Efficacy of Three Different Prophylactic Treatments for Postoperative Nausea and Vomiting after Vitrectomy: A Randomized Clinical Trial
Abstract
1. Introduction
2. Experimental Section
2.1. Study Design
2.2. Participants
- Patients younger than 18 years;
- an American Society of Anesthesiologists (ASA) physical status > III;
- diabetes mellitus;
- hypersensitivity to study drugs or rescue medication;
- patients suffering from acute or chronic nausea, motion sickness, and/or vomiting;
- a severe hepatic insufficiency (Child-Pugh score > 9);
- a clinically significant or unstable cardiac, respiratory, hepatic, renal, or other major organ system disease or a psychotic illness or depression;
- addiction to illicit substances or alcohol;
- patients who had taken antiemetic within 12 hours prior to surgery;
- chronic corticosteroid treatment;
- pregnancy; and
- subjects who, in the opinion of the investigator, would experience an unacceptable risk from the administration of the study drugs.
2.3. Randomization and Treatment
- Group A = placebo, received intravenously (IV) two syringes containing 10 mL of saline, one at the start of surgery and one 15 min before the end of surgery.
- Group B = one syringe of ondansetron, 4 mg diluted to 10 mL IV 15 min before the end of surgery, plus one syringe containing 10 mL of saline at the start of the surgery.
- Group C = one syringe of dexamethasone, 4 mg diluted to 10 mL IV, at the start of surgery plus one syringe containing 10 mL of saline 15 min before the end of surgery.
- Group D = one syringe of 4 mg dexamethasone diluted to 10 mL IV at the start of surgery and one syringe of 4 mg ondansetron diluted to 10 mL IV 15 min before the end of surgery.
2.4. Study Objectives
2.5. Statistical Analysis
3. Results
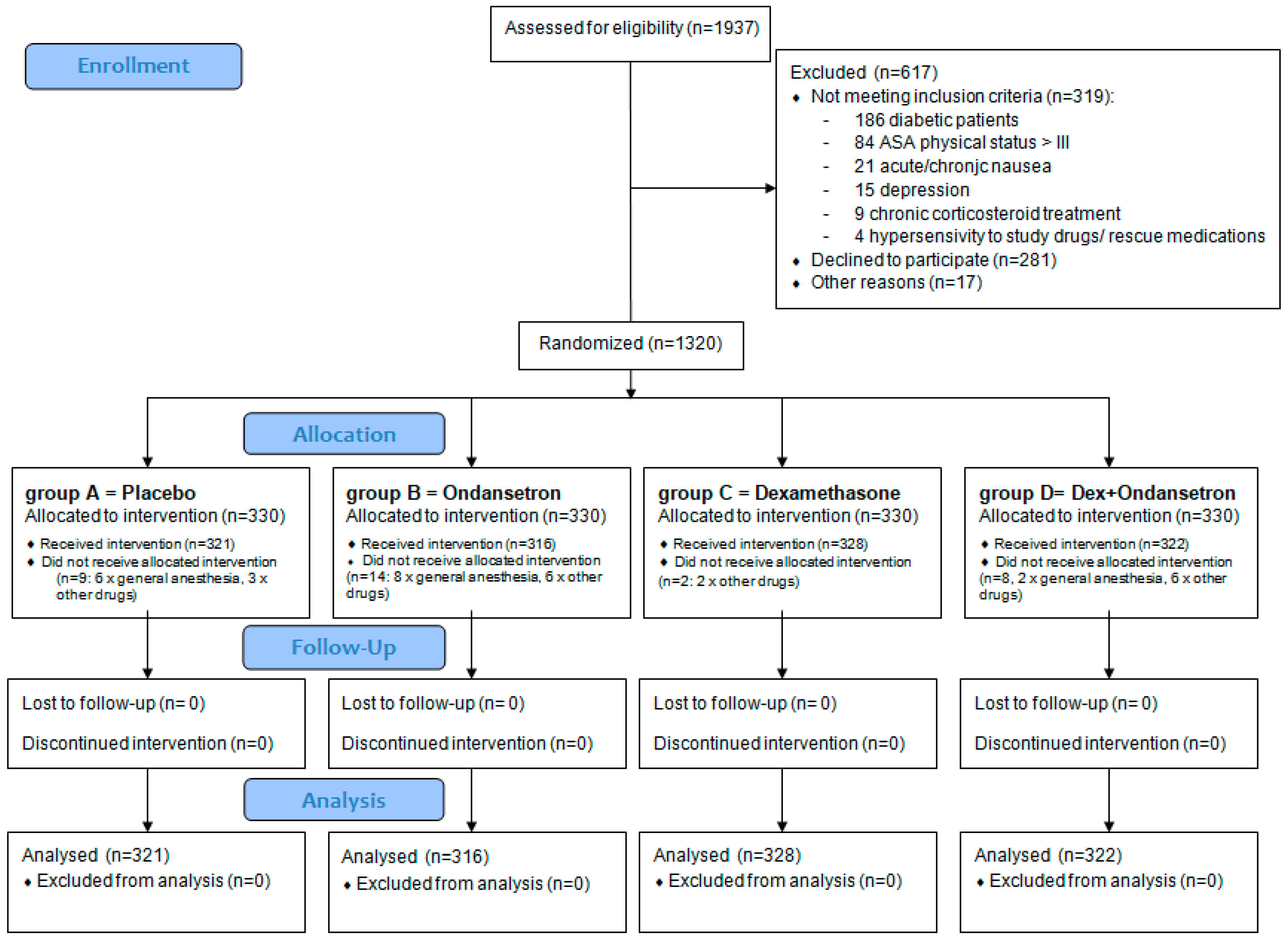
3.1. Patients’ Disposition and Baseline Characteristics
3.2. Primary Outcome Measures
3.3. Secondary Outcome Measures
3.4. Pain Score during the 24 h Post-Operative Period
3.5. Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Reibaldi, M.; Longo, A.; Romano, M.R.; Cennamo, G.; Mariotti, C.; Boscia, F.; Bonfiglio, V.; Avitabile, T. Delayed Suprachoroidal Hemorrhage After Pars Plana Vitrectomy: Five-Year Results of a Retrospective Multicenter Cohort Study. Am. J. Ophthalmol. 2015, 160, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Beyer, C.F.; Peyman, G.A.; Hill, J.M. Expulsive choroidalhemorrhage in rabbits. A histopathologic study. Arch. Ophthalmol. 1989, 107, 1648–1653. [Google Scholar] [CrossRef] [PubMed]
