UNR/CSDE1 Expression Is Critical to Maintain Invasive Phenotype of Colorectal Cancer through Regulation of c-MYC and Epithelial-to-Mesenchymal Transition
Abstract
1. Introduction
2. Experimental Section
2.1. Human Cell Lines
2.2. Patient Samples
2.3. Ethics Statement
2.4. Western Blot
2.5. Quantitative Real-Time PCR
2.6. RNA Interference
2.7. Cell Viability and Apoptosis
2.8. Invasion Assay
2.9. Ex Vivo Assay
2.10. Immunohistochemistry
2.11. Statistical Analysis
2.12. TCGA-Colorectal Cancer Dataset Analysis
3. Results
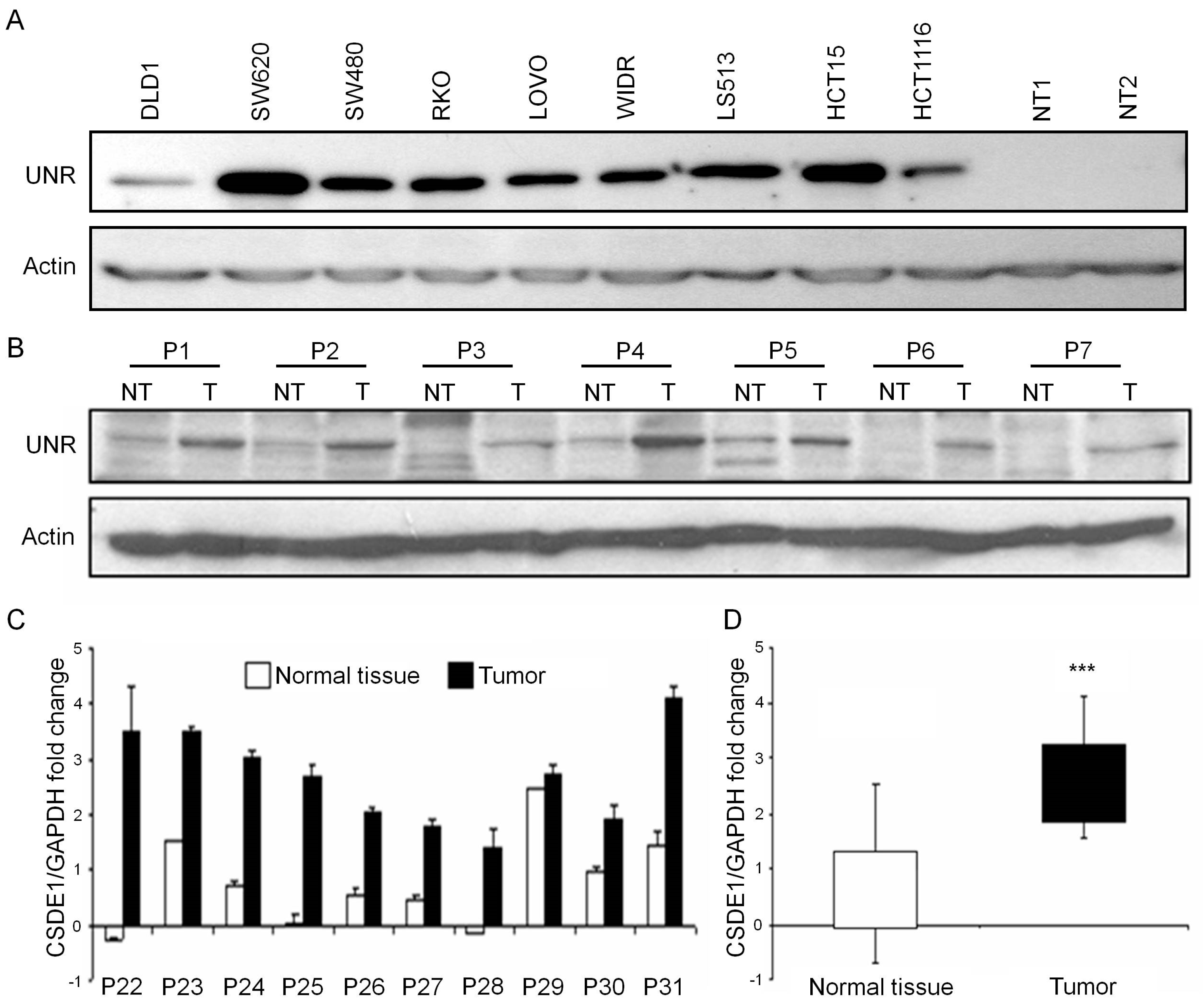
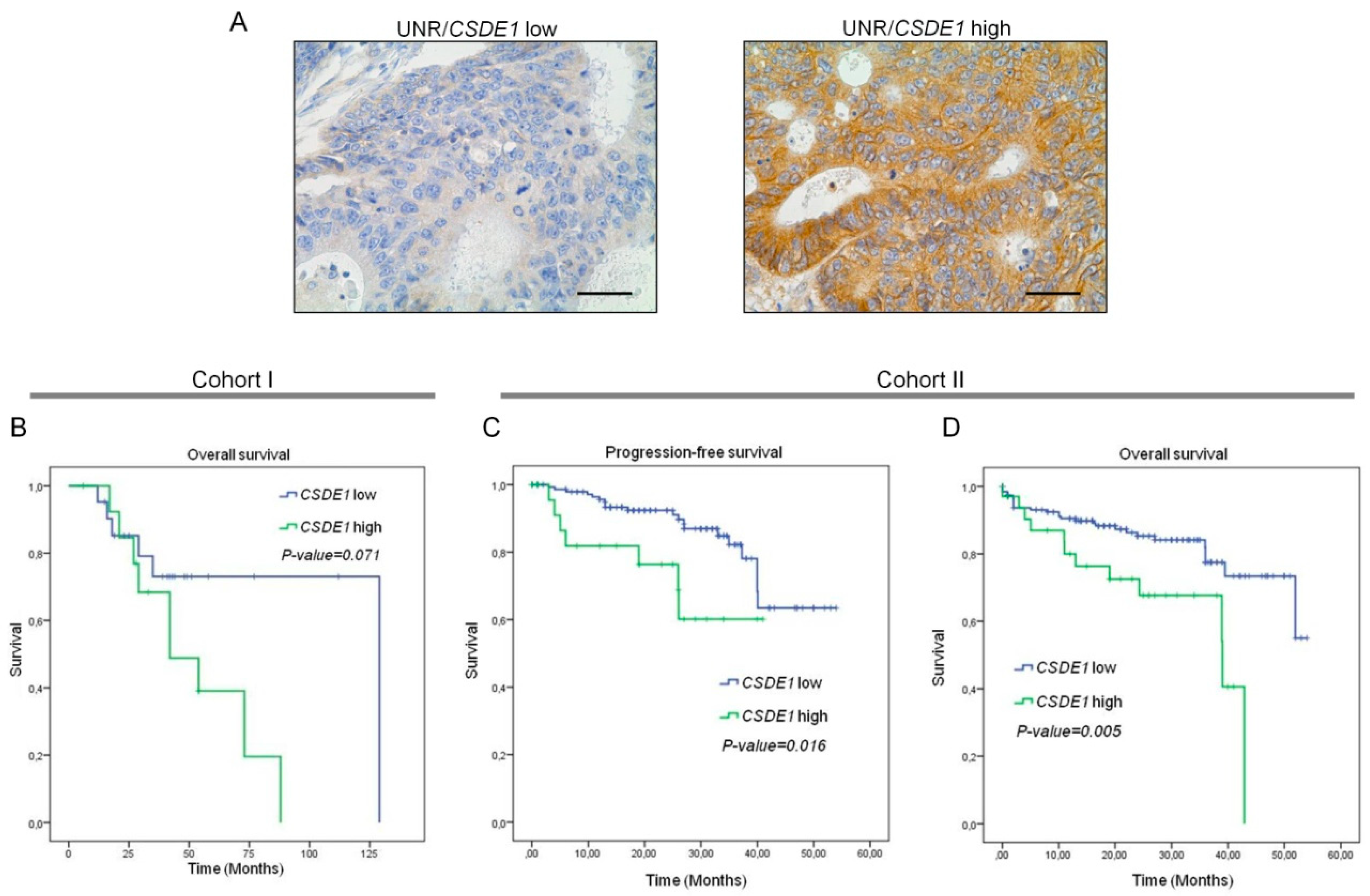
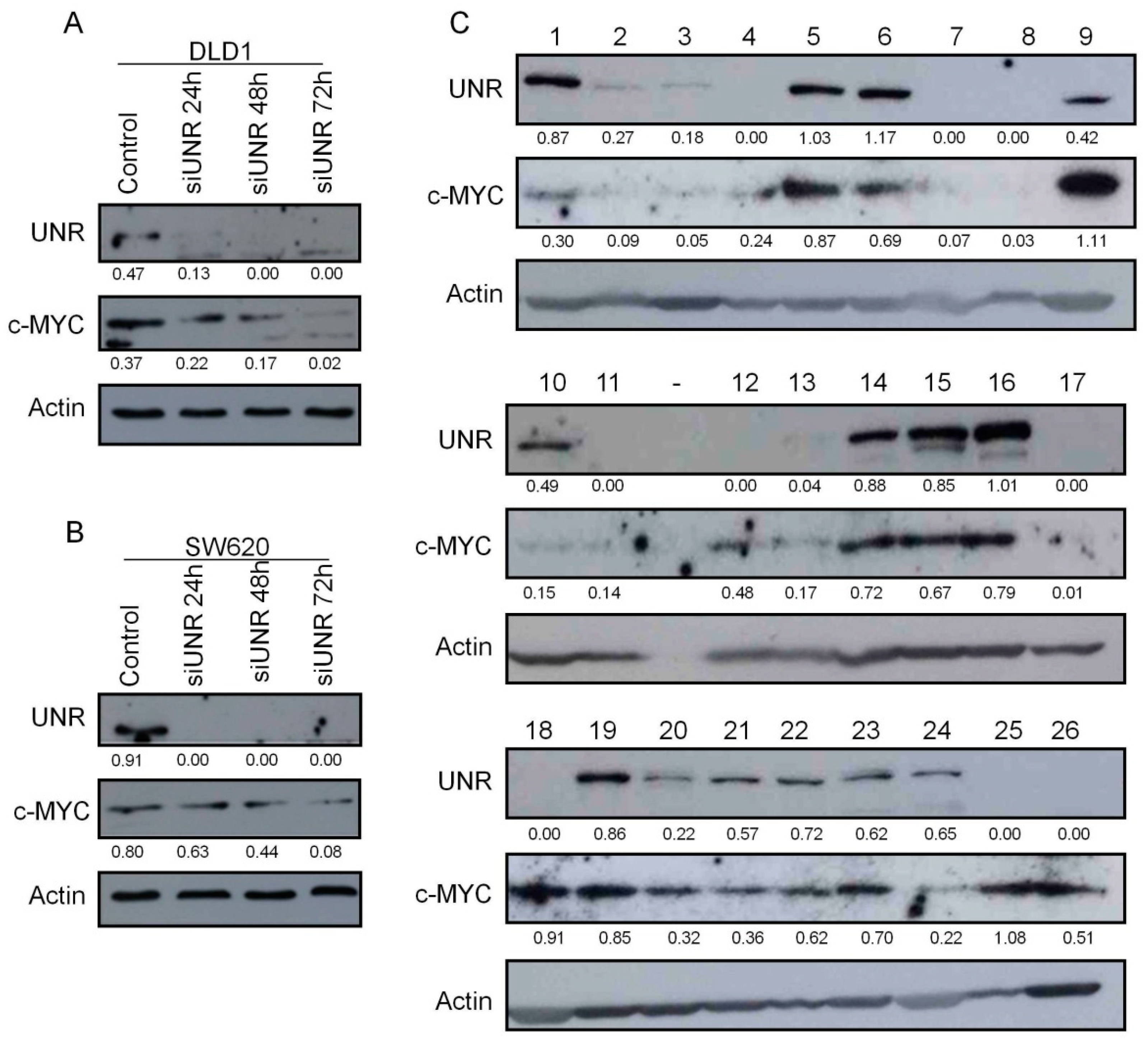
3.1. UNR/CSDE1 Is Overexpressed in CRC But Not in Untransformed Tissues
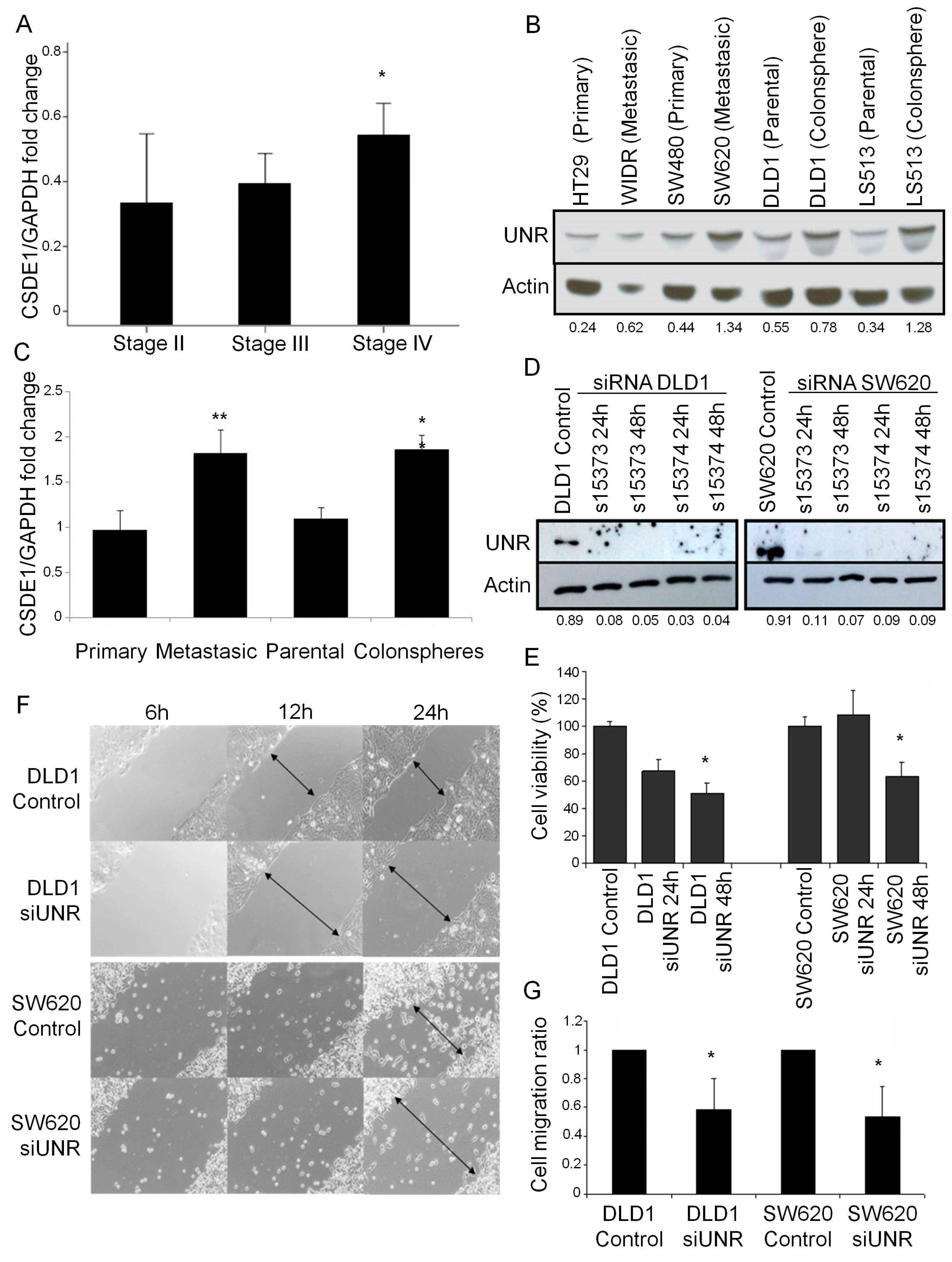
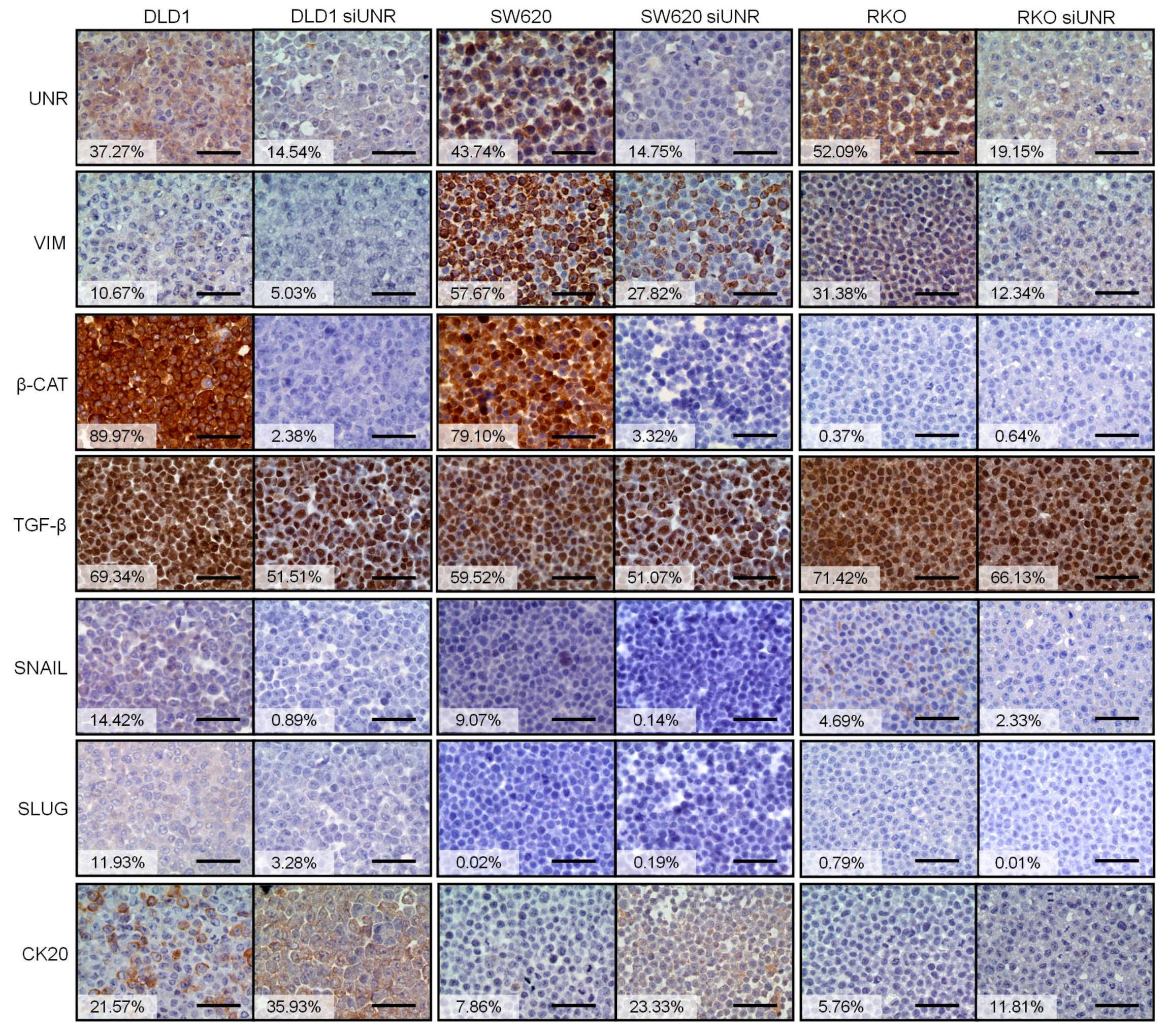
3.2. UNR/CSDE1 Knockdown Decreases Invasive Phenotype of CRC by Regulation of Epithelial-to-Mesenchymal Transition
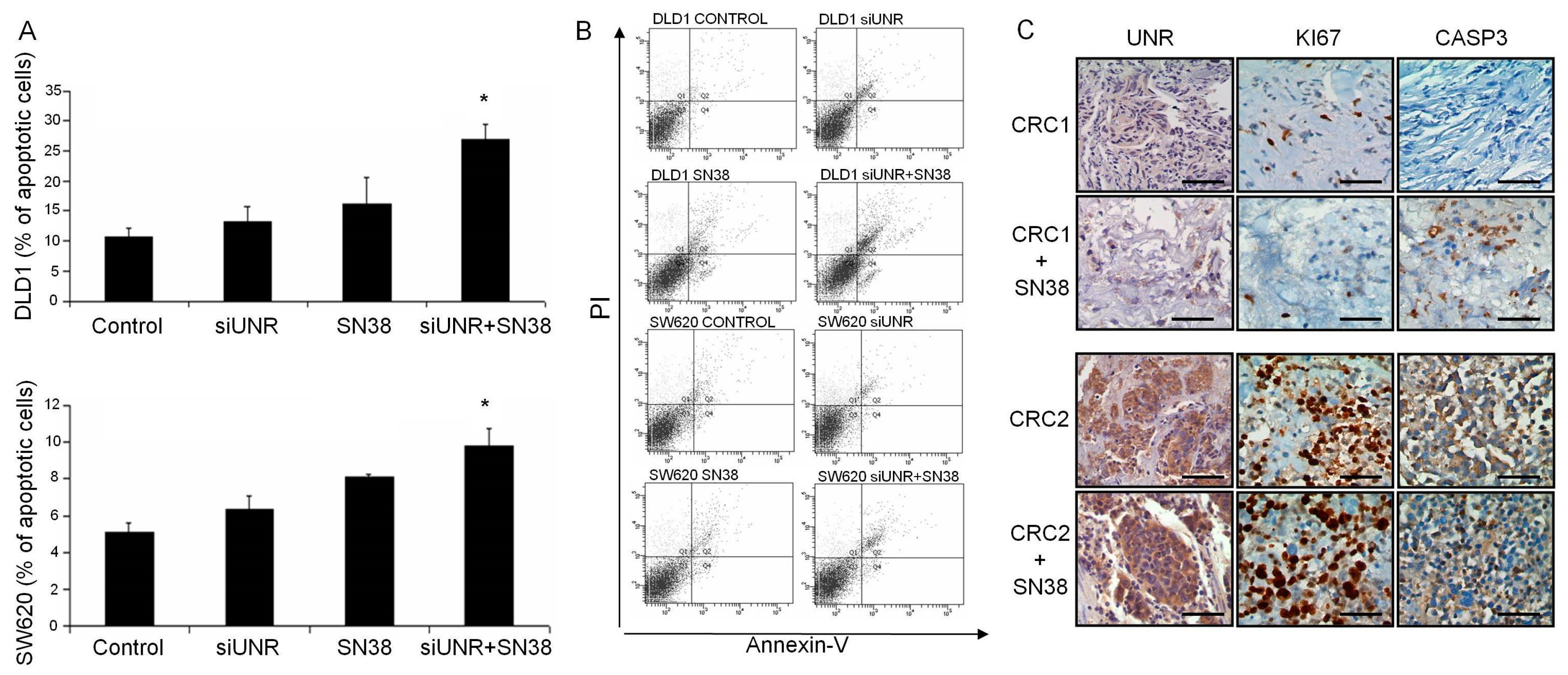
3.3. UNR/CSDE1 Downregulation Increases Response to Apoptosis Mediated by Camptothecin
3.4. High UNR/CSDE1 Expression Levels Predict Poor Outcome of CRC Patients
3.5. UNR/CSDE1 Expression Correlated with c-MYC Expression in CRC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Brenner, H.; Chen, C. The colorectal cancer epidemic: Challenges and opportunities for primary, secondary and tertiary prevention. Br. J. Cancer 2018, 119, 785–792. [Google Scholar] [CrossRef]
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef]
- Markowitz, S.D.; Bertagnolli, M.M. Molecular Basis of Colorectal Cancer. N. Engl. J. Med. 2009, 361, 2449–2460. [Google Scholar] [CrossRef]
- Wurth, L. Versatility of RNA-binding proteins in cancer. Comp. Funct. Genom. 2012, 2012, 178525. [Google Scholar] [CrossRef]
- Van Kouwenhove, M.; Kedde, M.; Agami, R. MicroRNA regulation by RNA-binding proteins and its implications for cancer. Nat. Rev. Cancer 2011, 9, 644–656. [Google Scholar] [CrossRef]
- Jeffers, M.; Paciucci, R.; Pellicer, A. Characterization of unr; a gene closely linked to N-ras. Nucleic Acids Res. 1990, 18, 4891–4899. [Google Scholar]
