Effects of Repetitive Transcranial Magnetic Stimulation over Prefrontal Cortex on Attention in Psychiatric Disorders: A Systematic Review
Abstract
1. Introduction
2. Transcranial Magnetic Stimulation
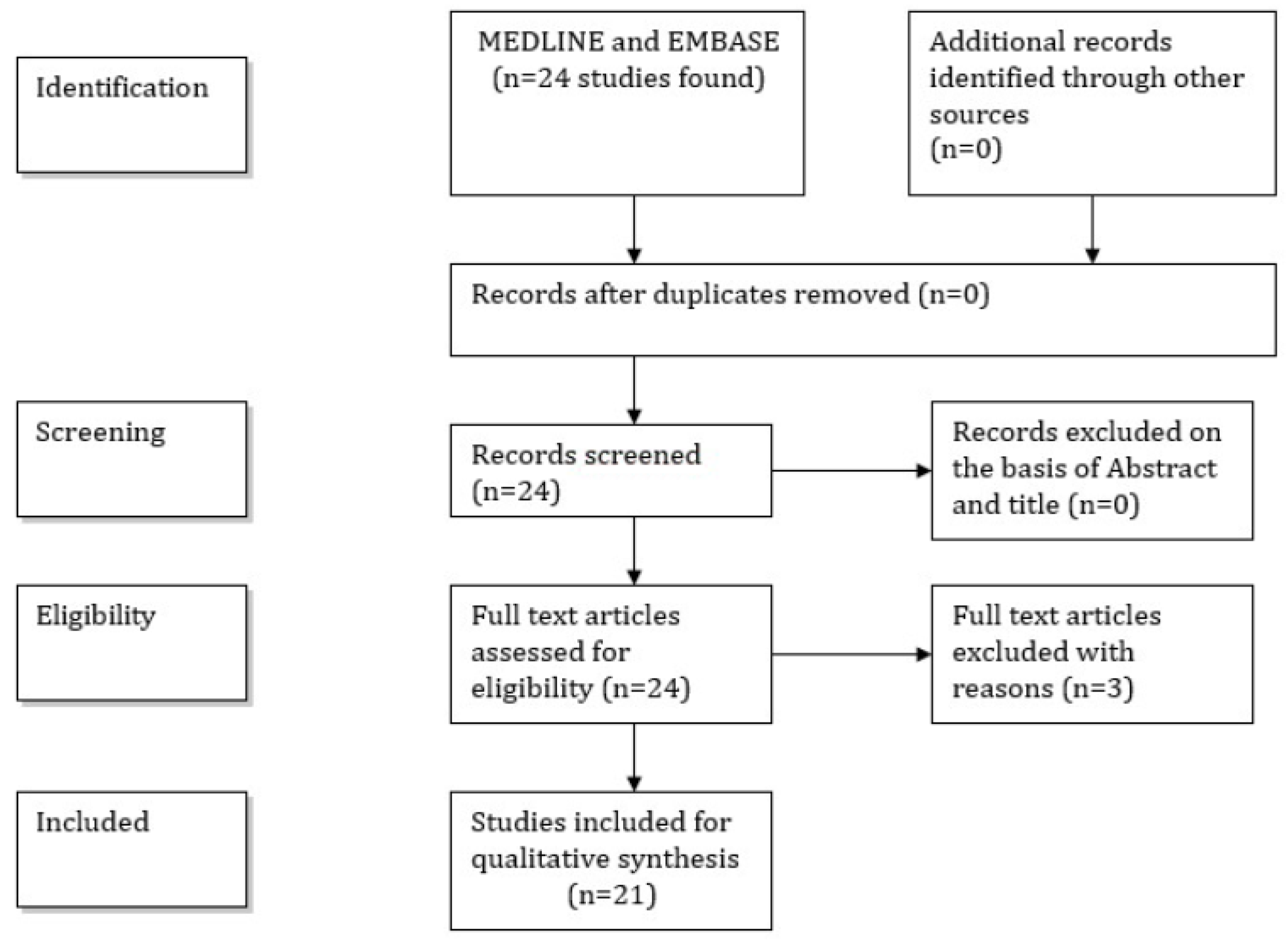
3. Material and Methods
4. Results
Healthy Individuals
5. Results
5.1. Depression
5.2. Schizophrenia
5.3. Autism Spectrum Disorder
5.4. Attention Deficit Hyperactivity Disorder
5.5. Addiction
5.6. Alzheimer’s Disease
6. Discussion
7. Conclusions
Author Contributions
Conflicts of Interest
References
- Treisman, A.M.; Gelade, G. A feature-integration theory of attention. Cogn. Psychol. 1980, 12, 97–136. [Google Scholar] [CrossRef]
- Treisman, A. Feature binding, attention and object perception. Philos. Trans. R Soc. Lond. B Biol. Sci. 1998, 353, 1295–1306. [Google Scholar] [CrossRef]
- Iimori, T.; Nakajima, S.; Miyazaki, T.; Tarumi, R.; Ogyu, K.; Wada, M.; Tsugawa, S.; Masuda, F.; Daskalakis, Z.J.; Blumberger, D.M.; et al. Effectiveness of the prefrontal repetitive transcranial magnetic stimulation on cognitive profiles in depression, schizophrenia, and Alzheimer’s disease: A systematic review. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 88, 31–40. [Google Scholar] [CrossRef]
- Pessoa, L.; Kastner, S.; Ungerleider, L.G. Neuroimaging studies of attention: From modulation of sensory processing to top-down control. J. Neurosci. 2003, 23, 3990–3998. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; McCandliss, B.D.; Fossella, J.; Flombaum, J.I.; Posner, M.I. The activation of attentional networks. Neuroimage 2005, 26, 471–479. [Google Scholar] [CrossRef]
- Hartlage, S.; Alloy, L.B.; Vazquez, C.; Dykman, B. Automatic and effortful processing in depression. Psychol. Bull. 1993, 113, 247–278. [Google Scholar] [CrossRef]
- Ochoa, E.L.; Lasalde-Dominicci, J. Cognitive deficits in schizophrenia: Focus on neuronal nicotinic acetylcholine receptors and smoking. Cell. Mol. Neurobiol. 2007, 27, 609–639. [Google Scholar] [CrossRef]
- Woods, S.P.; Lovejoy, D.W.; Ball, J.D. Neuropsychological characteristics of adults with ADHD: A comprehensive review of initial studies. Clin. Neuropsychol. 2002, 16, 12–34. [Google Scholar] [CrossRef] [PubMed]
- Tucha, O.; Mecklinger, L.; Laufkotter, R.; Klein, H.E.; Walitza, S.; Lange, K.W. Methylphenidate-induced improvements of various measures of attention in adults with attention deficit hyperactivity disorder. J. Neural. Transm. (Vienna) 2006, 113, 1575–1592. [Google Scholar] [CrossRef]
- Tucha, L.; Tucha, O.; Walitza, S.; Sontag, T.A.; Laufkotter, R.; Linder, M.; Lange, K.W. Vigilance and sustained attention in children and adults with ADHD. J. Atten. Disord. 2009, 12, 410–421. [Google Scholar] [CrossRef]
- Burack, J.A. Selective attention deficits in persons with autism: Preliminary evidence of an inefficient attentional lens. J. Abnorm. Psychol. 1994, 103, 535–543. [Google Scholar] [CrossRef]
- Belmonte, M.K.; Yurgelun-Todd, D.A. Anatomic dissociation of selective and suppressive processes in visual attention. Neuroimage 2003, 19, 180–189. [Google Scholar] [CrossRef]
- Belmonte, M.K.; Yurgelun-Todd, D.A. Functional anatomy of impaired selective attention and compensatory processing in autism. Cogn. Brain Res. 2003, 17, 651–664. [Google Scholar] [CrossRef]
