Environment and Male Fertility: Effects of Benzo-α-Pyrene and Resveratrol on Human Sperm Function In Vitro
Abstract
1. Introduction
2. Experimental Section
2.1. Patient Selection
2.2. Experimental Design
2.3. Flow Cytometry Analysis
2.4. Assessment of the Degree of Chromatin Compactness
2.4.1. Evaluation of Sperm Apoptosis/Vitality
2.4.2. Evaluation of the Mitochondrial Membrane Potential
2.4.3. Assessment of DNA Fragmentation
2.4.4. Evaluation of Lipoperoxidation
2.4.5. Measurement of Mitochondrial Superoxide Levels
2.5. Statistical Analysis
3. Results
3.1. Sperm Parameters
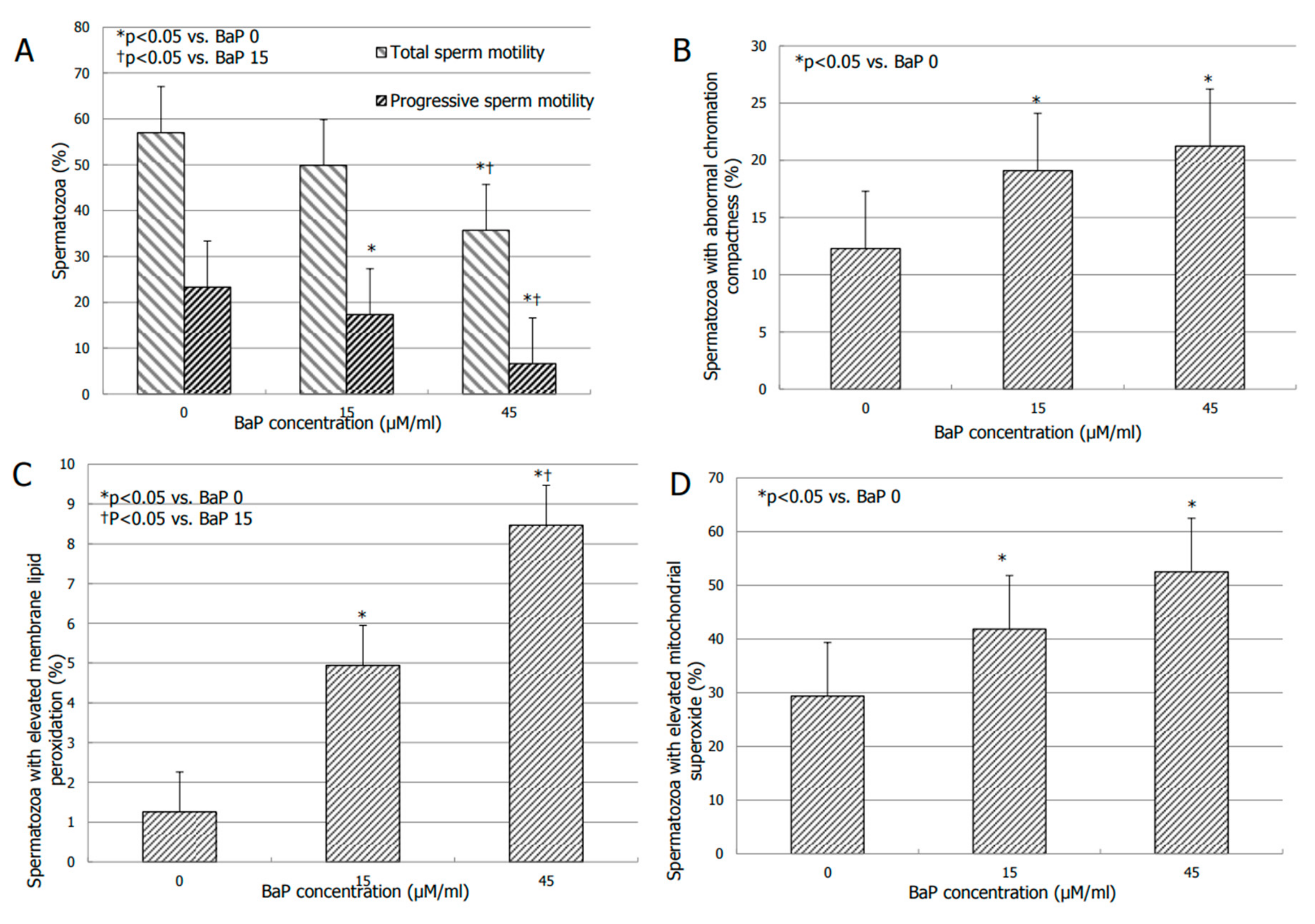
3.2. Effects of BaP on Sperm Motility
3.3. Effects of BaP on Bio-Functional Sperm Parameters
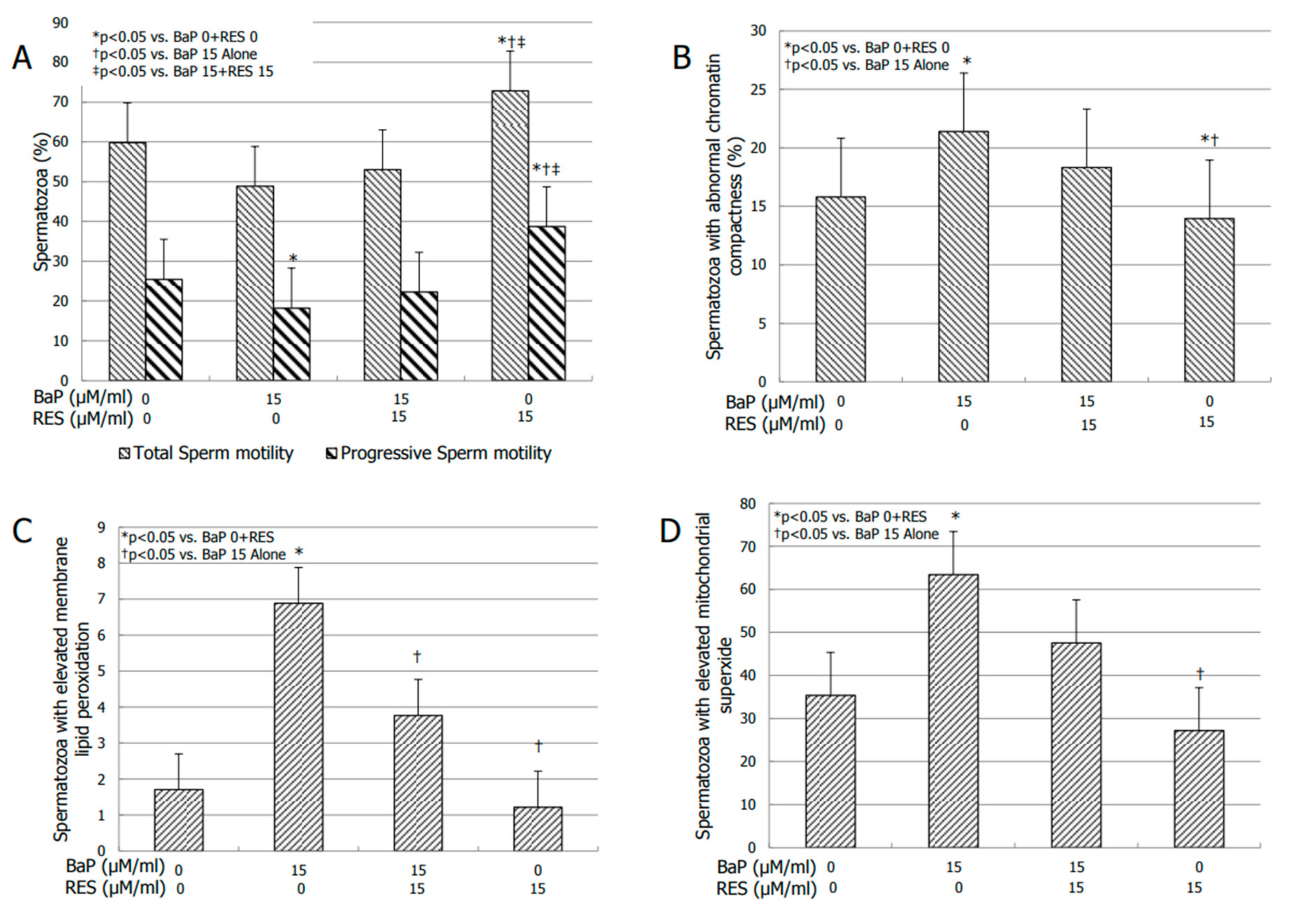
3.4. Effects of RES on Sperm Motility
3.5. Effect of RES on Bio-Functional Sperm Parameters
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Guerreiro, C.B.B.; Horálek, J.; De Leeuw, F.; Couvidat, F. Benzo(a)pyrene in Europe: Ambient air concentrations, population exposure and health effects. Environ. Pollut. 2016, 214, 657–667. [Google Scholar] [CrossRef] [PubMed]
- IARC Monographs. International Agency for Research on Cancer; International Agency for Research on Cancer (IARC): Lyon, France, 2012; Volume 100F. [Google Scholar]
- Gelboin, H.V. Benzo(a)pyrene metabolism, activation and carcinogenesis: Role and regulation of mixed function oxidases and related enzymes. Physiol. Rev. 1980, 60, 1107–1166. [Google Scholar] [CrossRef] [PubMed]
- Conney, A.H. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons. Cancer Res. 1982, 42, 4875–4917. [Google Scholar] [PubMed]
- Zenzes, M.T.; Bielecki, R.; Reed, T.E. Detection of benzo(a)pyrenediol epoxide DNA adducts in sperm of men exposed to cigarette smoke. Fertil. Steril. 1999, 72, 330–335. [Google Scholar] [CrossRef]
- Gaspari, L.; Chang, S.S.; Santella, R.M.; Garte, S.; Pedotti, P.; Taioli, E. Polycyclic aromatic hydrocarbon DNA adducts in human sperm as a marker of DNA damage and infertility. Mutat. Res. 2003, 535, 155–160. [Google Scholar] [CrossRef]
- Oliveri Conti, G.; Calogero, A.E.; Giacone, F.; Fiore, M.; Barchitta, M.; Agodi, A.; Ferrante, M. B(a)P adduct levels and fertility: A cross sectional study in a Sicilian population. Mol. Med. Rep. 2017, 15, 3398–3404. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.M.; Griffith, R.; Carey, A.; Butler, T.; Donne, S.W.; Beagley, K.W.; Aitken, R.J. The spermastatic and microbicidal actions of quinones and maleimides: Toward a dual-purpose contraceptive agent. Mol. Pharmacol. 2009, 76, 113–124. [Google Scholar] [CrossRef]
