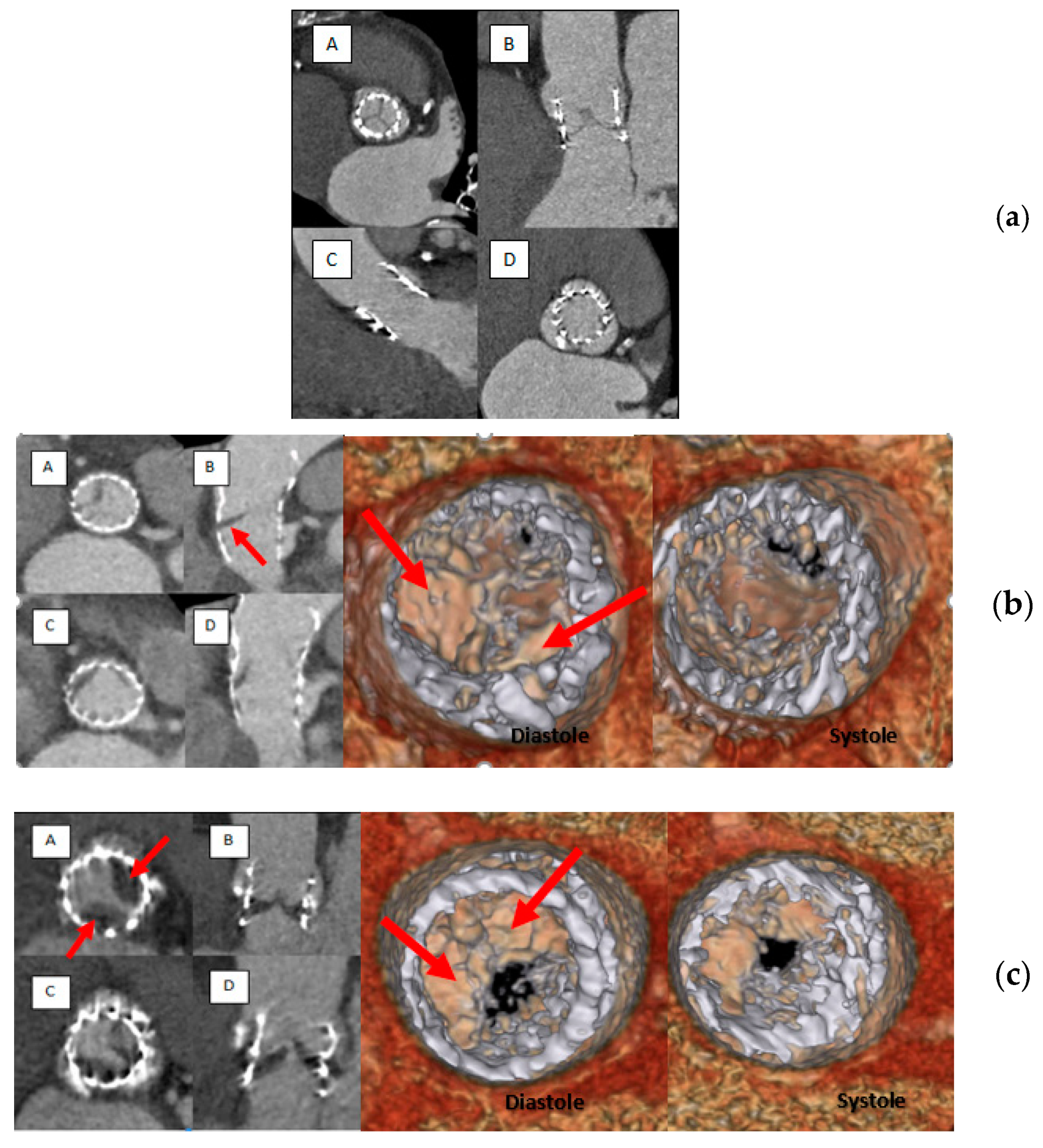
4.1. Main Results
The current report, drawn from a cohort of 90 patients, is among the first study to specifically evaluate the impact of platelet inhibition extent on the occurrence of subclinical leaflet thrombosis detected by MDCT. The salient results of the present study are (1) subclinical leaflet thrombosis is observed in a sizeable proportion of TAVR patients (15%) after a median follow-up of 114 (65–205) days; (2) lack of oral anticoagulant together with elevated Hb levels were evidenced as independent predictors of subclinical leaflet thrombosis; (3) occurrence of subclinical leaflet thrombosis was independent of the extent of platelet inhibition, as measured by the PRI-VASP or the CT-ADP assay; (4) no impact of subclinical leaflet thrombosis on clinical events could be established. Altogether, our findings suggest the main importance of OAC therapy in the prevention of subclinical leaflet thrombosis independently of platelet inhibition extent. The prevalence of HAM reported in the present study (15%), is consistent with recent data from SAVORY and RESOLVE large observational registries in which TAVR reduced leaflet motion could be evidenced in 11.9% of the cohort [
12]. In TAVR, important variations in the occurrence of SLT have been recently underlined, depending, in part, on the criteria used and the quality of MDCT acquisition. In the report by Sondergaard [
13], HALT was evidenced in 38.1% and complete HAM in 20.2% after 140 days. By contrast, other investigators have reported lower HALT rates ranging from 4% (Leetmaa T, Circ Cardiovasc Interv. 2015 [
14]), 7% (Hansson NC, JACC 2016 [
15]), to 10.3% (Pache G, Eur Heart J. 2016 [
16]). Of particular interest, recent data have underlined that leaflet thrombosis occurred more frequently in transcatheter bioprosthetic valves than surgical ones [
12].
