahashi, S.; Kawata, N.; Shibayama, T.; Hamada, N.; et al. Distance and oxygen desaturation in 6-min walk test predict prognosis in COPD patients. Respir. Med. 2007, 101, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Casanova, C.; Cote, C.; Marin, J.M.; Pinto-Plata, V.; de Torres, J.P.; Aguirre-Jaíme, A.; Vassaux, C.; Celli, B.R. Distance and oxygen desaturation during the 6-min walk test as predictors of long-term mortality in patients with COPD. Chest 2008, 134, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Golpe, R.; Perez-de-Llano, L.A.; Mendez-Marote, L.; Veres-Racamonde, A. Prognostic value of walk distance, work, oxygen saturation, and dyspnea during 6-min walk test in COPD patients. Respir. Care 2013, 58, 1329–1334. [Google Scholar] [CrossRef]
- Waatevik, M.; Johannessen, A.; Gomez Real, F.; Aanerud, M.; Hardie, J.A.; Bakke, P.S.; Lind Eagan, T.M. Oxygen desaturation in 6-min walk test is a risk factor for adverse outcomes in COPD. Eur. Respir. J. 2016, 48, 82–91. [Google Scholar] [CrossRef]
- Kent, B.D.; Mitchell, P.D.; McNicholas, W.T. Hypoxemia in patients with COPD: Cause, effects, and disease progression. Int. J. Chron. Obstr. Pulm. Dis. 2011, 6, 199–208. [Google Scholar]
- Van Meerhaeghe, A.; Sergysels, R. Control of breathing during exercise in patients with chronic airflow limitation with or without hypercapnia. Chest 1983, 84, 565–570. [Google Scholar] [CrossRef]
- Lacasse, Y.; Lecours, R.; Pelletier, C.; Bégin, R.; Maltais, F. Randomised trial of ambulatory oxygen in oxygen-dependent COPD. Eur. Respir. J. 2005, 25, 1032–1038. [Google Scholar] [CrossRef]
- Gorini, M.; Corrado, A.; Villella, G.; Ginanni, R.; Augustynen, A.; Tozzi, D. Physiologic effects of negative pressure ventilation in acute exacerbation of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2001, 163, 1614–1618. [Google Scholar] [CrossRef]
- Gorini, M.; Villella, G.; Ginanni, R.; Augustynen, A.; Tozzi, D.; Corrado, A. Effect of assist negative pressure ventilation by microprocessor based iron lung on breathing effort. Thorax 2002, 57, 258–262. [Google Scholar] [CrossRef]
- Ho, S.C.; Lin, H.C.; Kuo, H.P.; Chen, L.F.; Sheng, T.F.; Jao, W.C.; Wang, C.H.; Lee, K.Y. Exercise training with negative pressure ventilation improves exercise capacity in patients with severe restrictive lung disease: A prospective controlled study. Respir. Res. 2013, 14, 22. [Google Scholar] [CrossRef][Green Version]
- Huang, H.Y.; Chou, P.C.; Joa, W.C.; Chen, L.F.; Sheng, T.F.; Lin, H.C.; Yang, L.Y.; Pan, Y.B.; Chung, F.T.; Wang, C.H.; et al. Pulmonary rehabilitation coupled with negative pressure ventilation decreases decline in lung function, hospitalizations, and medical cost in COPD: A 5-year study. Medicine (Baltimore) 2016, 95, e5119. [Google Scholar] [CrossRef]