- Banerjee, B.; Chakraborty, S.; Ghosh, D.; Raha, S.; Sen, P.C.; Jana, K. Benzo(a)pyrene Induced p53 Mediated Male Germ Cell Apoptosis: Synergistic Protective Effects of Curcumin and Resveratrol. Front. Pharmacol. 2016, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Lanzafame, F.; La Vignera, S.; Vicari, E.; Calogero, A.E. Oxidative stress and medical antioxidant treatment in male infertility. Reprod. Biomed. Online 2009, 19, 638–659. [Google Scholar] [CrossRef] [PubMed]
- Sipinen, V.; Laubenthal, J.; Baumgartner, A.; Cemeli, E.; Linschooten, J.O.; Godschalk, R.W.L.; Van Schooten, F.J.; Anderson, D.; Brunborg, G. In vitro evaluation of baseline and induced DNA damage in human sperm exposed to benzo[a]pyrene or its metabolite benzo[a]pyrene-7,8-diol-9,10-epoxide, using the comet assay. Mutagenesis 2010, 25, 417–425. [Google Scholar] [CrossRef]
- Watanabe, S.; Kamiguchi, Y. Analysis of human spermatozoa following in vitro exposure to cyclophosphamide, benzo(a)pyrene and N-nitrosodimethylamine in the presence of rat liver S9. Mutat. Res. 2001, 491, 57–63. [Google Scholar] [CrossRef]
- Perrin, J.; Tassistro, V.; Mandon, M.; Grillo, J.M.; Botta, A.; Sari-Minodier, I. Tobacco consumption and benzo(a)pyrene-diol-epoxide-DNA adducts in spermatozoa: In smokers, swim-up procedure selects spermatozoa with decreased DNA damage. Fertil. Steril. 2011, 95, 2013–2017. [Google Scholar] [CrossRef]
- Mukhopadhyay, D.; Nandi, P.; Varghese, A.C.; Gutgutia, R.; Banerjee, S.; Bhattacharyya, A.K. The in vitro effect of benzo[a]pyrene on human sperm hyperactivation and acrosome reaction. Fertil. Steril. 2010, 94, 595–598. [Google Scholar] [CrossRef]
- Pervaiz, S. Resveratrol: From grapevines to mammalian biology. FASEB J. 2003, 17, 1975–1985. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.K.; Mustafi, S.B.; Ganguly, S.; Chatterjee, M.; Raha, S. Resveratrol induces apoptosis in K562 (chronic myelogenous leukemia) cells by targeting a key survival protein, heat shock protein 70. Cancer Sci. 2008, 99, 1109–1116. [Google Scholar] [CrossRef]