The study of mechanisms involved in thrombus formation during TAVR is far beyond the scope of the present study. Among various hypotheses, it is likely that the thrombus formation is the result of a complex interplay involving calcifications, native anatomy, hemodynamic, flow stasis, haemostatic factors, procedural factors, and valve type. Interaction between the prosthetic valve consisting in a metallic stent frame and three biologics leaflets, into a native or bioprosthetic (valve in valve) aortic valve, would create a new anatomic geometry characterized by modifications of shear stress regimen and turbulence. It is likely that the formation of a neosinus (region between the native and transcatheter aortic valve leaflet) favouring blood stasis would provide an ideal reservoir for thrombus formation. Moreover, exposure of procoagulant factors by the native valve (including tissue factor or procoagulant microparticles) may contribute to trigger thrombotic process. As pointed at by Midha et al. [
17] supra-annular position of the neosinus may reduce thrombotic potential owing to the reduction of blood stasis. Another important factor relies on the under expansion of the prosthesis as underlined in Sapiens 3 valves or in autopsy studies [
18]. Moreover, the possible contribution of valve injury mediated by balloon valvuloplasty or inflammatory response in thrombosis onset remains to be determined [
19]. Others authors have suggested that the structure composition of the valves may play a differential role in thrombus formation. Whilst CoreValve porcine pericardium valve contains a nickel and titanium alloy, the Edward Sapien bovine pericardium valve has a polyethylene terephthalate skirt and cobalt chromium stent that could trigger allergic reaction, IgE antibody formation, and the coagulation cascade [
20]. Finally, the contribution of paravalvular leak as a main determinant of platelet activation has recently be underlined [
21].