- Martinez, F.J.; Foster, G.; Curtis, J.L.; Criner, G.; Weinmann, G.; Fishman, A.; DeCamp, M.M.; Benditt, J.; Sciurba, F.; Make, B.; et al. Predictors of mortality in patients with emphysema and severe airflow obstruction. Am. J. Respir. Crit. Care Med. 2006, 173, 1326–1334. [Google Scholar] [CrossRef]
- Hjalmarsen, A.; Brenn, T.; Jongsma Risberg, M.; Meisler Antonsen, K.; Kristiansen Benum, E.; Aaseboe, U. Retrospective survival in elderly COPD patients receiving pulmonary rehabilitation; a study including maintenance rehabilitation. BMC Res. Notes. 2014, 7, 210. [Google Scholar] [CrossRef]
- Connor, M.C.; O’Shea, F.D.; O’Driscoll, M.F.; Concannon, D.; McDonnell, T.J. Efficacy of pulmonary rehabilitation in an Irish population. Ir. Med. J. 2001, 94, 46–48. [Google Scholar]
- Gerardi, D.A.; Lovett, L.; Benoit-Connors, M.L.; Reardon, J.Z.; ZuWallack, R.L. Variables related to increased mortality following out-patient pulmonary rehabilitation. Eur. Respir. J. 1996, 9, 431–435. [Google Scholar] [CrossRef]
- Hakamy, A.; Bolton, C.E.; McKeever, T.M. The effect of pulmonary rehabilitation on mortality, balance, and risk of fall in stable patients with chronic obstructive pulmonary disease. Chron. Respir. Dis. 2017, 14, 54–62. [Google Scholar] [CrossRef]
- Pauwells, R.A.; Buist, S.; Calverley, P.M.A.; Jenkins, C.R.; Hurd, S.S.; The GOLD Scientific Committee (NHLBI-WHO workshop summary). Global strategy for the diagnosis, management, and prevention of chronic obstructive disease. Am. J. Respir. Crit. Care. Med. 2001, 163, 1256–1276. [Google Scholar] [CrossRef]
- Witek, T.J., Jr.; Mahler, D.A. Minimal important difference of the transition dyspnoea index in a multinational clinical trial. Eur. Respir. J. 2003, 21, 267–272. [Google Scholar] [CrossRef]
- Lu, T.H.; Lee, M.C.; Chou, M.C. Accuracy of cause-of-death coding in Taiwan: Types of miscoding and effects on mortality statistics. Int. J. Epidemiol. 2000, 29, 336–343. [Google Scholar] [CrossRef]
- Lu, T.H.; Shau, W.Y.; Shih, T.P.; Lee, M.C.; Chou, M.C.; Lin, C.K. Factors associated with errors in death certificate completion. A national study in Taiwan. J. Clin. Epidemiol. 2001, 54, 232–238. [Google Scholar] [CrossRef]
- Balcells, E.; Anto, J.M.; Gea, J.; Gomez, F.P.; Rodriguez, E.; Marin, A.; Ferrer, A.; de Batlle, J.; Farrero, E.; Benet, M.; et al. Characteristics of patients admitted for the first time for COPD exacerbation. Respir. Med. 2009, 103, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Diaz, O.; Begin, P.; Torrealba, B.; Jover, E.; Lisboa, C. Effects of noninvasive ventilation on lung hyperinflation in stable hypercapnic COPD. Eur. Respir. J. 2002, 20, 1490–1498. [Google Scholar] [CrossRef] [PubMed]