- Cianciulli, A.; Calvello, R.; Cavallo, P.; Dragone, T.; Carofiglio, V.; Panaro, M.A. Modulation of NF-κB activation by resveratrol in LPS treated human intestinal cells results in downregulation of PGE2 production and COX-2 expression. Toxicol. In Vitro 2012, 26, 1122–1128. [Google Scholar] [CrossRef]
- Revel, A.; Raanani, H.; Younglai, E.; Xu, J.; Han, R.; Savouret, J.F.; Casper, R.F. Resveratrol, a natural aryl hydrocarbon receptor antagonist, protects sperm from DNA damage and apoptosis caused by benzo(a)pyrene. Reprod. Toxicol. 2001, 15, 479–486. [Google Scholar] [CrossRef]
- Meamar, M.; Zribi, N.; Cambi, M.; Tamburrino, L.; Marchiani, S.; Filimberti, E.; Fino, M.G.; Biggeri, A.; Menezo, Y.; Forti, G.; et al. Sperm DNA fragmentation induced by cryopreservation: New insights and effect of a natural extract from Opuntiaficus-indica. Fertil. Steril. 2012, 98, 326–333. [Google Scholar] [CrossRef]
- Silva, E.C.B.; Cajueiro, J.F.P.; Silva, S.V.; Soares, P.C.; Guerra, M.M.P. Effect of antioxidants resveratrol and quercetin on in vitro evaluation of frozen ram sperm. Theriogenology 2012, 77, 1722–1726. [Google Scholar] [CrossRef]
- Gadani, B.; Bucci, D.; Spinaci, M.; Tamanini, C.; Galeati, G. Resveratrol and Epigallocatechin-3-gallate addition to thawed boar sperm improves in vitro fertilization. Theriogenology 2017, 1, 88–93. [Google Scholar] [CrossRef]
- Kasdallah-Grissa, A.; Mornagui, B.; Aouani, E.; Hammami, M.; Gharbi, N.; Kamoun, A.; El-Fazaâ, S. Protective effect of resveratrol on ethanol-induced lipid peroxidation in rats. Alcohol Alcohol. 2006, 41, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Collodel, G.; Federico, M.G.; Geminiani, M.; Martini, S.; Bonechi, C.; Rossi, C.; Figura, N.; Moretti, E. Effect of transresveratrol on induced oxidative stress in human sperm and in rat germinal cells. Reprod. Toxicol. 2011, 31, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Jing, X.; Wu, X.; Yan, M. Protective effect of resveratrol on spermatozoa function in male infertility induced by excess weight and obesity. Mol. Med. Rep. 2016, 14, 4659–4665. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Barbonetti, A.; Vassallo, M.R.; Di Rosa, A.; Leombruni, Y.; Felzani, G.; Gandini, L.; Lenzi, A.; Necozione, S.; Francavilla, S.; Francavilla, F. Involvement of mitochondrial dysfunction in the adverse effect exerted by seminal plasma from men with spinal cord injury on sperm motility. Andrology 2013, 1, 456–463. [Google Scholar] [CrossRef]