- Boussadia, O.; Amiot, F.; Cases, S.; Triqueneaux, G.; Jacquemin-Sablon, H.; Dautry, F. Transcription of unr (upstream of N-ras) down-modulates N-ras expression in vivo. FEBS Lett. 1997, 420, 20–24. [Google Scholar] [CrossRef]
- Martín-Hernández, J.; Sørensen, A.B.; Pedersen, F.S. Murine Leukemia Virus Proviral Insertions between the N-ras and unr Genes in B-Cell Lymphoma DNA Affect the Expression of N-ras Only. J. Virol. 2001, 75, 11907–11912. [Google Scholar] [CrossRef]
- Triqueneaux, G.; Velten, M.; Franzon, P.; Dautry, F.; Jacquemin-Sablon, H. RNA binding specificity of Unr, a protein with five cold shock domains. Nucleic Acids Res. 1999, 27, 1926–1934. [Google Scholar] [CrossRef]
- Mitchell, S.A.; Spriggs, K.A.; Coldwell, M.J.; Jackson, R.J.; Willis, A.E. The Apaf-1 internal ribosome entry segment attains the correct structural conformation for function via interactions with PTB and unr. Mol. Cell 2003, 11, 757–771. [Google Scholar] [CrossRef]
- Grosset, C.; Chen, C.Y.A.; Xu, N.; Sonenberg, N.; Jacquemin-Sablon, H.; Shyu, A. Bin A mechanism for translationally coupled mRNA turnover: Interaction between the poly(A) tail and a c-fos RNA coding determinant via a protein complex. Cell 2000, 103, 29–40. [Google Scholar] [CrossRef]
- Chang, T.C.; Yamashita, A.; Chen, C.Y.A.; Yamashita, Y.; Zhu, W.; Durdan, S.; Kahvejian, A.; Sonenberg, N.; Shyu, A. Bin UNR, a new partner of poly(A)-binding protein, plays a key role in translationally coupled mRNA turnover mediated by the c-fos major coding-region determinant. Genes Dev. 2004, 18, 2010–2023. [Google Scholar] [CrossRef]
- Evans, J.R.; Mitchell, S.A.; Spriggs, K.A.; Ostrowski, J.; Bomsztyk, K.; Ostarek, D.; Willis, A.E. Members of the poly (rC) binding protein family stimulate the activity of the c-myc internal ribosome entry segment in vitro and in vivo. Oncogene 2003, 22, 8012–8020. [Google Scholar] [CrossRef]
- Wurth, L.; Papasaikas, P.; Olmeda, D.; Bley, N.; Calvo, G.T.; Guerrero, S.; Cerezo-Wallis, D.; Martinez-Useros, J.; García-Fernández, M.; Hüttelmaier, S.; et al. UNR/CSDE1 Drives a Post-transcriptional Program to Promote Melanoma Invasion and Metastasis. Cancer Cell 2016, 30, 694–707. [Google Scholar] [CrossRef]