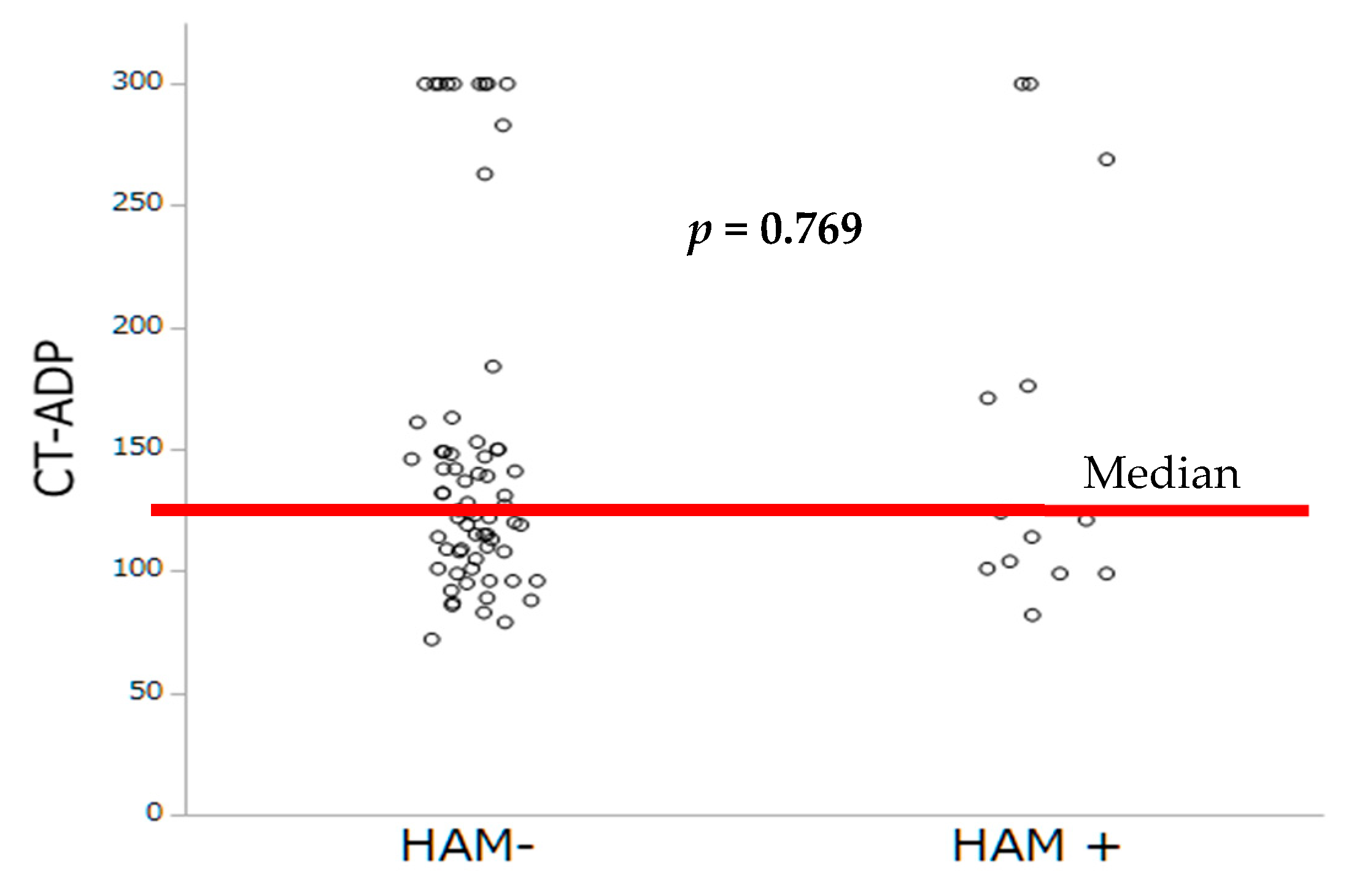
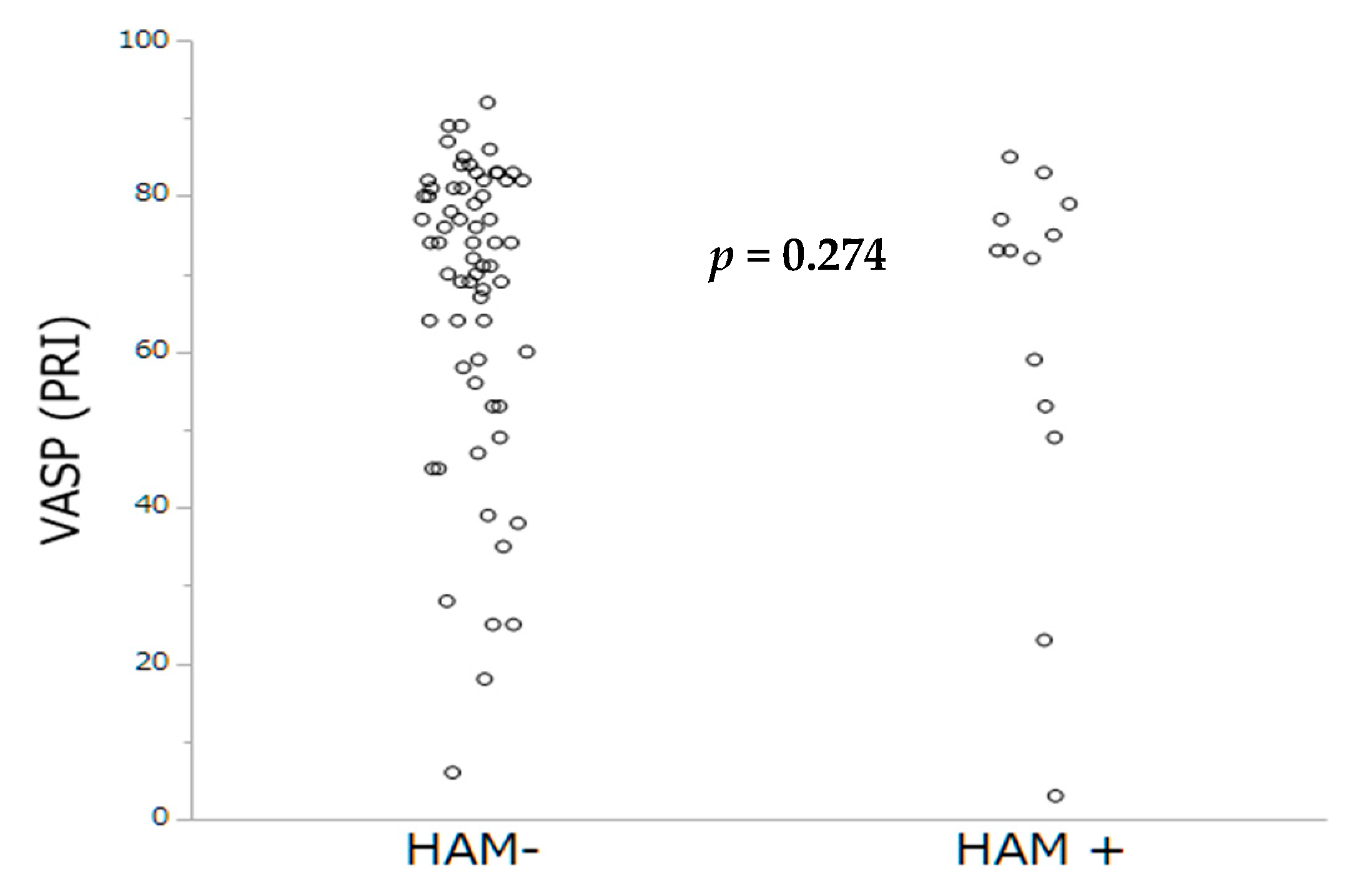
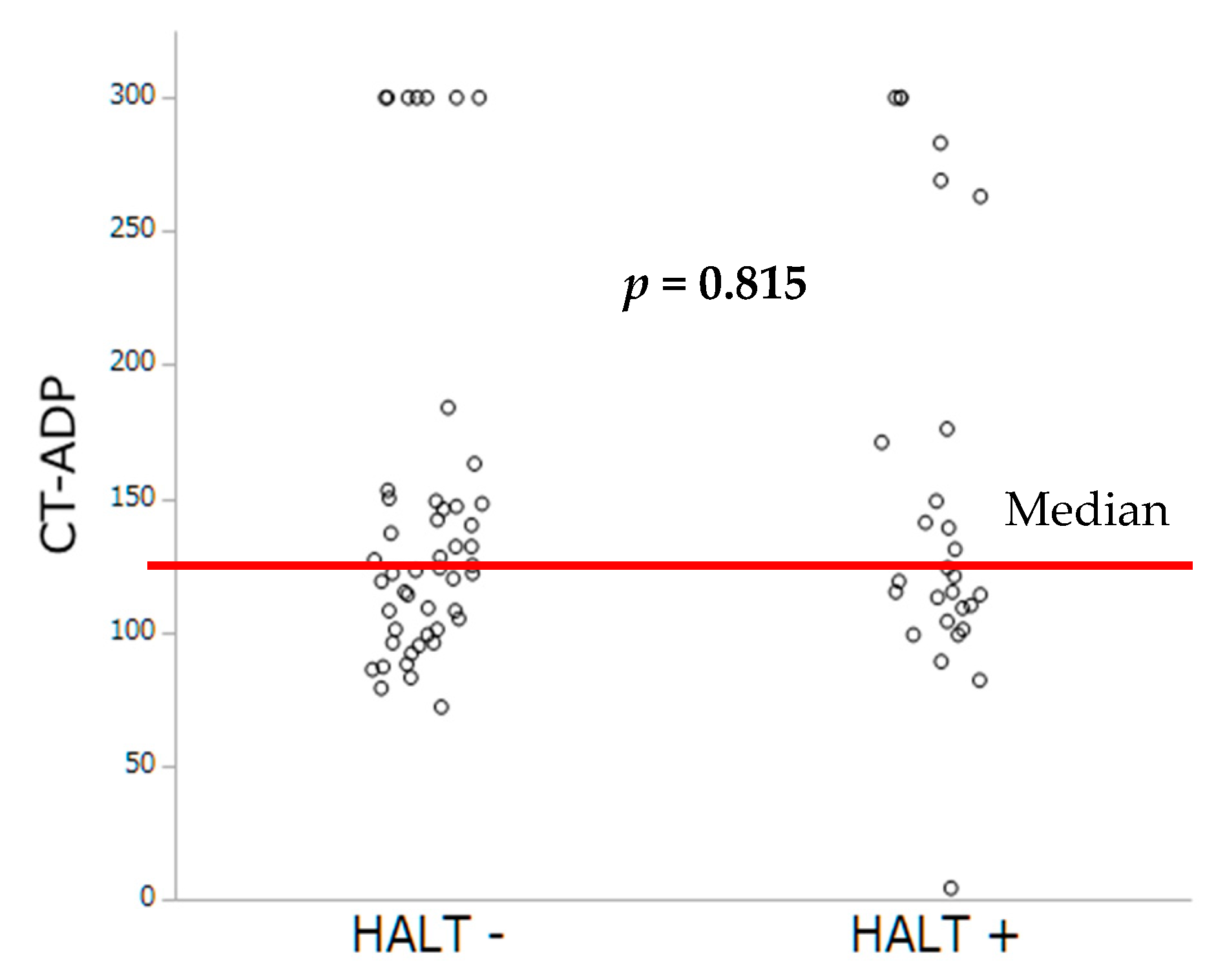
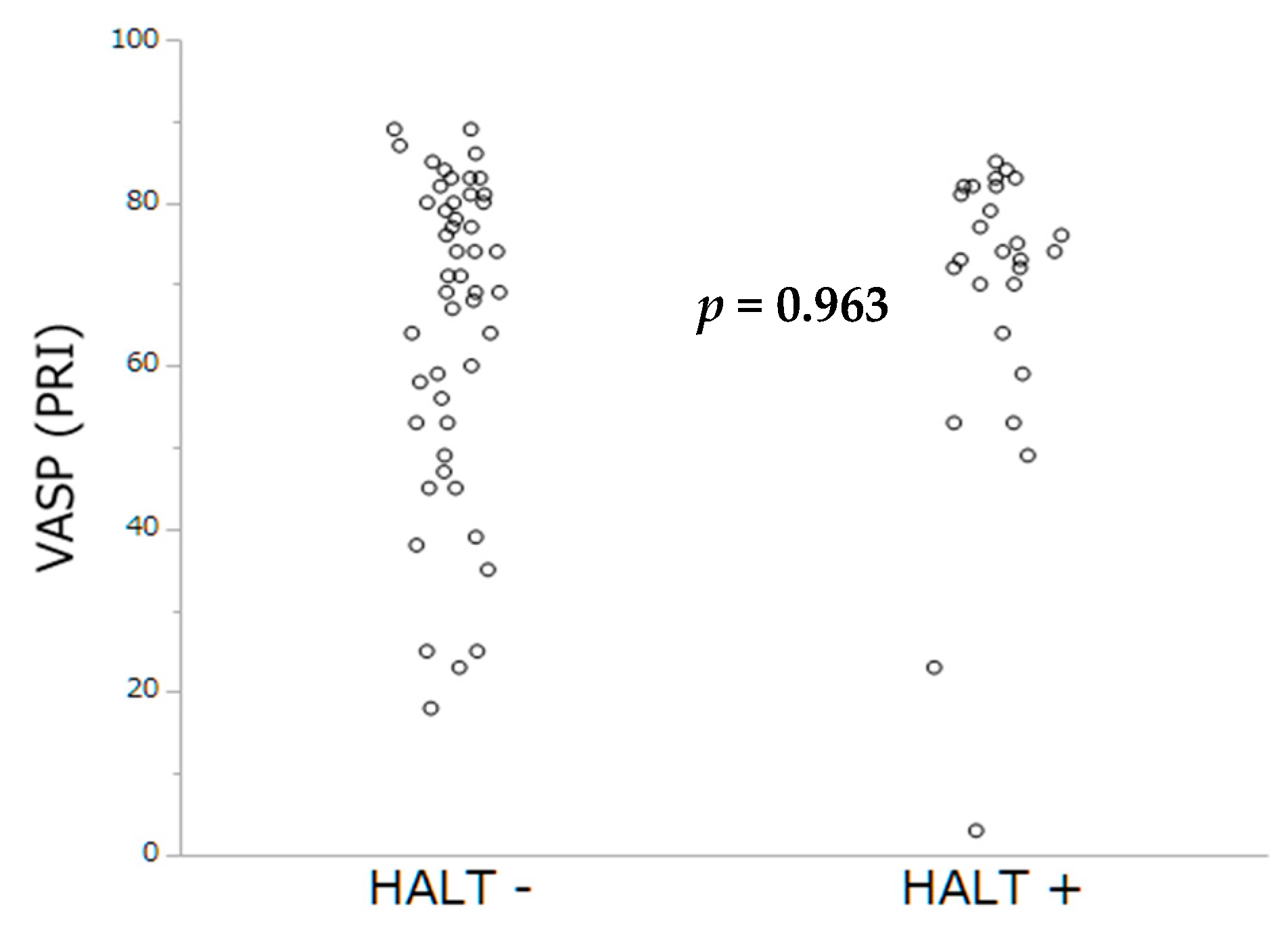
As extensively demonstrated in PCI, we sought to investigate whether impaired platelet inhibition evaluated either by PRI-VASP or by CT-ADP could contribute to subclinical leaflet thrombosis. In TAVR, we have previously demonstrated that prolonged CT-ADP (>180 s) is not only a marker or paravalvular leakage (through enhanced proteolysis of high molecular weight von Willebrand factors) but also constitutes an integrate marker of enhanced periprocedural and late bleeding risk [
22,
23]. In our hand no impact of impaired platelet inhibition of HAM and HALT phenomenon could be established. This result is consistent with the recent study from Nührenberg et al. [
24], which did not find significant association between the occurrence of HALT and impaired platelet inhibition assessed by ADP test. In the present study, the mean CT-ADP at discharge was 149 s which is comparable with data from Nührenberg and coworkers, but also with those from patients with coronary artery disease [
7]. Altogether, our finding suggests that the development of bioprosthetic valve thrombosis is mainly platelet independent, consistent with a primary role of contact phase activation and more likely to be targeted by OAC.
