Plasma Malondialdehyde and Risk of New-Onset Diabetes after Transplantation in Renal Transplant Recipients: A Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Data Collection
2.3. Measurements and Definitions
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Prospective Analyses on NODAT
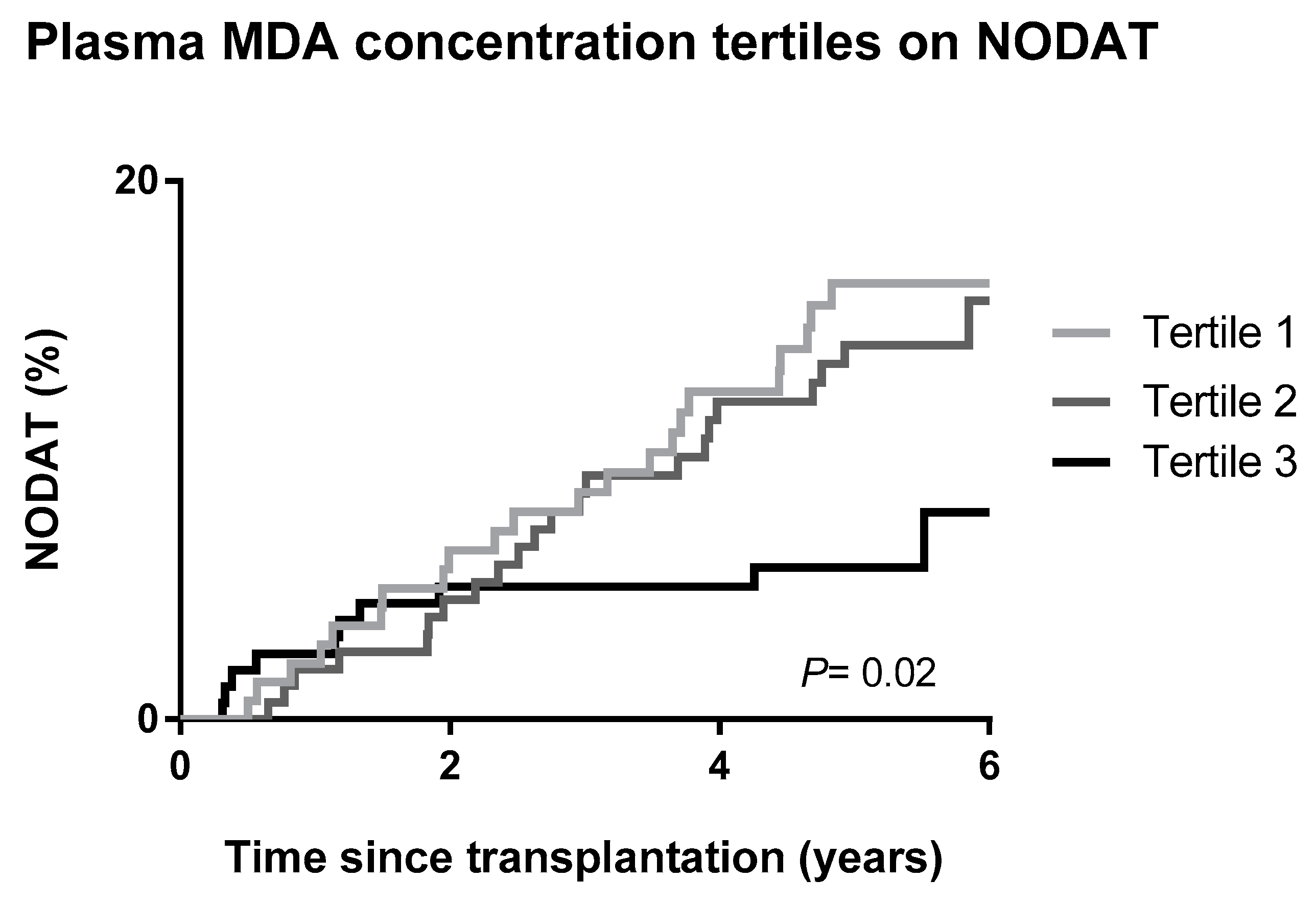
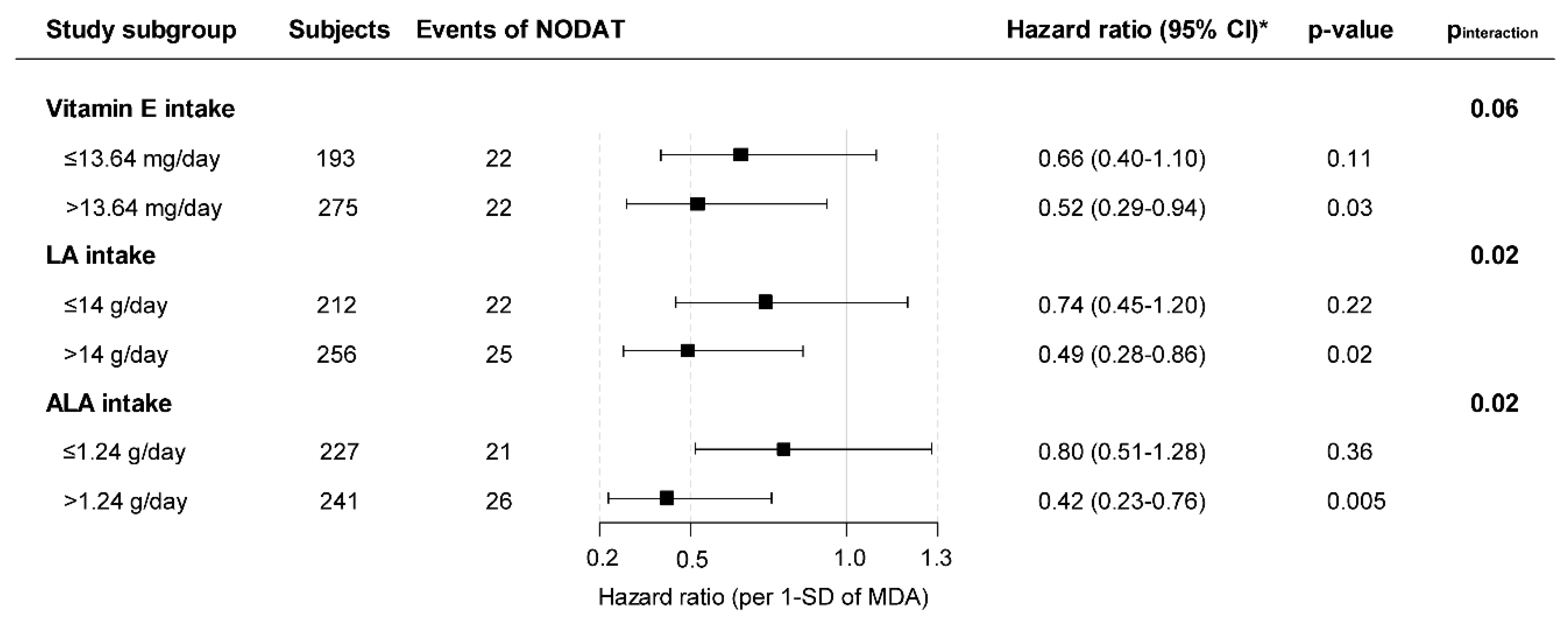
3.3. Secondary Analysis on MDA and NODAT
3.4. Prospective Analysis on All-Cause Mortality, Cardiovascular Mortality, and Graft Failure
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Montori, V.M.; Basu, A.; Erwin, P.J.; Velosa, J.A.; Gabriel, S.E.; Kudva, Y.C. Posttransplantation diabetes: A systematic review of the literature. Diabetes Care 2002, 25, 583–592. [Google Scholar] [CrossRef]
- Rodrigo, E.; Fernandez-Fresnedo, G.; Valero, R.; Ruiz, J.C.; Pinera, C.; Palomar, R.; González-Cotorruelo, J.; Gómez-Alamillo, C.; Arias, M. New-Onset Diabetes after Kidney Transplantation: Risk Factors. J. Am. Soc. Nephrol. 2006, 17, S291–S295. [Google Scholar] [CrossRef]
- Kaposztas, Z.; Gyurus, E.; Kahan, B.D. New-onset diabetes after renal transplantation: Diagnosis; incidence; risk factors; impact on outcomes; and novel implications. Transplant. Proc. 2011, 43, 1375–1394. [Google Scholar] [CrossRef]
- Woodward, R.S.; Schnitzler, M.A.; Baty, J.; Lowell, J.A.; Lopez-Rocafort, L.; Haider, S.; Woodworth, T.G.; Brennan, D.C. Incidence and cost of new onset diabetes mellitus among U.S. wait-listed and transplanted renal allograft recipients. Am. J. Transplant. 2003, 3, 590–598. [Google Scholar] [CrossRef]
- Cosio, F.G.; Pesavento, T.E.; Osei, K.; Henry, M.L.; Ferguson, R.M. Post-transplant diabetes mellitus: Increasing incidence in renal allograft recipients transplanted in recent years. Kidney Int. 2001, 59, 732–737. [Google Scholar] [CrossRef]
- Pérez Fernandez, R.; Martín Mateo, M.C.; De Vega, L.; Bustamante Bustamante, J.; Herrero, M.; Bustamante Munguira, E. Antioxidant enzyme determination and a study of lipid peroxidation in renal transplantation. Ren. Fail 2002, 24, 353–359. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples, Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Noberasco, G.; Odetti, P.; Boeri, D.; Maiello, M.; Adezati, L. Malondialdehyde (MDA) level in diabetic subjects. Relationship with blood glucose and glycosylated hemoglobin. Biomed. Pharmacother. 1991, 45, 193–196. [Google Scholar] [CrossRef]