- Diaz, O.; Begin, P.; Andresen, M.; Jover, E.; Lisboa, C. Physiological and clinical effects of diurnal noninvasive ventilation in hypercapnic COPD. Eur. Respir. J. 2005, 26, 1016–1023. [Google Scholar] [CrossRef]
- Kohnlein, T.; Windisch, W.; Kohler, D.; Drabik, A.; Geiseler, J.; Hartl, S.; Karg, O.; Laier-Groeneveld, G.; Nava, S.; Schonhofer, B.; et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: A prospective, multicentre, randomised, controlled clinical trial. Lancet Respir. Med. 2014, 2, 698–705. [Google Scholar] [CrossRef]
- Budweiser, S.; Heinemann, F.; Fischer, W.; Dobroschke, J.; Pfeifer, M. Long-term reduction of hyperinflation in stable COPD by non-invasive nocturnal home ventilation. Respir. Med. 2005, 99, 976–984. [Google Scholar] [CrossRef]
- Bowen, J.B.; Votto, J.J.; Thrall, R.S.; Haggerty, M.C.; Stockdale-Woolley, R.; Bandyopadhyay, T.; ZuWallack, R.L. Functional status and survival following pulmonary rehabilitation. Chest 2000, 118, 697–703. [Google Scholar] [CrossRef]
- Ries, A.L.; Kaplan, R.M.; Limberg, T.M.; Prewitt, L.M. Effects of pulmonary rehabilitation on physiologic and psychosocial outcomes in patients with chronic obstructive pulmonary disease. Ann. Intern. Med. 1995, 122, 823–832. [Google Scholar] [CrossRef]
- Barbera, J.A.; Ramirez, J.; Roca, J.; Wagner, P.D.; Sanchez-Lloret, J.; Rodriguez-Roisin, R. Lung structure and gas exchange in mild chronic obstructive pulmonary disease. Am. Rev. Respir. Dis. 1990, 141, 895–901. [Google Scholar] [CrossRef]
- Thompson, A.A.; Dickinson, R.S.; Murphy, F.; Thomson, J.P.; Marriott, H.M.; Tavares, A.; Willson, J.; Williams, L.; Lewis, A.; Mirchandani, A.; et al. Hypoxia determines survival outcomes of bacterial infection through HIF-1alpha dependent re-programming of leukocyte metabolism. Sci. Immunol. 2017, 2, eaal2861. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, S.F.; Tambuwala, M.M.; Bruning, U.; Schaible, B.; Scholz, C.C.; Byrne, A.; O’Connor, A.; Gallagher, W.M.; Lenihan, C.R.; Garvey, J.F.; et al. An intact canonical NF-kappaB pathway is required for inflammatory gene expression in response to hypoxia. J. Immunol. 2011, 186, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am. J. Respir. Crit. Care Med. 2017, 195, 557–582. [Google Scholar] [CrossRef] [PubMed]
- Pitta, F.; Troosters, T.; Probst, V.S.; Langer, D.; Decramer, M.; Gosselink, R. Are patients with COPD more active after pulmonary rehabilitation? Chest 2008, 134, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Camillo, C.A.; Langer, D.; Osadnik, C.R.; Pancini, L.; Demeyer, H.; Burtin, C.; Gosselink, R.; Decramer, M.; Janssens, W.; Troosters, T. Survival after pulmonary rehabilitation in patients with COPD: Impact of functional exercise capacity and its changes. Int. J. Chron. Obstr. Pulm. Dis. 2016, 11, 2671–2679. [Google Scholar] [CrossRef] [PubMed]