- Sun, X.; Fang, H.; Li, X.; Rossin, R.; Welch, M.J.; Taylor, J.S. MicroPET imaging of MCF-7 tumors in mice via unr mRNA-targeted peptide nucleic acids. Bioconjug. Chem. 2005, 16, 294–305. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, M.; Wu, Q.; Peng, J.; Ruan, Y.; Gu, J. Hepsin inhibits CDK11p58 IRES activity by suppressing unr expression and eIF-2α phosphorylation in prostate cancer. Cell. Signal. 2015, 27, 789–797. [Google Scholar] [CrossRef]
- Martinez-Useros, J.; Georgiev-Hristov, T.; Fernández-Aceñero, M.J.; Borrero-Palacios, A.; Indacochea, A.; Guerrero, S.; Li, W.; Cebrián, A.; Del Pulgar, T.G.; Puime-Otin, A.; et al. UNR/CDSE1 expression as prognosis biomarker in resectable pancreatic ductal adenocarcinoma patients: A proof-of-concept. PLoS ONE 2017, 12, e0182044. [Google Scholar] [CrossRef]
- Bitarte, N.; Bandres, E.; Boni, V.; Zarate, R.; Rodriguez, J.; Gonzalez-Huarriz, M.; Lopez, I.; Javier Sola, J.; Alonso, M.M.; Fortes, P.; et al. MicroRNA-451 is involved in the self-renewal, tumorigenicity, and chemoresistance of colorectal cancer stem cells. Stem Cells 2011, 29, 1661–1671. [Google Scholar] [CrossRef]
- Souza, V.; Dong, Y. Bin; Zhou, H.S.; Zacharias, W.; McMasters, K.M. SW-620 cells treated with topoisomerase I inhibitor SN-38: Gene expression profiling. J. Transl. Med. 2005, 23, 44. [Google Scholar] [CrossRef][Green Version]
- Martinez-Useros, J.; Rodriguez-Remirez, M.; Borrero-Palacios, A.; Moreno, I.; Cebrian, A.; Gomez del Pulgar, T.; del Puerto-Nevado, L.; Vega-Bravo, R.; Puime-Otin, A.; Perez, N.; et al. DEK is a potential marker for aggressive phenotype and irinotecan-based therapy response in metastatic colorectal cancer. BMC Cancer 2014, 14, 965. [Google Scholar] [CrossRef]
- Fuhrich, D.G.; Lessey, B.A.; Savaris, R.F. Comparison of HSCORE assessment of endometrial β3 integrin subunit expression with digital HSCORE using computerized image analysis (ImageJ). Anal. Quant. Cytol. Histol. 2013, 35, 210–216. [Google Scholar]