- Zavar-Reza, J.; Shahmoradi, H.; Mohammadyari, A.; Mohammadbeigi, M.; Hosseini, R.; Vakili, M.; Barabadi, T.; DaneshPouya, F.; Tajik-kord, M.; Shahmoradi, E. Evaluation of Malondialdehyde (MDA) in type 2 diabetic Patients with Coronary Artery Disease (CAD). J. Biol. Today’s World 2014, 3, 129–132. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, S.; Jiang, X.; Wang, Y.H.; Li, F.; Wang, Y.G.; Zheng, Y.; Cai, L. The role of the Nrf2/Keap1 pathway in obesity and metabolic syndrome. Rev. Endocr. Metab. Disord. 2015, 16, 35–45. [Google Scholar] [CrossRef]
- Sottero, B.; Gargiulo, S.; Russo, I.; Barale, C.; Poli, G.; Cavalot, F. Postprandial Dysmetabolism and Oxidative Stress in Type 2 Diabetes: Pathogenetic Mechanisms and Therapeutic Strategies. Med. Res. Rev. 2015, 35, 968–1031. [Google Scholar] [CrossRef] [PubMed]
- Schoenmakers, E.; Agostini, M.; Mitchell, C.; Schoenmakers, N.; Papp, L.; Rajanayagam, O.; Padidela, R.; Ceron-Gutierrez, L.; Doffinger, R.; Prevosto, C.; et al. Mutations in the selenocysteine insertion sequence-binding protein 2 gene lead to a multisystem selenoprotein deficiency disorder in humans. J. Clin. Investig. 2010, 120, 4220–4235. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, X.; Yin, Y.; Zheng, J.T.; Jiang, C.F.; Wang, J.; Shen, H.; Li, C.Y.; Wang, M.; Liu, L.Z.; et al. Insulin Regulates Glucose Consumption and Lactate Production through Reactive Oxygen Species and Pyruvate Kinase M2. Oxid. Med. Cell. Longev. 2014, 2014, 504953. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.D. Type 2 diabetes as a redox disease. Lancet 2014, 383, 841–843. [Google Scholar] [CrossRef]
- Meigs, J.B.; Larson, M.G.; Fox, C.S.; Keaney, J.F.; Vasan, R.S.; Benjamin, E.J. Association of Oxidative Stress, Insulin Resistance, and Diabetes Risk Phenotypes: the Framingham Offspring Study. Diabetes Care 2007, 30, 2529–2535. [Google Scholar] [CrossRef] [PubMed]