- Wedzicha, J.A.; Banerji, D.; Chapman, K.R.; Vestbo, J.; Roche, N.; Ayers, R.T.; Thach, C.; Fogel, R.; Patalano, F.; Vogelmeier, C.F.; et al. Indacaterol-Glycopyrronium versus Salmeterol-Fluticasone for COPD. N. Engl. J. Med. 2016, 374, 2222–2234. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.T.; Wang, C.H.; Lin, H.C.; Lin, S.M.; Lee, K.Y.; Lo, Y.L.; Hung, S.H.; Chang, Y.M.; Chung, K.F.; Kuo, H.P. Efficacy of a cell phone-based exercise programme for COPD. Eur. Respir. J. 2008, 32, 651–659. [Google Scholar] [CrossRef]
- Hsieh, M.H.; Fang, Y.F.; Chung, F.T.; Lee, C.S.; Chang, Y.C.; Liu, Y.Z.; Wu, C.H.; Lin, H.C. Distance-saturation product of the 6-min walk test predicts mortality of patients with non-cystic fibrosis bronchiectasis. J. Thorac. Dis. 2017, 9, 3168–3176. [Google Scholar] [CrossRef]
- Lettieri, C.J.; Nathan, S.D.; Browning, R.F.; Barnett, S.D.; Ahmad, S.; Shorr, A.F. The distance-saturation product predicts mortality in idiopathic pulmonary fibrosis. Respir. Med. 2006, 100, 1734–1741. [Google Scholar] [CrossRef]

| NPV | Non-NPV | p-Value | |
|---|---|---|---|
| (n = 163) | (n = 178) | ||
| Age, years | 69 ± 9.7 | 71 ± 8.1 | 0.018 |
| Gender, male | 145 (89%) | 167 (93.8%) | 0.108 |
| Smoking exposure | 0.556 | ||
| Current smoker | 115 | 137 | |
| Ex-smoker | 32 | 28 | |
| Non-smoker | 16 | 17 | |
| Smoking (PKY) | 28.4 ± 33.2 | 35.1 ± 28.5 | 0.050 |
| Body mass index, kg/m2 | 22.7 ± 4 | 22.5 ± 3.7 | 0.516 |
| GOLD stage | 0.999 | ||
| I | 18 (11%) | 19 (10.7%) | |
| II | 53 (32.5%) | 58 (32.6%) | |
| III | 62 (38%) | 69 (38.8%) | |
| IV | 30 (18.4%) | 32 (18%) | |
| Charlson index | 3.0 (1.9) | 2.7 (1.9) | 0.094 |
| Appearance of comorbidities during follow-up | 0.712 | ||
| Ischemic heart disease | 24 | 33 | 0.346 |
| Cerebrovascular disease | 9 | 8 | 0.663 |
| Diabetes | 26 | 21 | 0.266 |
| Liver disease | 4 | 3 | 0.617 |
| Chronic kidney disease | 1 | 1 | 0.950 |
| Walking distance (6MWD), M | 369.5 ± 107.2 | 360 ± 100.5 | 0.400 |
| FVC, L | 1.9 ± 0.7 | 1.8 ± 0.5 | 0.500 |
| FVC, % pred. | 62.6 ± 22.3 | 60.4 ± 18.8 | 0.324 |
| FEV1, L | 1.1 ± 0.5 | 1.1 ± 0.4 | 0.162 |
| FEV1, % pred. | 52.2 ± 23.3 | 50.2 ± 21.8 | 0.425 |
| FEV1/FVC, % | 62.7 ± 38.7 | 57.3 ± 11.1 | 0.077 |
| O2 saturation | |||
| Pre-exercise, % | 94.7 ± 2.7 | 95 ± 2.4 | 0.235 |
| During-exercise, % | 85.6 ± 7.8 | 85.5 ± 7.8 | 0.928 |
| Medications | |||
| At baseline | |||
| LAMA alone | 14 | 8 | 0.101 |
| LABA + ICS | 34 | 27 | |
| LAMA + LABA + ICS | 108 | 133 | |
| At end of follow-up | 0.210 | ||
| LAMA alone | 13 | 9 | |