- Cohen, L.H. Measurement of life events. In Life Events and Psychological Functioning: Theoretical and Methodological Issues; Cohen, L.H., Ed.; Sage: Newbury Park, CA, USA, 1988; pp. 11–30. [Google Scholar]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, l1. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio Cancer Genomics Portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Weiswald, L.B.; Richon, S.; Validire, P.; Briffod, M.; Lai-Kuen, R.; Cordelières, F.P.; Bertrand, F.; Dargere, D.; Massonnet, G.; Marangoni, E.; et al. Newly characterised ex vivo colospheres as a three-dimensional colon cancer cell model of tumour aggressiveness. Br. J. Cancer 2009, 101, 473–482. [Google Scholar] [CrossRef]
- Wang, X.; Gong, W.; Qing, H.; Geng, Y.; Wang, X.; Zhang, Y.; Peng, L.; Zhang, H.; Jiang, B. P21-activated kinase 5 inhibits camptothecin-induced apoptosis in colorectal carcinoma cells. Tumor Biol. 2010, 31, 575–582. [Google Scholar] [CrossRef]
- Matter, N.; Herrlich, P.; König, H. Signal-dependent regulation of splicing via phosphorylation of Sam68. Nature 2002, 420, 691–695. [Google Scholar] [CrossRef]
- Busà, R.; Paronetto, M.P.; Farini, D.; Pierantozzi, E.; Botti, F.; Angelini, D.F.; Attisani, F.; Vespasiani, G.; Sette, C. The RNA-binding protein Sam68 contributes to proliferation and survival of human prostate cancer cells. Oncogene 2007, 26, 4372–4382. [Google Scholar] [CrossRef]
- Rajan, P.; Gaughan, L.; Dalgliesh, C.; El-Sherif, A.; Robson, C.N.; Leung, H.Y.; Elliott, D.J. Regulation of gene expression by the RNA-binding protein Sam68 in cancer. Biochem. Soc. Trans. 2008, 36, 505–507. [Google Scholar] [CrossRef]
- Topisirovic, I.; Sonenberg, N. mRNA translation and energy metabolism in cancer: The role of the MAPK and mtorc1 Pathways. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 355–367. [Google Scholar] [CrossRef]
- Hsieh, A.C.; Ruggero, D. Targeting eukaryotic translation initiation factor 4E (eIF4E) in cancer. Clin. Cancer Res. 2010, 20, 4914–4920. [Google Scholar] [CrossRef]
- Coleman, L.J.; Peter, M.B.; Teall, T.J.; Brannan, R.A.; Hanby, A.M.; Honarpisheh, H.; Shaaban, A.M.; Smith, L.; Speirs, V.; Verghese, E.T.; et al. Combined analysis of eIF4E and 4E-binding protein expression predicts breast cancer survival and estimates eIF4E activity. Br. J. Cancer 2009, 100, 1393–1399. [Google Scholar] [CrossRef]