- Montonen, J.; Knekt, P.; Järvinen, R.; Reunanen, A. Dietary Antioxidant Intake and Risk of Type 2 Diabetes. Diabetes Care 2004, 27, 362–366. [Google Scholar] [CrossRef]
- Osté, M.C.J.; Corpeleijn, E.; Navis, G.J.; Keyzer, C.A.; Soedamah-Muthu, S.S.; van den Berg, E.; Postmus, D.; de Borst, M.H.; Kromhout, D.; Bakker, S.J.L. Mediterranean style diet is associated with low risk of new-onset diabetes after renal transplantation. BMJ Open Diabetes Res. Care 2017, 5, e000283. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO Clinical Practice Guideline for the Care of Kidney Transplant Recipients. Am. J. Transplant. 2009, 9, S1–S155. [Google Scholar] [CrossRef]
- van den Berg, E.; Engberink, M.F.; Brink, E.J.; van Baak, M.A.; Gans, R.O.; Navis, G.; Bakker, S.J. Dietary protein, blood pressure and renal function in renal transplant recipients. Br. J. Nutr. 2013, 109, 1463–1470. [Google Scholar] [CrossRef]
- van den Berg, E.; Geleijnse, J.M.; Brink, E.J.; van Baak, M.A.; Homan van der Heide, J.J.; Gans, R.O.; Navis, G.; Bakker, S.J. Sodium intake and blood pressure in renal transplant recipients. Nephrol. Dial. Transplant. 2012, 27, 3352–3359. [Google Scholar] [CrossRef]
- Wendel-Vos, G.C.W.; Schuit, A.J.; Saris, W.H.; Kromhout, D. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J. Clin. Epidemiol. 2003, 56, 1163–1169. [Google Scholar] [CrossRef]
- Feunekes, G.I.; Van Staveren, W.A.; De Vries, J.H.; Burema, J.; Hautvast, J.G. Relative and biomarker-based validity of a food-frequency questionnaire estimating intake of fats and cholesterol. Am. J. Clin. Nutr. 1993, 58, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Stichting, N. Nederlands Voedingsstoffen Bestand, NEVO Tabel 2006; Dutch Nutrient Database; Voorlichtingsbureau voor de voiding: The Hague, The Netherlands, 2006. [Google Scholar]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S. [Google Scholar] [CrossRef]
- De Vries, A.P.; Bakker, S.J.; Van Son, W.J.; Van Der Heide, J.J.; Ploeg, R.J.; The, H.T.; de Jong, P.E.; Gans, R.O. Metabolic Syndrome Is Associated with Impaired Long-term Renal Allograft Function; Not All Component criteria Contribute Equally. Am. J. Transplant. 