- Ramesh, A.; Inyang, F.; Lunstra, D.D.; Niaz, M.S.; Kopsombut, P.; Jones, K.M.; Hood, D.B.; Hills, E.R.; Archibong, A.E. Alteration of fertility endpoints in adult male F-344 rats by subchronic exposure to inhaled benzo(a)pyrene. Exp. Toxicol. Pathol. 2008, 60, 269–280. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hou, L.; Zhang, X.; Dioni, L.; Barretta, F.; Dou, C.; Zheng, Y.; Hoxha, M.; Bertazzi, P.A.; Schwartz, J.; Wu, S.; et al. Inhalable particulate matter and mitochondrial DNA copy number in highly exposed individuals in Beijing, China: A repeated-measure study. Part. Fibre Toxicol. 2013, 10, 17. [Google Scholar] [CrossRef]
- Calogero, A.; Polosa, R.; Perdichizzi, A.; Guarino, F.; La Vignera, S.; Scarfia, A.; Fratantonio, E.; Condorelli, R.A.; Bonanno, O.; Barone, N.; et al. Cigarette smoke extract immobilizes human spermatozoa and induces sperm apoptosis. Reprod. Biomed. Online 2009, 19, 564–571. [Google Scholar] [CrossRef]
- Gandini, L.; Lombardo, F.; Lenzi, A.; Culasso, F.; Pacifici, R.; Zuccaro, P.; Dondero, F. The in-vitro effects of nicotine and cotinine on sperm motility. Hum. Reprod. 1997, 12, 727–733. [Google Scholar] [CrossRef][Green Version]
- Arabi, M.; Shareghi, B. Anti-fertility effect of nicotine. Zhonghua Nan Ke Xue 2005, 11, 323–330. [Google Scholar]
- Condorelli, R.A.; La Vignera, S.; Giacone, F.; Iacoviello, L.; Vicari, E.; Mongioì, L.; Calogero, A.E. In vitro effects of nicotine on sperm motility and bio-functional flow cytometry sperm parameters. Int. J. Immunopathol. Pharmacol. 2013, 26, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Zhang, G.; Sun, L.; Wang, Z.; Zou, P.; Gao, J.; Peng, K.; Chen, Q.; Yang, H.; Zhou, N.; et al. Polycyclic aromatic hydrocarbons exposure decreased sperm mitochondrial DNA copy number: A cross-sectional study (marhcs) in chongqing, china. Environ. Pollut. 2017, 220, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.W.; Liu, V.W.; Tsao, G.S.; Yao, K.M.; Furukawa, T.; Chan, K.K.; Ngan, H.Y. Loss of MKP3 mediated by oxidative stress enhances tumorigenicity and chemoresistance of ovarian cancer cells. Carcinogenesis 2008, 29, 1742–1750. [Google Scholar] [CrossRef]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef]
- Aitken, R.J.; Baker, M.A.; Nixon, B. Are sperm capacitation and apoptosis the opposite ends of a continuum driven by oxidative stress? Asian J. Androl. 2015, 17, 633–639. [Google Scholar] [CrossRef]