- Dumstorf, C.A.; Konicek, B.W.; McNulty, A.M.; Parsons, S.H.; Furic, L.; Sonenberg, N.; Graff, J.R. Modulation of 4E-BP1 Function as a Critical Determinant of Enzastaurin-Induced Apoptosis. Mol. Cancer Ther. 2010, 9, 3158–3163. [Google Scholar] [CrossRef]
- Bergalet, J.; Fawal, M.; Lopez, C.; Desjobert, C.; Lamant, L.; Delsol, G.; Morello, D.; Espinos, E. HuR-Mediated Control of C/EBP mRNA Stability and Translation in ALK-Positive Anaplastic Large Cell Lymphomas. Mol. Cancer Res. 2011, 9, 485–496. [Google Scholar] [CrossRef]
- Kakuguchi, W.; Kitamura, T.; Kuroshima, T.; Ishikawa, M.; Kitagawa, Y.; Totsuka, Y.; Shindoh, M.; Higashino, F. HuR Knockdown Changes the Oncogenic Potential of Oral Cancer Cells. Mol. Cancer Res. 2010, 8, 520–528. [Google Scholar] [CrossRef]
- Nowotarski, S.L.; Shantz, L.M. The RNA binding protein HuR stabilizes the ornithine decarboxylase (ODC) transcript in non-melanoma skin cancer. Cancer Res. 2010, 70, 3161. [Google Scholar] [CrossRef]
- Elatmani, H.; Dormoy-Raclet, V.; Dubus, P.; Dautry, F.; Chazaud, C.; Jacquemin-Sablon, H. The RNA-binding protein Unr prevents mouse embryonic stem cells differentiation toward the primitive endoderm lineage. Stem Cells 2011, 29, 1504–1516. [Google Scholar] [CrossRef]
- Dormoy-Raclet, V.; Markovits, J.; Malato, Y.; Huet, S.; Lagarde, P.; Montaudon, D.; Jacquemin-Sablon, A.; Jacquemin-Sablon, H. Unr, a cytoplasmic RNA-binding protein with cold-shock domains, is involved in control of apoptosis in ES and HuH7 cells. Oncogene 2007, 26, 2595–2605. [Google Scholar] [CrossRef]
- Hsiang, Y.H.; Liu, L.F. Identification of mammalian dna topoisomerase i as an intracellular target of the anticancer drug camptothecin. Cancer Res. 1988, 48, 1722–1726. [Google Scholar]
- Liu, C.-Y.; Lin, H.-H.; Tang, M.-J.; Wang, Y.-K. Vimentin contributes to epithelial-mesenchymal transition cancer cell mechanics by mediating cytoskeletal organization and focal adhesion maturation. Oncotarget 2015, 6, 15966–15983. [Google Scholar] [CrossRef]