2004, 4, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Esatbeyoglu, T.; Bayram, B.; Rimbach, G. A fast and validated method for the determination of malondialdehyde in fish liver using high-performance liquid chromatography with a photodiode array detector. J. Food Sci. 2014, 79, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.; Wilkinson, A.; Dantal, J.; Dotta, F.; Haller, H.; Hernandez, D.; Kasiske, B.L.; Kiberd, B.; Krentz, A.; Legendre, C.; et al. New-onset diabetes after transplantation: 2003 international concensus guidelines. Transplantation 2003, 75, S3–S24. [Google Scholar] [CrossRef] [PubMed]
- Sharif, A.; Hecking, M.; De Vries, A.P.J.; Porrini, E.; Hornum, M.; Rasoul-Rockenschaub, S.; Berlakovich, G.; Krebs, M.; Kautzky-Willer, A.; Schernthaner, G.; et al. Proceedings From an International Consensus Meeting on Posttransplantation Diabetes Mellitus, Recommendations and Future Directions. Am. J. Transpl. 2014, 14, 1992–2000. [Google Scholar] [CrossRef]
- Zelle, D.M.; Corpeleijn, E.; Stolk, R.P.; de Greef, M.H.; Gans, R.O.; van der Heide, J.J.; Navis, G.; Bakker, S.J. Low Physical Activity and Risk of Cardiovascular and All-Cause Mortality in Renal Transplant Recipients. Clin. J. Am. Soc. Nephrol. 2011, 6, 898–905. [Google Scholar] [CrossRef]
- Selvin, S. Statistical Analysis of Epidemiologic Data, 3rd ed.; Oxford University Press: New York, NY, USA, 2004. [Google Scholar]
- Ristow, M.; Zarse, K.; Oberbach, A.; Klöting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Blüher, M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8665–8670. [Google Scholar] [CrossRef]
- Rodrigo, R.; Prieto, J.C.; Castillo, R. Cardioprotection against ischaemia/reperfusion by vitamins C and E plus n-3 fatty acids: molecular mechanisms and potential clinical applications. Clin. Sci. (Lond) 2013, 124, 1–15. [Google Scholar] [CrossRef]
- Ristow, M.; Schmeisser, S. Extending life span by increasing oxidative stress. Free Radic. Biol. Med. 2011, 51, 327–336. [Google Scholar] [CrossRef]
- Blackhall, M.L.; Coombes, J.S.; Fassett, R. The Relationship between Antioxidant Supplements and Oxidative Stress in Renal Transplant Recipients: A Review. ASAIO J. 2004, 50, 451–457. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M. Factors influencing fatty acids in meat and the role of antioxidants in improving meat quality. Br J. Nutr 1997, 78, S49–S60. [Google Scholar] [CrossRef]