Among various factors possibly involved in the development of SLT, converging evidences have highlighted a key role of anticoagulant therapy in the prevention or treatment of silent valve thrombosis. In the RESOLVE and SAVORY registries, subclinical leaflet thrombosis was less frequent among patients treated by OAC (4%) than patients receiving DAPT (15%). In this report, SLT resolved in all patients receiving anticoagulant either by VKA or NOACS, whereas it persisted in 91% of patients under antiplatelet therapy alone. Similarly, in our experience, HAM could only be evidenced in 7.7% patients under OAC. Multivariable analysis confirmed the independent association between lack of OAC at hospital discharge and HAM phenomenon. Accordingly, in a larger registry by Hansson [
15] and coworkers, lack of warfarin treatment was also pointed out as an independent predictor of valve thrombosis. To date, important controversies remain on the impact of valve thrombosis on aortic valve pressure gradients, valve deterioration and adverse clinical outcomes. In the present report, mean aortic gradient at 30-day and one-year follow-up were not significantly different among groups. However, significant elevation of the mean aortic transvalvular gradient could be evidenced in 4 HAM patients (30.7%). Accordingly, in the report by Chakravarty [
12], an increase in aortic valve gradient could be evidenced in 14% of patients with subclinical leaflet thrombosis. Other authors have emphasized the view that the treatment with OAC leads not only to regression of valve thickening but also on changes in transvalvular pressure gradients [
25]. In the setting of TAVR, the question of valve durability remains of paramount importance. From a pathophysiological point of view, it is likely that endothelial damage and subsequent thrombosis may contribute to the infiltration of inflammatory cells within the valvular tissue leading to adverse remodeling and deterioration. Consistent with this paradigm, recent pathological analysis of 23 explanted transcatheter heart valves has underlined the existence of a time-dependent degeneration of heart valve consisting of thrombus formation, endothelial hyperplasia, fibrosis, tissue remodeling, proteinase expression, and calcification [
26]. In line with this view, registry data from a cohort of 1521 TAVI patients has elegantly depicted that the rate of valve deterioration, as assessed by elevation in mean transvalvular gradient was 2.8% at one year following TAVR. Of paramount importance, the absence of anticoagulation therapy at discharge was evidenced as a key factor of valve deterioration, together with valve in valve procedure and the use of small 23-mm valve [
27]. Although the contribution of confounding factors is difficult to delineate precisely in a prospective registry (OAC is mainly given in AF patients, AF could lead to gradient underestimation, and subsequently could underestimate valve deterioration in OAC receiving patients), those findings suggest that OAC are of paramount importance in the development of valve deterioration overt time.
Another crucial issue relies on the clinical impact of valve thrombosis on thromboembolic events. The pioneering work by Chakravarty [
12] has suggested that SLT was associated with increased rates of transient ischemic attack (TIA). Conversely, in a large registry comprising 754 patients (among them 120 patients with valve thrombosis), no differences in overall mortality and stroke/TIA rates could be demonstrated after a 406-day follow-up. However, it should be emphasized that rates of stroke/TIA were surprisingly extremely low (<2%) for a TAVR population [
28], which probably reflects the German policy characterized by a more liberal use of TAVR implantation that includes lower risk patients. By contrast, insights from the US FDA MAUDE database has highlighted that leaflet thrombosis is associated with adverse outcomes including stroke, cardiogenic shock and death [
29].
The definition of optimal antithrombotic therapies following TAVR remains a matter of important controversies. Whereas the frequency of subclinical leaflet thrombosis and the possible link between SLT and valve deterioration advocate for a liberal use of OAC, safety concerns and the assessment of bleeding events remain key elements when prescribing OAC. Several groups including ours have recently underlined the paramount importance of bleeding events that significantly outweighed ischemic concerns [
23,
24,
25,
26,
27,
28,
29,
30]. Of major importance, late bleeding is considered as a main determinant of adverse outcome in the frail TAVR population. Ongoing trials (ATLANTIS trial NCT02943785, POPular-TAVI trial NCT02247128, ENVISAGE-TAVI trial NCT02943785, AUREA trial NCT01642134, and AVATAR trial NCT02735902) will provide important insights on the efficacy but also safety profile of OAC including NOACS. However, alerting signal was very recently provided by the GALILEO trial which was prematurely halted. In this study, RIVAROXABAN, a Xa inhibitor was associated with greater risk of all-cause mortality, thromboembolic events, and bleeding in TAVR patients.