- Peinado, H.; Olmeda, D.; Cano, A. Snail, ZEB and bHLH factors in tumour progression: An alliance against the epithelial phenotype? Nat. Rev. Cancer 2007, 6, 415–428. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- Chen, K.; Liu, Q.; Tsang, L.L.; Ye, Q.; Chan, H.C.; Sun, Y.; Jiang, X. Human MSCs promotes colorectal cancer epithelial-mesenchymal transition and progression via CCL5/β-catenin/Slug pathway. Cell Death Dis. 2017, 25, e2819. [Google Scholar] [CrossRef]
- Chu, P.; Wu, E.; Weiss, L.M. Cytokeratin 7 and Cytokeratin 20 expression in epithelial neoplasms: A survey of 435 cases. Mod. Pathol. 2000, 13, 962–972. [Google Scholar] [CrossRef]
- Land, H.; Parada, L.F.; Weinberg, R.A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature 1983, 304, 596–602. [Google Scholar] [CrossRef]
- Keath, E.J.; Caimi, P.G.; Cole, M.D. Fibroblast lines expressing activated c-myc oncogenes are tumorigenic in nude mice and syngeneic animals. Cell 1984, 39, 339–348. [Google Scholar] [CrossRef]
- Kelekar, A.; Cole, M.D. Tumorigenicity of fibroblast lines expressing the adenovirus E1a, cellular p53, or normal c-myc genes. Mol. Cell. Biol. 1986, 6, 7–14. [Google Scholar] [CrossRef]
- Anderson, E.C.; Catnaigh, P.O. Regulation of the expression and activity of Unr in mammalian cells. Biochem. Soc. Trans. 2015, 43, 1241–1246. [Google Scholar] [CrossRef]
- Gatti, G.; Maresca, G.; Natoli, M.; Florenzano, F.; Nicolin, A.; Felsani, A.; D’Agnano, I. Myc prevents apoptosis and enhances endoreduplication induced by paclitaxel. PLoS ONE 2009, 4, e5442. [Google Scholar] [CrossRef]
- Bucci, B.; D’Agnano, I.; Amendola, D.; Citti, A.; Raza, G.H.; Miceli, R.; De Paula, U.; Marchese, R.; Albini, S.; Felsani, A.; et al. Myc down-regulation sensitizes melanoma cells to radiotherapy by inhibiting MLH1 and MSH2 mismatch repair proteins. Clin. Cancer Res. 2005, 11, 2756–2767. [Google Scholar] [CrossRef]
- D’Agnano, I.; Valentini, A.; Fornari, C.; Bucci, B.; Starace, G.; Felsani, A.; Citro, G. Myc down-regulation induces apoptosis in M14 melanoma cells by increasing p27kip1 levels. Oncogene 2001, 20, 2814–2825. [Google Scholar] [CrossRef]