- Alvarez, J.A.; Emory, E. Executive function and the frontal lobes: A meta-analytic review. Neuropsychol. Rev. 2006, 16, 17–42. [Google Scholar] [CrossRef]
- Johnson, J.A.; Strafella, A.P.; Zatorre, R.J. The role of the dorsolateral prefrontal cortex in bimodal divided attention: Two transcranial magnetic stimulation studies. J. Cogn. Neurosci. 2007, 19, 907–920. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, P.B.; Fountain, S.; Daskalakis, Z.J. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin. Neurophysiol. 2006, 117, 2584–2596. [Google Scholar] [CrossRef]
- Cotelli, M.; Calabria, M.; Manenti, R.; Rosini, S.; Zanetti, O.; Cappa, S.F.; Miniussi, C. Improved language performance in Alzheimer disease following brain stimulation. J. Neurol. Neurosurg. Psychiatry 2011, 82, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Cotelli, M.; Manenti, R.; Cappa, S.F.; Geroldi, C.; Zanetti, O.; Rossini, P.M.; Miniussi, C. Effect of transcranial magnetic stimulation on action naming in patients with Alzheimer disease. Arch. Neurol. 2006, 63, 1602–1604. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Darwish, E.S.; Khedr, E.M.; El Serogy, Y.M.; Ali, A.M. Effects of low versus high frequencies of repetitive transcranial magnetic stimulation on cognitive function and cortical excitability in Alzheimer’s dementia. J. Neurol. 2012, 259, 83–92. [Google Scholar] [CrossRef]
- Devi, G.; Voss, H.U.; Levine, D.; Abrassart, D.; Heier, L.; Halper, J.; Martin, L.; Lowe, S. Open-label, short-term, repetitive transcranial magnetic stimulation in patients with Alzheimer’s disease with functional imaging correlates and literature review. Am. J. Alzheimers Dis. Other Dement. 2014, 29, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Padala, P.R.; Padala, K.P.; Lensing, S.Y.; Jackson, A.N.; Hunter, C.R.; Parkes, C.M.; Dennis, R.A.; Bopp, M.M.; Caceda, R.; Mennemeier, M.S.; et al. Repetitive transcranial magnetic stimulation for apathy in mild cognitive impairment: A double-blind, randomized, sham-controlled, cross-over pilot study. Psychiatry Res. 2018, 261, 312–318. [Google Scholar] [CrossRef]
- Pascual-Leone, A.; Tormos, J.M.; Keenan, J.; Tarazona, F.; Canete, C.; Catala, M.D. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J. Clin. Neurophysiol. 1998, 15, 333–343. [Google Scholar] [CrossRef]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009, 120, 2008–2039. [Google Scholar] [CrossRef]
- Lefaucheur, J.P.; Andre-Obadia, N.; Antal, A.; Ayache, S.S.; Baeken, C.; Benninger, D.H.; Cantello, R.M.; Cincotta, M.; de Carvalho, M.; De Ridder, D.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin. Neurophysiol. 2014, 125, 2150–2206. [Google Scholar] [CrossRef]
- Zangen, A.; Roth, Y.; Voller, B.; Hallett, M. Transcranial magnetic stimulation of deep brain regions: Evidence for efficacy of the H-coil. Clin. Neurophysiol. 2005, 116, 775–779. [Google Scholar] [CrossRef]
- Wagner, M.; Rihs, T.A.; Mosimann, U.P.; Fisch, H.U.; Schlaepfer, T.E. Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex affects divided attention immediately after cessation of stimulation. J. Psychiatr. Res. 2006, 40, 315–321. [Google Scholar] [CrossRef]