- Risérus, U.; Willett, W.C.; Hu, F.B. Dietary fats and prevention of type 2 diabetes. Prog. Lipid Res. 2009, 48, 44–51. [Google Scholar] [CrossRef]
- Sotomayor, C.G.; Cortés, I.A.; Gormaz, J.G.; Vera, S.; Libuy, M.; Valls, N.; Rodrigo, R. Role of Oxidative Stress in Renal TransplantationBases for an n-3 PUFA Strategy Against Delayed Graft Function. Curr. Med. Chem. 2017, 24, 1469–1485. [Google Scholar] [CrossRef]
- Rodrigo, R.; Korantzopoulos, P.; Cereceda, M.; Asenjo, R.; Zamorano, J.; Villalabeitia, E.; Baeza, C.; Aguayo, R.; Castillo, R.; Carrasco, R.; et al. A Randomized Controlled Trial to Prevent Post-Operative Atrial Fibrillation by Antioxidant Reinforcement. J. Am. Coll. Cardiol. 2013, 62, 1457–1465. [Google Scholar] [CrossRef]
- Koenig, W.; Sund, M.; Frohlich, M.; Lowel, H.; Hutchinson, W.L.; Pepys, M.B. Refinement of the Association of Serum C-Reactive Protein Concentration and Coronary Heart Disease Risk by Correction for within-Subject Variation Over Time: The MONICA Augsburg Studies, 1984 and 1987. Am. J. Epidemiol. 2003, 158, 357–364. [Google Scholar] [CrossRef]
- Danesh, J.; Wheeler, J.G.; Hirschfield, G.M.; Eda, S.; Eiriksdottir, G.; Rumley, A.; Lowe, G.D.; Pepys, M.B.; Gudnason, V. C-Reactive Protein and Other Circulating Markers of Inflammation in the Prediction of Coronary Heart Disease. N. Engl. J. Med. 2004, 350, 1387–1397. [Google Scholar] [CrossRef]
| Baseline Characteristics | Plasma MDA, Ln | ||||
|---|---|---|---|---|---|
| Linear Regression ¥ | Adjusted Linear Regression † | Backwards Linear Regression § | |||
| Std. β | Std. β | Std. β | |||
| Plasma MDA, µmol/L | 2.55 (1.92–3.66) | – | – | – | |
| Demographic and anthropometric | |||||
| Age, years | 52 ± 13 | 0.01 | 0.02 | ||
| Male sex, n (%) | 292 (57) | −0.06 * | −0.07 * | ~ | |
| Weight, kg | 79.0 ± 15.4 | −0.04 | −0.01 | ||
| Height, cm | 174 ± 10 | −0.04 | 0.02 | ||
| BMI, kg/m2 | 26.0 ± 4.4 | −0.03 | −0.02 | ||
| Waist, cm a | 96.4 ± 13.7 | −0.07 * | −0.05 | ||
| Glucose and lipids metabolism | |||||