- Gu, A.; Ji, G.; Long, Y.; Zhou, Y.; Shi, X.; Song, L.; Wang, X. Assessment of an association between an aryl hydrocarbon receptor gene (ahr) polymorphism and rick of male infertility. Toxicol. Sci. 2011, 122, 415–421. [Google Scholar] [CrossRef]
- Štramová, X.; Čegan, A.; Hampl, R.; Kanďár, R. Effects of smoking on fatty acid composition of phospholipid sperm membrane and the malondialdehyde levels in human seminal plasma. Andrologia 2015, 47, 967–973. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, J.; Shi, J.S. Anti-inflammatory activities of resveratrol in the brain: Role of resveratrol in microglial activation. Eur. J. Pharmacol. 2010, 636, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 9, 6. [Google Scholar]
- Wang, G.; Hu, Z.; Song, X.; Cui, Q.; Fu, Q.; Jia, R.; Zou, Y.; Li, L.; Yin, Z. Analgesic and anti-inflammatory activities of resveratrol through classic models in mice and rats. Evid. Based Complement. Altern. Med. 2017, 2017, 9. [Google Scholar] [CrossRef]
- Kuršvietienė, L.; Stanevičienė, I.; Mongirdienė, A.; Bernatonienė, J. Multiplicity of effects and health benefits of resveratrol. Medicina 2016, 52, 148–155. [Google Scholar] [CrossRef]
- Zhou, Z.X.; Mou, S.F.; Chen, X.Q.; Gong, L.L.; Ge, W.S. Anti-inflammatory activity of resveratrol prevents inflammation by inhibiting NF-kB in animal models of acute pharyngitis. Mol. Med. Rep. 2018, 17, 1269–1274. [Google Scholar]
- Carré, J.; Gatimel, N.; Moreau, J.; Parinaud, J.; Léandri, R. Does air pollution play a role in infertility?: A systematic review. Environ. Health 2017, 28, 82. [Google Scholar] [CrossRef]
- Piver, B.; Berthou, F.; Dreano, Y.; Lucas, D. Inhibition of CYP3A, CYP1A and CYP2E1 activities by resveratrol and other non volatile red wine components. Toxicol. Lett. 2001, 125, 83–91. [Google Scholar] [CrossRef]
- Lopes, S.; Jurisicova, A.; Sun, J.G.; Casper, R.F. Reactive oxygen species: Potential cause for DNA fragmentation in human spermatozoa. Hum. Reprod. 1998, 13, 896–900. [Google Scholar] [CrossRef] [PubMed]
- Calogero, A.E.; Fishel, S.; Hall, J.; Ferrara, E.; Vicari, E.; Green, S.; Hunter, A.; Burrello, N.; Thornton, S.; D’Agata, R. Correlation between intracellular cAMP content, kinematic parameters and hyperactivation of human spermatozoa after incubation with pentoxifylline. Fertil. Steril. 1998, 13, 911–915. [Google Scholar] [CrossRef]