- Bersani, F.S.; Minichino, A.; Enticott, P.G.; Mazzarini, L.; Khan, N.; Antonacci, G.; Raccah, R.N.; Salviati, M.; Delle Chiaie, R.; Bersani, G.; et al. Deep transcranial magnetic stimulation as a treatment for psychiatric disorders: A comprehensive review. Eur. Psychiatry 2013, 28, 30–39. [Google Scholar] [CrossRef]
- Coulthard, E.; Singh-Curry, V.; Husain, M. Treatment of attention deficits in neurological disorders. Curr. Opin. Neurol. 2006, 19, 613–618. [Google Scholar] [CrossRef]
- Sharma, K.; Davis, T.; Coulthard, E. Enhancing attention in neurodegenerative diseases: Current therapies and future directions. Transl. Neurosci. 2016, 7, 98–109. [Google Scholar] [CrossRef]
- Nebel, K.; Wiese, H.; Stude, P.; de Greiff, A.; Diener, H.C.; Keidel, M. On the neural basis of focused and divided attention. Cogn. Brain Res. 2005, 25, 760–776. [Google Scholar] [CrossRef]
- Blanchet, S.; Gagnon, G.; Schneider, C. The contribution of the dorsolateral prefrontal cortex in full and divided encoding: A paired-pulse transcranial magnetic stimulation study. Behav. Neurol. 2010, 23, 107–115. [Google Scholar] [CrossRef]
- Sabatino, M.; Di Nuovo, S.; Sardo, P.; Abbate, C.S.; La Grutta, V. Neuropsychology of selective attention and magnetic cortical stimulation. Int. J. Psychophysiol. 1996, 21, 83–89. [Google Scholar] [CrossRef]
- Rounis, E.; Stephan, K.E.; Lee, L.; Siebner, H.R.; Pesenti, A.; Friston, K.J.; Rothwell, J.C.; Frackowiak, R.S. Acute changes in frontoparietal activity after repetitive transcranial magnetic stimulation over the dorsolateral prefrontal cortex in a cued reaction time task. J. Neurosci. 2006, 26, 9629–9638. [Google Scholar] [CrossRef]
- Vohn, R.; Fimm, B.; Weber, J.; Schnitker, R.; Thron, A.; Spijkers, W.; Willmes, K.; Sturm, W. Management of attentional resources in within-modal and cross-modal divided attention tasks: An fMRI study. Hum. Brain Mapp. 2007, 28, 1267–1275. [Google Scholar] [CrossRef]
- Vanderhasselt, M.A.; De Raedt, R.; Baeken, C.; Leyman, L.; Clerinx, P.; D’Haenen, H. The influence of rTMS over the right dorsolateral prefrontal cortex on top-down attentional processes. Brain Res. 2007, 1137, 111–116. [Google Scholar] [CrossRef]
- Zanto, T.P.; Rubens, M.T.; Thangavel, A.; Gazzaley, A. Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nat. Neurosci. 2011, 14, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Han, H.J.; Ahn, H.M.; Kim, S.A.; Kim, S.E. Effects of five daily high-frequency rTMS on Stroop task performance in aging individuals. Neurosci. Res. 2012, 74, 256–260. [Google Scholar] [CrossRef]
- Hoppner, J.; Schulz, M.; Irmisch, G.; Mau, R.; Schlafke, D.; Richter, J. Antidepressant efficacy of two different rTMS procedures. High frequency over left versus low frequency over right prefrontal cortex compared with sham stimulation. Eur. Arch. Psychiatry Clin. Neurosci. 2003, 253, 103–109. [Google Scholar] [CrossRef]
- Januel, D.; Dumortier, G.; Verdon, C.M.; Stamatiadis, L.; Saba, G.; Cabaret, W.; Benadhira, R.; Rocamora, J.F.; Braha, S.; Kalalou, K.; et al. A double-blind sham controlled study of right prefrontal repetitive transcranial magnetic stimulation (rTMS): Therapeutic and cognitive effect in medication free unipolar depression during 4 weeks. Prog. Neuropsychopharmacol. Biol. Psychiatry 2006, 30, 126–130. [Google Scholar] [CrossRef]