- Greco, C.; D’Agnano, I.; Vitelli, G.; Vona, R.; Marino, M.; Mottolese, M.; Zuppi, C.; Capoluongo, E.; Ameglio, F. c-MYC deregulation is involved in melphalan resistance of multiple myeloma: Role of PDGF-BB. Int. J. Immunopathol. Pharmacol. 2006, 19, 67–79. [Google Scholar] [CrossRef]
- Pilling, A.B.; Kim, J.; Estrada-Bernal, A.; Zhou, Q.; Le, A.T.; Singleton, K.R.; Heasley, L.E.; Tan, A.C.; DeGregori, J.; Doebele, R.C. ALK is a critical regulator of the MYC-signaling axis in ALK positive lung cancer. Oncotarget 2018, 9, 8823–8835. [Google Scholar] [CrossRef]
- Cho, K. Bin; Cho, M.K.; Lee, W.Y.; Kang, K.W. Overexpression of c-myc induces epithelial mesenchymal transition in mammary epithelial cells. Cancer Lett. 2010, 293, 230–239. [Google Scholar] [CrossRef]
- Li, Y.; El-Kady, A.; Sun, Y.; Liao, Dj. Cyclin D1 inhibits whereas c-Myc enhances the cytotoxicity of cisplatin in mouse pancreatic cancer cells via regulation of several members of the NF-κB and Bcl-2 families. J. Carcinog. 2011, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Choi, I.J.; Kim, C.G.; Kim, H.S.; Oshima, A.; Yamada, Y.; Arao, T.; Nishio, K.; Michalowski, A.; Green, J.E. Three-gene predictor of clinical outcome for gastric cancer patients treated with chemotherapy. Pharmacogenom. J. 2012, 12, 119–127. [Google Scholar] [CrossRef]
- Niimi, S.; Nakagawa, K.; Yokota, J.; Tsunokawa, Y.; Nishio, K.; Terashima, Y.; Shibuya, M.; Terada, M.; Saijo, N. Resistance to anticancer drugs in NIH3T3 cells transfected with c-myc and/or c-H-ras genes. Br. J. Cancer 1991, 63, 237–241. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | N (%) | Characteristics | N (%) |
|---|---|---|---|
| Median Age (range) | 64 years (37–83) | Mestastasic disease | |
| Gender | Synchronous | 23 (66%) | |
| Female | 13 (37%) | Metachronous | 12 (34%) |
| Male | 18 (52%) | Differentiation grade | |
| N/A | 4 (11%) | Well | 9 (26%) |
| Family history of cancer | Moderate | 16 (46%) | |
| No | 25 (72%) | Poor | 3 (8%) |
| Yes | 5 (14%) | N/A | 7 (20%) |
| N/A | 5 (14%) | Inflammation | |
| Localization of primary tumor | No | 11 (32%) | |
| Right | 6 (17%) | Low | 14 (40%) |
| Transverse | 2 (6%) | Moderate | 4 (11%) |
| Left | 2 (6%) | N/A | 6 (17%) |
| Sigma | 11 (32%) | Vascular Invasion | |