| Glucose, mmol/L(mg/dL) b | 5.16 (93) ± 0.64 (11) | 0.10 ** | 0.11 ** | 0.12 ** | |
| HbA1c, % c | 5.67 ± 0.36 | 0.05 | 0.05 | ||
| Impaired fasting glucose, n (%) | 122 (24) | 0.06 * | 0.07 * | ~ | |
| Total cholesterol, mmol/L | 5.12 ± 1.11 | 0.05 | 0.05 | ||
| HDL cholesterol, mmol/L d | 1.3 (1.1–1.7) | 0.09 ** | 0.06 * | 0.09 ** | |
| LDL cholesterol, mmol/L d | 3.0 ± 0.9 | −0.04 | −0.04 | ||
| Triglycerides, mmol/L e | 1.62 (1.21–2.16) | 0.03 | 0.05 | ||
| Transplantation-related data | |||||
| Time after transplant, years | 5.2 (2.0–12.2) | 0.03 | 0.02 | ||
| Living donor, n (%) | 187 (36) | 0.07 * | 0.07 * | ~ | |
| Pre-emptive, n (%) | 92 (18) | 0.03 | 0.02 | ||
| Immunosuppressive therapy | |||||
| Acute rejection treatment, n (%) | 124 (24) | 0.06 * | 0.08 * | ~ | |
| Use of calcineurin inhibitors | |||||
| Tacrolimus, n (%) | 89 (17) | −0.01 | 0.02 | ||
| Cyclosporine, n (%) | 194 (38) | −0.02 | −0.01 | ||
| Use of proliferation inhibitors | |||||
| Azathriopine, n (%) | 95 (18) | 0.01 | 0.01 | ||
| Mycophenolic acid, n (%) | 340 (66) | 0.03 | 0.03 | ||
| Prednisolone cumulative dose, g | 16.9 (5.8–36.3) | 0.02 | 0.02 | ||
| Cardiovascular history | |||||
| History of CV disease, n (%) f | 204 (40) | 0.01 | 0.01 | ||
| SBP, mmHg e | 135 ± 17 | −0.03 | −0.01 | ||
| DBP, mmHg e | 83 ± 11 | 0.05 | 0.08 * | ~ | |
| Use of antihypertensive medication, n (%) | 448 (87) | −0.05 | −0.02 | ||
| Graft function and inflammation | |||||
| Serum creatinine, µmol/L d | 123 (100–159) | −0.07 * | 0.12 * | ~ | |
| eGFR (CKD-EPI), mL/mind d | 53 ± 20 | 0.10 ** | 0.10 ** | ~ | |
| Protein excretion, g/day | 0.18 (0.02–0.32) | 0.01 | 0.04 | ||
| hs-CRP, mg/L g | 1.4 (0.6–3.8) | <0.01 | <0.01 | ||
| Leucocytes, × 109/L e | 7.8 (6.3–9.6) | 0.10 ** | 0.09 ** | 0.12 ** | |
| Nutrition | |||||
| Plasma albumin, g/L d | 43.3 ± 3.0 | 0.002 | −0.003 | ||
| Kcal intake, kcal/day h | 2189 ± 617 | −0.002 | 0.01 | ||
| Fatty acids intake h | |||||
| n-6 LA, g/day ∧ | 15 (13–19) | 0.03 | 0.05 | ||
| n-6 AA, g/day ∧ | 0.05 (0.04–0.06) | 0.02 | 0.02 | ||
| n-3 ALA, g/day ∧ | 1.25 (1.02–1.60) | 0.01 | 0.03 | ||
| n-3 EPA, g/day ∧ | 0.04 (0.01–0.09) | 0.05 | 0.05 | ||
| n-3 DHA, g/day ∧ | 0.06 (0.03–0.13) | 0.06 | 0.06 | ||