- Speer, A.M.; Repella, J.D.; Figueras, S.; Demian, N.K.; Kimbrell, T.A.; Wasserman, E.M.; Post, R.M. Lack of adverse cognitive effects of 1 Hz and 20 Hz repetitive transcranial magnetic stimulation at 100% of motor threshold over left prefrontal cortex in depression. J. ECT 2001, 17, 259–263. [Google Scholar] [CrossRef]
- Vanderhasselt, M.A.; De Raedt, R.; Leyman, L.; Baeken, C. Acute effects of repetitive transcranial magnetic stimulation on attentional control are related to antidepressant outcomes. J. Psychiatry Neurosci. 2009, 34, 119–126. [Google Scholar]
- Vanderhasselt, M.A.; De Raedt, R.; Baeken, C.; Leyman, L.; D’Haenen, H. A single session of rTMS over the left dorsolateral prefrontal cortex influences attentional control in depressed patients. World J. Biol. Psychiatry 2009, 10, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Mittrach, M.; Thunker, J.; Winterer, G.; Agelink, M.W.; Regenbrecht, G.; Arends, M.; Mobascher, A.; Kim, S.J.; Wolwer, W.; Brinkmeyer, J.; et al. The tolerability of rTMS treatment in schizophrenia with respect to cognitive function. Pharmacopsychiatry 2010, 43, 110–117. [Google Scholar] [CrossRef]
- Ullrich, H.; Kranaster, L.; Sigges, E.; Andrich, J.; Sartorius, A. Ultra-high-frequency left prefrontal transcranial magnetic stimulation as augmentation in severely ill patients with depression: A naturalistic sham-controlled, double-blind, randomized trial. Neuropsychobiology 2012, 66, 141–148. [Google Scholar] [CrossRef]
- Levkovitz, Y.; Harel, E.V.; Roth, Y.; Braw, Y.; Most, D.; Katz, L.N.; Sheer, A.; Gersner, R.; Zangen, A. Deep transcranial magnetic stimulation over the prefrontal cortex: Evaluation of antidepressant and cognitive effects in depressive patients. Brain Stimul. 2009, 2, 188–200. [Google Scholar] [CrossRef]
- Martin, D.M.; McClintock, S.M.; Forster, J.J.; Lo, T.Y.; Loo, C.K. Cognitive enhancing effects of rTMS administered to the prefrontal cortex in patients with depression: A systematic review and meta-analysis of individual task effects. Depress Anxiety 2017, 34, 1029–1039. [Google Scholar] [CrossRef]
- Kavanaugh, B.C.; Aaronson, S.T.; Clarke, G.N.; Holtzheimer, P.E.; Johnson, C.W.; McDonald, W.M.; Schneider, M.B.; Carpenter, L.L. Neurocognitive effects of repetitive transcranial magnetic stimulation with a 2-coil device in treatment-resistant major depressive disorder. J. ECT 2018, 34, 258–265. [Google Scholar] [CrossRef]
- Naim-Feil, J.; Bradshaw, J.L.; Sheppard, D.M.; Rosenberg, O.; Levkovitz, Y.; Dannon, P.; Fitzgerald, P.B.; Isserles, M.; Zangen, A. Neuromodulation of attentional control in major depression: A pilot deep TMS study. Neural Plast. 2016, 2016, 5760141. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.A.; Zatorre, R.J. Neural substrates for dividing and focusing attention between simultaneous auditory and visual events. Neuroimage 2006, 31, 1673–1681. [Google Scholar] [CrossRef]
- Wolwer, W.; Lowe, A.; Brinkmeyer, J.; Streit, M.; Habakuck, M.; Agelink, M.W.; Mobascher, A.; Gaebel, W.; Cordes, J. Repetitive transcranial magnetic stimulation (rTMS) improves facial affect recognition in schizophrenia. Brain Stimul. 2014, 7, 559–563. [Google Scholar] [CrossRef]