- Obuchowska, I.; Mariak, Z. Risk factors of massive suprachoroidalhemorrhage during extracapsular cataract extraction surgery. Eur. J. Ophthalmol. 2005, 15, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Tuli, S.S.; WuDunn, D.; Ciulla, T.A.; Cantor, L.B. Delayed suprachoroidalhemorrhage after glaucoma filtration procedures. Ophthalmology 2001, 108, 1808–1811. [Google Scholar] [CrossRef]
- Mei, H.; Xing, Y.; Yang, A.; Wang, J.; Xu, Y.; Heiligenhaus, A. Suprachoroidalhemorrhage during pars plana vitrectomy in traumatized eyes. Retina 2009, 29, 473–476. [Google Scholar] [CrossRef]
- Chandra, A.; Xing, W.; Kadhim, M.R.; Williamson, T.H. Suprachoroidalhemorrhage in pars plana vitrectomy: Risk factors and outcomes over 10 years. Ophthalmology 2014, 121, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.D.; Grossman, D.S.; Mundy, K.M.; Sugar, A.; Sloan, F.A. Severe adverse events after cataract surgery among medicare beneficiaries. Ophthalmology 2011, 118, 1716–1723. [Google Scholar] [CrossRef] [PubMed]
- Eberhart, L.H.; Morin, A.M.; Hoerle, S.; Wulf, H.; Geldner, G. Droperidol and dolasetron alone or in combination for prevention of postoperative nausea and vomiting after vitrectomy. Ophthalmology 2004, 111, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Costen, M.T.; Newsom, R.S.; Wainwright, A.C.; Luff, A.J.; Canning, C.R. Expanding role of local anaesthesia in vitreoretinal surgery. Eye 2005, 19, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomized trials. Ann. Intern. Med. 2010, 152, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Reibaldi, M.; Parravano, M.; Varano, M.; Longo, A.; Avitabile, T.; Uva, M.G.; Zagari, M.; Toro, M.; Boscia, F.; Boccassini, B.; et al. Foveal microstructure and functional parameters in lamellar macular hole. Am. J. Ophthalmol. 2012, 154, 974–980.e1. [Google Scholar] [CrossRef] [PubMed]
- Laoos, M.J.A.; Houterman, S.; Scheltinga, M.R.M.; Roumen, R.M.H. Evaluating postherniorrhaphy groin pain: Visual analogue scale or verbal rating scale? Hernia 2008, 12, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Ye, W.; Sun, N.; Jin, X. Analgesic and sedative effects of dezocine and midazolam during vitrectomy. Curr. Eye Res. 2016, 41, 1460–1464. [Google Scholar] [CrossRef]