- Calogero, A.E.; Burrello, N.; Ferrara, E.; Hall, J.; Fishel, S.; D’Agata, R. γ-Aminobutyric acid (GABA) A and B receptors mediate the stimulatory effects of GABA on the human sperm acrosome reaction: Interaction with progesterone. Fertil. Steril. 1999, 71, 930–936. [Google Scholar] [CrossRef]
| Sperm Parameter | Values | 5th Pecentile |
|---|---|---|
| Concentration (million/mL) | 75 ± 7.3 | 15 |
| Total count (million/ejaculate) | 256 ± 32.6 | 39 |
| Progressive motility (%) | 33.0 ± 0.3 | 32 |
| Total motility (%) | 68.7 ± 1.9 | 40 |
| Normal forms (%) | 6.9 ± 0.53 | 4 |
| Leukocytes (million/mL) | 0.7 ± 0.04 | <1 |
| 0 | 15 µM/mL | 45 µM/mL | Normal Values | |
|---|---|---|---|---|
| Alive spermatozoa (%) | 69.6 ± 3.9 | 65.7 ± 4.5 | 60.4 ± 7.7 | >60 |
| Spermatozoa with phosphatidylserine externalization (%) | 1.7 ± 0.4 | 1.3 ± 0.4 | 1.2 ± 0.3 | <10.7 |
| Spermatozoa in late apoptosis (%) | 8.9 ± 2.4 | 9.1 ± 2.8 | 16.7 ± 6.3 | <24.1 |
| Spermatozoa with low mitochondrial membrane potential (%) | 26.1 ± 6.2 | 29 ± 7.4 | 43.7 ± 9.5 | <11.9 |
| Spermatozoa with DNA fragmentation (%) | 9.2 ± 3.7 | 10.1 ± 5.1 | 11.5 ± 4.3 | <4.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alamo, A.; Condorelli, R.A.; Mongioì, L.M.; Cannarella, R.; Giacone, F.; Calabrese, V.; La Vignera, S.; Calogero, A.E. Environment and Male Fertility: Effects of Benzo-α-Pyrene and Resveratrol on Human Sperm Function In Vitro. J. Clin. Med. 2019, 8, 561. https://doi.org/10.3390/jcm8040561
Alamo A, Condorelli RA, Mongioì LM, Cannarella R, Giacone F, Calabrese V, La Vignera S, Calogero AE. Environment and Male Fertility: Effects of Benzo-α-Pyrene and Resveratrol on Human Sperm Function In Vitro. Journal of Clinical Medicine. 2019; 8(4):561. https://doi.org/10.3390/jcm8040561
Chicago/Turabian StyleAlamo, Angela, Rosita A. Condorelli, Laura M. Mongioì, Rossella Cannarella, Filippo Giacone, Vittorio Calabrese, Sandro La Vignera, and Aldo E. Calogero. 2019. "Environment and Male Fertility: Effects of Benzo-α-Pyrene and Resveratrol on Human Sperm Function In Vitro" Journal of Clinical Medicine 8, no. 4: 561. https://doi.org/10.3390/jcm8040561
APA StyleAlamo, A., Condorelli, R. A., Mongioì, L. M., Cannarella, R., Giacone, F., Calabrese, V., La Vignera, S., & Calogero, A. E. (2019). Environment and Male Fertility: Effects of Benzo-α-Pyrene and Resveratrol on Human Sperm Function In Vitro. Journal of Clinical Medicine, 8(4), 561. https://doi.org/10.3390/jcm8040561