- Guse, B.; Falkai, P.; Gruber, O.; Whalley, H.; Gibson, L.; Hasan, A.; Obst, K.; Dechent, P.; McIntosh, A.; Suchan, B.; et al. The effect of long-term high frequency repetitive transcranial magnetic stimulation on working memory in schizophrenia and healthy controls--a randomized placebo-controlled, double-blind fMRI study. Behav. Brain Res. 2013, 237, 300–307. [Google Scholar] [CrossRef]
- Prikryl, R.; Ustohal, L.; Prikrylova Kucerova, H.; Kasparek, T.; Venclikova, S.; Vrzalova, M.; Ceskova, E. A detailed analysis of the effect of repetitive transcranial magnetic stimulation on negative symptoms of schizophrenia: A double-blind trial. Schizophr. Res. 2013, 149, 167–173. [Google Scholar] [CrossRef]
- Sokhadze, E.; Baruth, J.; Tasman, A.; Mansoor, M.; Ramaswamy, R.; Sears, L.; Mathai, G.; El-Baz, A.; Casanova, M.F. Low-frequency repetitive transcranial magnetic stimulation (rTMS) affects event-related potential measures of novelty processing in autism. Appl. Psychophysiol. Biofeedback 2010, 35, 147–161. [Google Scholar] [CrossRef]
- Casanova, M.F.; Baruth, J.M.; El-Baz, A.; Tasman, A.; Sears, L.; Sokhadze, E. Repetitive Transcranial Magnetic Stimulation (rTMS) Modulates event-related potential (ERP) indices of attention in autism. Transl. Neurosci. 2012, 3, 170–180. [Google Scholar] [CrossRef]
- Sokhadze, E.M.; Lamina, E.V.; Casanova, E.L.; Kelly, D.P.; Opris, I.; Tasman, A.; Casanova, M.F. Exploratory study of rTMS neuromodulation effects on electrocortical functional measures of performance in an oddball test and behavioral symptoms in autism. Front. Syst. Neurosci. 2018, 12, 20. [Google Scholar] [CrossRef]
- Bloch, Y.; Harel, E.V.; Aviram, S.; Govezensky, J.; Ratzoni, G.; Levkovitz, Y. Positive effects of repetitive transcranial magnetic stimulation on attention in ADHD Subjects: A randomized controlled pilot study. World J. Biol. Psychiatry 2010, 11, 755–758. [Google Scholar] [CrossRef]
- Paz, Y.; Friedwald, K.; Levkovitz, Y.; Zangen, A.; Alyagon, U.; Nitzan, U.; Segev, A.; Maoz, H.; Koubi, M.; Bloch, Y. Randomised sham-controlled study of high-frequency bilateral deep transcranial magnetic stimulation (dTMS) to treat adult attention hyperactive disorder (ADHD): Negative results. World J. Biol. Psychiatry 2018, 19, 561–566. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, X.; Liang, Q.; Li, X.; Yang, J.; Yuan, J. High-frequency repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex restores attention bias to negative information in methamphetamine addicts. Psychiatry Res. 2018, 265, 151–160. [Google Scholar] [CrossRef]
- Herremans, S.C.; Van Schuerbeek, P.; De Raedt, R.; Matthys, F.; Buyl, R.; De Mey, J.; Baeken, C. The impact of accelerated right prefrontal high-frequency repetitive transcranial magnetic stimulation (rTMS) on cue-reactivity: An fMRI study on craving in recently detoxified alcohol-dependent patients. PLoS ONE 2015, 10, e0136182. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, W.; Liu, X.; Xu, Q.; Tang, L.; Wu, S. Adjunctive treatment with high frequency repetitive transcranial magnetic stimulation for the behavioral and psychological symptoms of patients with Alzheimer’s disease: A randomized, double-blind, sham-controlled study. Shanghai Arch. Psychiatry 2015, 27, 280–288. [Google Scholar] [CrossRef]