| LABA + ICS | 36 | 30 | |
| LAMA + LABA + ICS | 112 | 137 | |
| Overall mortality events | |||
| All-cause death, n (%) | 44 (27.0) | 74 (41.6) | 0.006 |
| Cardiovascular death, n (%) | 4 (2.5) | 7 (3.9) | 0.440 |
| Lung cancer death, n (%) | 3 (1.8) | 9 (5.1) | 0.107 |
| Other death, n (%) | 9 (5.5) | 9 (5.1) | 0.848 |
| Disease-specific death (pulmonary, cardiovascular, and lung cancer), n (%) | 35 (13.3) | 65 (37.3) | 0.002 |
| NPV + D | NPV + ND | Non-NPV + D | Non-NPV + ND | p-Value | |
|---|---|---|---|---|---|
| (n = 83) | (n = 80) | (n = 93) | (n = 85) | ||
| Age, years | 69 ± 9.1 | 68.7 ± 10.3 | 71 ± 9.1 | 71.4 ± 6.8 | 0.108 a |
| Gender, male | 76 (91.6%) | 69 (86.3%) | 87 (93.5%) | 80 (94.1%) | 0.253 |
| Smoking exposure | 0.244 | ||||
| Current smoker | 63 | 52 | 76 | 61 | |
| Ex-smoker | 15 | 17 | 12 | 16 | |
| Non-smoker | 5 | 11 | 5 | 8 | |
| Smoking (PKY) | 31.3 ± 36.2 | 25.3 ± 29.6 | 37.8 ± 31.5 | 32 ± 24.6 | 0.078 |
| Body mass index, kg/m2 | 22.3 ± 4 | 23.2 ± 3.9 | 21.8 ± 3.8 | 23.2 ± 3.3 | 0.049 |
| GOLD stage | <0.001 | ||||
| I | 3 (3.6%) | 15 (18.8%) | 6 (6.5%) | 13 (15.3%) | |
| II | 17 (20.5%) | 36 (45%) | 21 (22.6%) | 37 (43.5%) | |
| III | 37 (44.6%) | 25 (31.3%) | 42 (45.2%) | 27 (31.8%) | |
| IV | 26 (31.3%) | 4 (5%) | 24 (25.8%) | 8 (9.4%) | |
| Charlson index | 3.2 ± 2.1 | 2.8 ± 1.8 | 2.6 ± 1.7 | 2.8 ± 2.1 | 0.260 |
| Appearance of comorbidities during follow-up | 0.586 | ||||
| Ischemic heart disease | 14 | 10 | 19 | 20 | 0.293 |
| Cerebrovascular disease | 8 | 1 | 6 | 2 | 0.051 |
| Diabetes | 18 | 8 | 10 | 11 | 0.107 |
| Liver disease | 3 | 1 | 1 | 2 | 0.627 |
| Chronic kidney disease | 1 | 0 | 1 | 0 | 0.584 |
| Walking distance (6MWD), M | 357.6 ± 114 | 381.8 ± 98.9 | 363.8 ± 98.4 | 355.9 ± 103.1 | 0.366 |
| FVC, L | 1.6 ± 0.5 | 2.1 ± 0.7 | 1.7 ± 0.5 | 2 ± 0.6 | <0.001 a |
| FVC, % pred. | 54.1 ± 18.3 | 71.4 ± 22.7 | 55.9 ± 16.8 | 65.3 ± 19.7 | <0.001 a |
| FEV1, L | 0.9 ± 0.4 | 1.3 ± 0.5 | 0.9 ± 0.4 | 1.2 ± 0.5 | <0.001 a |
| FEV1, % pred. | 43.3 ± 19.3 | 61.4 ± 23.5 | 44.1 ± 18.5 | 57 ± 23.2 | <0.001 a |
| FEV1/FVC, % | 63.3 ± 13.2 | 62 ± 11.4 | 54.7 ± 11.4 | 60.2 ± 10.1 | 0.175 |
| O2 saturation | |||||
| Pre-exercise, % | 93.5 ± 3.0 | 95.9 ± 1.8 | 94.5 ± 2.8 | 95.6 ± 1.7 | <0.001 a |
| During-exercise, % | 80.6 ± 7.4 | 90.8 ± 3.8 | 80.3 ± 7.2 | 91.2 ± 2.6 | <0.001 a |
| Medications | |||||
| At baseline | 0.574 | ||||
| LAMA alone | 7 | 7 | 4 | 4 | |
| LABA + ICS | 18 | 16 | 15 | 12 | |
| LAMA + LABA + ICS | 58 | 50 | 70 | 63 | |
| At end of follow-up | 0.376 | ||||
| LAMA alone | 7 | 6 | 5 | 4 | |
| LABA + ICS | 14 | 22 | 16 | 14 | |
| LAMA + LABA + ICS | 62 | 50 | 72 | 65 | |
| Overall mortality events | |||||