| Rectum | 8 (23%) | No | 25 (72%) |
| Cecum | 3 (8%) | Yes | 4 (11%) |
| N/A | 3 (8%) | N/A | 6 (17%) |
| pT | MMR | ||
| T1 | 2 (6%) | Deficient | 3 (9%) |
| T2 | 2 (6%) | Proficient | 29 (82%) |
| T3 | 29 (82%) | N/A | 3 (9%) |
| T4 | 1 (3%) | RAS status | |
| N/A | 1 (3%) | wild-type | 24 (69%) |
| pN | Mutated | 6 (17%) | |
| N0 | 18 (52%) | N/A | 5 (14%) |
| N1 | 11 (31%) | UNR | |
| N2 | 5 (14%) | High | 14 (40%) |
| N/A | 1 (3%) | Low | 21 (60%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Useros, J.; Garcia-Carbonero, N.; Li, W.; Fernandez-Aceñero, M.J.; Cristobal, I.; Rincon, R.; Rodriguez-Remirez, M.; Borrero-Palacios, A.; Garcia-Foncillas, J. UNR/CSDE1 Expression Is Critical to Maintain Invasive Phenotype of Colorectal Cancer through Regulation of c-MYC and Epithelial-to-Mesenchymal Transition. J. Clin. Med. 2019, 8, 560. https://doi.org/10.3390/jcm8040560
Martinez-Useros J, Garcia-Carbonero N, Li W, Fernandez-Aceñero MJ, Cristobal I, Rincon R, Rodriguez-Remirez M, Borrero-Palacios A, Garcia-Foncillas J. UNR/CSDE1 Expression Is Critical to Maintain Invasive Phenotype of Colorectal Cancer through Regulation of c-MYC and Epithelial-to-Mesenchymal Transition. Journal of Clinical Medicine. 2019; 8(4):560. https://doi.org/10.3390/jcm8040560
Chicago/Turabian StyleMartinez-Useros, Javier, Nuria Garcia-Carbonero, Weiyao Li, Maria J. Fernandez-Aceñero, Ion Cristobal, Raul Rincon, Maria Rodriguez-Remirez, Aurea Borrero-Palacios, and Jesus Garcia-Foncillas. 2019. "UNR/CSDE1 Expression Is Critical to Maintain Invasive Phenotype of Colorectal Cancer through Regulation of c-MYC and Epithelial-to-Mesenchymal Transition" Journal of Clinical Medicine 8, no. 4: 560. https://doi.org/10.3390/jcm8040560
APA StyleMartinez-Useros, J., Garcia-Carbonero, N., Li, W., Fernandez-Aceñero, M. J., Cristobal, I., Rincon, R., Rodriguez-Remirez, M., Borrero-Palacios, A., & Garcia-Foncillas, J. (2019). UNR/CSDE1 Expression Is Critical to Maintain Invasive Phenotype of Colorectal Cancer through Regulation of c-MYC and Epithelial-to-Mesenchymal Transition. Journal of Clinical Medicine, 8(4), 560. https://doi.org/10.3390/jcm8040560