- Marazziti, D.; Consoli, G.; Picchetti, M.; Carlini, M.; Faravelli, L. Cognitive impairment in major depression. Eur. J. Pharmacol. 2010, 626, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Lyketsos, C.G.; DelCampo, L.; Steinberg, M.; Miles, Q.; Steele, C.D.; Munro, C.; Baker, A.S.; Sheppard, J.M.; Frangakis, C.; Brandt, J.; et al. Treating depression in Alzheimer disease: Efficacy and safety of sertraline therapy, and the benefits of depression reduction: The DIADS. Arch. Gen. Psychiatry 2003, 60, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, G.; Gole, R.; Moussavi, Z. rTMS as a treatment of Alzheimer’s disease with and without comorbidity of depression: A review. Neurosci. J. 2013, 2013, 679389. [Google Scholar] [CrossRef]
- Stogmann, E.; Moser, D.; Klug, S.; Gleiss, A.; Auff, E.; Dal-Bianco, P.; Pusswald, G.; Lehrner, J. Activities of Daily Living and Depressive Symptoms in Patients with Subjective Cognitive Decline, Mild Cognitive Impairment, and Alzheimer’s Disease. J. Alzheimers Dis. 2016, 49, 1043–1050. [Google Scholar] [CrossRef]
- Early, T.S.; Posner, M.I.; Reiman, E.M.; Raichle, M.E. Hyperactivity of the left striato-pallidal projection. Part I: Lower level theory. Psychiatr. Dev. 1989, 7, 85–108. [Google Scholar] [PubMed]
- Fernandez-Duque, D.; Posner, M.I. Brain imaging of attentional networks in normal and pathological states. J. Clin. Exp. Neuropsychol. 2001, 23, 74–93. [Google Scholar] [CrossRef]
- Okonkwo, O.C.; Wadley, V.G.; Ball, K.; Vance, D.E.; Crowe, M. Dissociations in visual attention deficits among persons with mild cognitive impairment. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 2008, 15, 492–505. [Google Scholar] [CrossRef]
- Dannhauser, T.M.; Walker, Z.; Stevens, T.; Lee, L.; Seal, M.; Shergill, S.S. The functional anatomy of divided attention in amnestic mild cognitive impairment. Brain 2005, 128, 1418–1427. [Google Scholar] [CrossRef] [PubMed]
- Nakaaki, S.; Murata, Y.; Sato, J.; Shinagawa, Y.; Tatsumi, H.; Hirono, N.; Furukawa, T.A. Greater impairment of ability in the divided attention task is seen in Alzheimer’s disease patients with depression than in those without depression. Dement. Geriatr. Cogn. Disord. 2007, 23, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, P.; Wan, C.; Jin, Z.; Zhang, J.; Li, L. Evaluating the role of the dorsolateral prefrontal cortex and posterior parietal cortex in memory-guided attention with repetitive transcranial magnetic stimulation. Front. Hum. Neurosci. 2018, 12, 236. [Google Scholar] [CrossRef]
| Studies | No | Gender | Mean Age | Disease Duration | Education |
|---|---|---|---|---|---|
| M/F | (y) | (y) | (y) | ||
| Depression | |||||
| Speer et al., 2001 [28] | 18 | - | 45 ± 7 | - | - |
| Höppner et al., 2003 [29] | 30 | 8/22 | 56.4 ± 11.1 | - | - |
| Januel et al., 2006 [30] | 27 | 6/21 | 37.78 ± 11.27 | 77.77 ± 90.82 mo | - |
| Levkovitz et al., 2009 [31] | 23 H1 | 12/11 | 45.57 ± 13.34 | 13.96 ± 2.96 | - |
| 22 H2 | 11/11 | 45.77 ± 11.99 | 13.00 ± 2.12 | - | |
| 11 HIL 110% | 3/5 | 44.27 ± 11.36 | 15.45 ± 2.02 | - | |
| 8 HIL 120% | 10/10 | 49.88 ± 9.52 | 13.13 ± 2.81 | - | |