| Lifestyle | |||||
| Current smokers, n (%) i | 67 (13) | −0.01 | 0.002 | ||
| Alcohol intake, g/day h | 2.92 (0.04–11.52) | −0.08 * | −0.08 * | ~ | |
| SQUASH-score, intensity × hours | 5555 (2640–8513) | −0.02 | <0.01 | ||
| NODAT | HR (95% CI) Per 1-SD | p |
|---|---|---|
| Crude model | 0.61 (0.41–0.92) | 0.02 |
| Model 1 | 0.63 (0.42–0.94) | 0.02 |
| Model 2 | 0.54 (0.36–0.83) | <0.01 |
| Model 3 | 0.54 (0.35–0.82) | <0.01 |
| Model 4 | 0.56 (0.37–0.85) | <0.01 |
| Model 5 | 0.55 (0.36–0.83) | <0.01 |
| Model 6 | 0.55 (0.36–0.83) | <0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yepes-Calderón, M.; Sotomayor, C.G.; Gomes-Neto, A.W.; Gans, R.O.B.; Berger, S.P.; Rimbach, G.; Esatbeyoglu, T.; Rodrigo, R.; Geleijnse, J.M.; Navis, G.J.; et al. Plasma Malondialdehyde and Risk of New-Onset Diabetes after Transplantation in Renal Transplant Recipients: A Prospective Cohort Study. J. Clin. Med. 2019, 8, 453. https://doi.org/10.3390/jcm8040453
Yepes-Calderón M, Sotomayor CG, Gomes-Neto AW, Gans ROB, Berger SP, Rimbach G, Esatbeyoglu T, Rodrigo R, Geleijnse JM, Navis GJ, et al. Plasma Malondialdehyde and Risk of New-Onset Diabetes after Transplantation in Renal Transplant Recipients: A Prospective Cohort Study. Journal of Clinical Medicine. 2019; 8(4):453. https://doi.org/10.3390/jcm8040453
Chicago/Turabian StyleYepes-Calderón, Manuela, Camilo G. Sotomayor, António W. Gomes-Neto, Rijk O.B. Gans, Stefan P. Berger, Gerald Rimbach, Tuba Esatbeyoglu, Ramón Rodrigo, Johanna M. Geleijnse, Gerjan J. Navis, and et al. 2019. "Plasma Malondialdehyde and Risk of New-Onset Diabetes after Transplantation in Renal Transplant Recipients: A Prospective Cohort Study" Journal of Clinical Medicine 8, no. 4: 453. https://doi.org/10.3390/jcm8040453
APA StyleYepes-Calderón, M., Sotomayor, C. G., Gomes-Neto, A. W., Gans, R. O. B., Berger, S. P., Rimbach, G., Esatbeyoglu, T., Rodrigo, R., Geleijnse, J. M., Navis, G. J., & Bakker, S. J. L. (2019). Plasma Malondialdehyde and Risk of New-Onset Diabetes after Transplantation in Renal Transplant Recipients: A Prospective Cohort Study. Journal of Clinical Medicine, 8(4), 453. https://doi.org/10.3390/jcm8040453