| All-cause death, n (%) | 31 (37.3) | 13 (16.2) * | 45 (48.3) | 29 (34.1) | 0.0001 |
| Pulmonary death, n (%) | 22 (26.5) | 6 (7.5) | 35 (37.6) | 14 (16.5) | <0.0001 |
| Cardiovascular death, n (%) | 2 (2.4) | 2 (2.5) | 2 (2.2) | 5 (5.9) | 0.4613 |
| Lung cancer death, n (%) | 1 (1.2) | 2 (2.5) | 4 (4.3) | 5 (5.9) | 0.3735 |
| Other death, n (%) | 6 (7.2) | 3 (3.8) | 4 (4.3) | 5 (5.9) | 0.7422 |
| Disease-specific death (pulmonary, cardiovascular and lung cancer), n (%) | 25 (30.1) | 10 (12.5) * | 41 (44.1) | 24 (28.2) | 0.0001 |
| Variables | Hazard Ratios | 95% Confidence Interval | p-Value |
|---|---|---|---|
| COPD groups | |||
| NPV + ND | 1 | ||
| NPV + D | 2.02 | 0.89–4.60 | 0.09 |
| Non-NPV + ND | 2.42 | 1.13–5.19 | 0.02 |
| Non-NPV + D | 2.52 | 1.11–5.72 | 0.03 |
| Age (year) | 1.02 | 0.99–1.05 | 0.19 |
| Gender | |||
| Female | 1 | ||
| Male | 3.57 | 0.81–15.7 | 0.09 |
| Smoking | |||
| No | 1 | ||
| Yes | 1.37 | 0.76–2.48 | 0.30 |
| FEV1 (%) per 10% decrease | 1.01 | 0.99–1.02 | 0.06 |
| Annual admission rate | 1.13 | 0.85–1.50 | 0.41 |
| Charlson index | |||
| ≤2 | 1 | ||
| ≥3 | 1.17 | 0.61–2.26 | 0.63 |
| 6MWD per 10-m increase | 0.98 | 0.96–1.00 | 0.03 |
| Body mass index | 0.99 | 0.93–1.05 | 0.73 |
| Nadir saturation during 6MWT | |||
| SpO2: ≥90 | 1 | ||
| SpO2: 80–89 | 2.67 | 1.35–5.28 | <0.01 |
| SpO2: <80 | 3.13 | 1.33–7.36 | <0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.-Y.; Lo, C.-Y.; Yang, L.-Y.; Chung, F.-T.; Sheng, T.-F.; Lin, H.-C.; Lin, C.-W.; Huang, Y.-C.; Chang, C.-J.; Chung, K.F.; et al. Maintenance Negative Pressure Ventilation Improves Survival in COPD Patients with Exercise Desaturation. J. Clin. Med. 2019, 8, 562. https://doi.org/10.3390/jcm8040562
Huang H-Y, Lo C-Y, Yang L-Y, Chung F-T, Sheng T-F, Lin H-C, Lin C-W, Huang Y-C, Chang C-J, Chung KF, et al. Maintenance Negative Pressure Ventilation Improves Survival in COPD Patients with Exercise Desaturation. Journal of Clinical Medicine. 2019; 8(4):562. https://doi.org/10.3390/jcm8040562
Chicago/Turabian StyleHuang, Hung-Yu, Chun-Yu Lo, Lan-Yan Yang, Fu-Tsai Chung, Te-Fang Sheng, Horng-Chyuan Lin, Chang-Wei Lin, Yu-Chen Huang, Chee-Jen Chang, Kian Fan Chung, and et al. 2019. "Maintenance Negative Pressure Ventilation Improves Survival in COPD Patients with Exercise Desaturation" Journal of Clinical Medicine 8, no. 4: 562. https://doi.org/10.3390/jcm8040562
APA StyleHuang, H.-Y., Lo, C.-Y., Yang, L.-Y., Chung, F.-T., Sheng, T.-F., Lin, H.-C., Lin, C.-W., Huang, Y.-C., Chang, C.-J., Chung, K. F., & Wang, C.-H. (2019). Maintenance Negative Pressure Ventilation Improves Survival in COPD Patients with Exercise Desaturation. Journal of Clinical Medicine, 8(4), 562. https://doi.org/10.3390/jcm8040562