| Vanderhasselt et al., 2009a [32] | 16 | 6/10 | 42 ± 11.2 | ||
| Vanderhasselt et al., 2009b [33] | 15 | 6/9 | 45.6 ± 5.87 | - | - |
| Ullrich et al., 2012 [34] | Active 22 | 31.8/68.2% | 56.9/10.2% | - | - |
| Sham 21 | 42.9/57.1% | 54.1/7.8% | |||
| Naim-Feil et al., 2016 [35] | 21 | 10/11 | 44 ± 9 | 15 ± 3 | - |
| Kavanaugh et al., 2018 [36] | Active 43 | 10/31 | 45.84 ± 11.87 | 17.94 ± 3.7 | - |
| Sham 41 | 12/31 | 47.95 ± 12.78 | 15.59 ± 9.17 | ||
| Schizophrenia | |||||
| Mittrach et al., 2010 [37] | Active 18 | 14/4 | 34.5 ± 0.5 | 5.7 ± 5.2 | - |
| Sham 14 | 11/3 | 34.4 ± 10.5 | 5.6 ± 8.7 | ||
| Guse et al., 2013 [38] | Active 13 | 10/3 | 37 (22-58) | 15.5 | - |
| Sham 12 | 9/3 | 36 (20-51 | 12.6 | ||
| Prikryl et al., 2013 [39] | Active 23 | 23/0 | 31.6 ± 8.04 | 4.91 ± 5.09 y | 12.43 ± 2.06 y |
| Control 17 | 17/0 | 33.94 ± 9.98 | 5.89 ± 7.91 y | 12.44 ± 1.97 | |
| Wölwer et al., 2014 [40] | Active 18 | 14/4 | 34.3 ± 5.7 | 5.7 ± 5.2 | - |
| Sham 14 | 11/3 | 34.4 ± 5.6 | 5.6 ± 8.7 | ||
| Attention deficit hyperactivity disorder | |||||
| Bloch et al., 2010 [41] | 13 | 7/6 | - | - | - |
| Active 9 | 6/3 | 32 ± 11 | |||
| Paz. et al., 2017 [42] | Sham 13 | 8/5 | 30.85 ± 6.82 | - | - |
| Alzheimer disease | |||||
| Wu et al., 2015 [43] | Active 26 | 10/16 | 71.4 ± 4.9 | 5.1 ± 1.5 | 11.4 ± 2.7 y |
| Control 26 | 11/15 | 71.9 ± 4.8 | 5.1 ± 1.5 | 11.5 ± 2.1 y | |
| Autism | |||||
| Sokhadze et al., 2010 [44] | 13 | 12/1 | 15.6 ± 5.8 | - | - |
| Casanova et al., 2012 [45] | 45 | 39/6 | 13 ± 2.7 | - | - |
| Sokhadze et al., 2018 [46] | 112 | 93/19 | 13.1 ± 1.78 | - | - |
| Addiction | |||||
| Herremans et al, 2015 [47] | 26 | 17/9 | 45.2 ± 9.3 | - | - |
| Zang et al., 2018 [48] | 31 | 31/0 | 43 ± 9.15 | 13 ± 7.45 | - |
| Studies | Stimulation Parameters | Outcome Measures | Principal Findings | ||||
|---|---|---|---|---|---|---|---|
| Position | Intensitity | Frequency | Total Pulses Per Session | No. Sessions | |||
| Depression | |||||||
| Speer et al., 2001 [28] | L DLPFC | 100% MT | 20 Hz 1 Hz | 1600 | 10 | Continuous Performance Task | No significant changes |
| Hoeppner et al., 2003 [29] | L DLPFC R DLPFC | 80% MT | 20 Hz 1 Hz | ? | 10 | d2 Test | No significant changes |
| Januel et al., 2006 [30] | R DLPFC | 90% MT | 1 Hz | ? | 16 | Auditory and visual attention span | No significant differences |
| Levkovitz et al., 2009 [31] | H-Coil DLPFC | 120% MT | 20 Hz | 1689 | 20 | CANTAB, RVP | ↑ RVP performances |
| Vanderhasselt et al., 2009a [32] | L DLPFC | 110% MT | 10 Hz | 1560 | 10 | VAS Self-paced switching task | ↑ Attentional processes |
| Vanderhasselt et al., 2009b [33] | L DLPFC | 110% MT | 10 Hz | 1560 | 10 | Self-paced switching task | ↑ Attentional control |
| Ullrich et al.; 2012 [34] | L DLPFC | 110% MT | 30 Hz 1 Hz | 1800 990 | 15 | ZVT, SKT | ↑ Processing speed performance ↑ |
| Naim-Feil et al., 2016 [35] | H-Coil L > R DLPFC | 120% MT | 20 Hz | 1680 | 1 (n = 21) 20 (n = 13) | BDI, SART | ↓ Sustained attention deficits |
| Kavanaugh et al., 2018 [36] | 2-coil L > R DLPF | 120% MT | 10 Hz | 3000 | 20 | CDR System | ↑ Continuity and power of attention |
| Schizophrenia | |||||||
| Mittrach et al., 2010 [37] | L DLPFC | 110% MT | 10 Hz | 1000 | 10 | d2 Test | No significant changes |
| Guse et al., 2013 [38] | L DLPFC | 110% MT | 10 Hz | 1000 | 15 | TAP | Significant time-by-stimulation interaction in divided attention |
| Prikryl et al., 2013 [39] | L DLPFC | 110% MT | 10 Hz | 2000 | 15 | SANS | ↓ SANS total score + all domains of negative symptoms |
| Woelwer et al., 2014 [40] | L DLPFC | 110% MT | 10 Hz | 10000 | 10 | d2 Test | No significant changes |
| Attention deficit hyperactivity disorder | |||||||
| Bloch et al., 2010 [41] | R DLPFC | 100% MT | 20 Hz | ? | ? | PANAS, VAS attention/mood CANTAB | ↑ VAS for attention |
| Paz. et al., 2017 [42] | H-Coil L/R DLPFC | 120% MT | 18 Hz | 1980 | 20 | TOVA, CAARS | No differences sham/active rTMS |
| Alzheimer disease | |||||||
| Wu et al., 2015 [43] | L DLPFC | 80% RMT | 20 Hz | 1200 | 20 | BEHAVE-AD, ADAS-Cog scores | Improvement in all ADAS-Cog scores |
| Autism | |||||||
| Sokhadze et al., 2010 [44] | L DLPFC | 90 % RMT | 0,5 Hz | 150 | 6 | ABC, SCR, RBS Early and late ERP components | Improvement of error percentage to targets P50 parieto-occipital↓, frontal ↑ |
| Casanova et al., 2012 [45] | L/R DLPFC | 90 % RMT | ≤ 1 Hz | 150 | 12 | Selective attention illusory figures ERP indices of selective attention | ↓ in response errors ↑ N200 and P300 components |
| Sokhadze et al., 2018 [46] | L/R DLPFC | 90 % RMT | 1 Hz | 180 | 18 | Visual oddball with Kanizsa figures Stimulus and response-locked ERP | ↑ Motor responses accuracy ↑ Early and later-stage ERP indices |
| Addiction | |||||||
| Herremans et al, 2015 [47] | R DLPFC | 110 % RMT | 20 Hz | 1560 | 15 | AUQ, OCDS | Cue-induced alcohol craving was not altered |
| Zang et al., 2018 [48] | L DLPFC | 90 % RMT | 10 Hz | 2000 | 14 | Chinese Affective Picture System | Improvement of emotional attention in meth addicts |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hauer, L.; Sellner, J.; Brigo, F.; Trinka, E.; Sebastianelli, L.; Saltuari, L.; Versace, V.; Höller, Y.; Nardone, R. Effects of Repetitive Transcranial Magnetic Stimulation over Prefrontal Cortex on Attention in Psychiatric Disorders: A Systematic Review. J. Clin. Med. 2019, 8, 416. https://doi.org/10.3390/jcm8040416
Hauer L, Sellner J, Brigo F, Trinka E, Sebastianelli L, Saltuari L, Versace V, Höller Y, Nardone R. Effects of Repetitive Transcranial Magnetic Stimulation over Prefrontal Cortex on Attention in Psychiatric Disorders: A Systematic Review. Journal of Clinical Medicine. 2019; 8(4):416. https://doi.org/10.3390/jcm8040416
Chicago/Turabian StyleHauer, Larissa, Johann Sellner, Francesco Brigo, Eugen Trinka, Luca Sebastianelli, Leopold Saltuari, Viviana Versace, Yvonne Höller, and Raffaele Nardone. 2019. "Effects of Repetitive Transcranial Magnetic Stimulation over Prefrontal Cortex on Attention in Psychiatric Disorders: A Systematic Review" Journal of Clinical Medicine 8, no. 4: 416. https://doi.org/10.3390/jcm8040416
APA StyleHauer, L., Sellner, J., Brigo, F., Trinka, E., Sebastianelli, L., Saltuari, L., Versace, V., Höller, Y., & Nardone, R. (2019). Effects of Repetitive Transcranial Magnetic Stimulation over Prefrontal Cortex on Attention in Psychiatric Disorders: A Systematic Review. Journal of Clinical Medicine, 8(4), 416. https://doi.org/10.3390/jcm8040416