- Heinke, W.; Frank, T.; Meier, P.; Wiegel, M.; Korth, D. Postoperative vomiting after pars plana vitrectomy. Anaesthesiol. Reanim. 1996, 21, 47–50. (In German) [Google Scholar]
- Iwamoto, K.; Schwartz, H. Antiemtetic effect of droperidol after ophthalmic surgery. Arch. Ophthalmol. 1978, 96, 1378–1379. [Google Scholar] [CrossRef] [PubMed]
- FDA. Inapsine (Droperidol) Dear Healthcare Professional Letter Dec 2001. 2001. Available online: https://wayback.archive-it.org/7993/20170113165136/http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm173778.htm (accessed on 8 February 2019).
- Sinclair, D.R.; Chung, F.; Mezei, G. Can postoperative nausea and vomiting be predicted? Anesthesiology 1999, 91, 109–118. [Google Scholar] [CrossRef]
- Horn, C.C.; Wallisch, W.J.; Homanics, G.E.; Williams, J.P. Pathophysiological and neurochemical mechanisms of postoperative nausea and vomiting. Eur. J. Pharmacol. 2014, 722, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Ahn, E.J.; Kang, H.; Choi, G.J.; Baek, C.W.; Jung, Y.H.; Woo, Y.C. The Effectiveness of Midazolam for Preventing Postoperative Nausea and Vomiting: A Systematic Review and Meta-Analysis. Anesth. Analg. 2016, 122, 664–676. [Google Scholar] [CrossRef]
- Shen, Y.D.; Chen, C.Y.; Wu, C.H.; Cherng, Y.G.; Tam, K.W. Dexamethasone, ondansetron, and their combination and postoperative nausea and vomiting in children undergoing strabismus surgery: A meta-analysis of randomized controlled trials. Paediatr. Anaesth. 2014, 24, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Chandrakantan, A.; Glass, P.S. Multimodal therapies for postoperative nausea and vomiting, and pain. Br. J. Anaesth. 2011, 107 (Suppl. 1), i27–i40. [Google Scholar] [CrossRef]
- Goodin, S.; Cunningham, R. 5-HT3-Receptor Antagonists for the Treatment of Nausea and Vomiting: A Reappraisal of Their Side-Effect Profile. Oncologist 2002, 7, 424–436. [Google Scholar] [CrossRef] [PubMed]
- FDA Drug Safety Communication: New Information Regarding QT Prolongation with Ondansetron (Zofran). Available online: http://www.fda.gov/Drugs/DrugSafety/ucm310190.htm (accessed on 8 March 2019).
- Doggrell, S.A.; Hancox, J.C. Cardiac safety concerns for ondansetron, an antiemetic commonly used for nausea linked to cancer treatment and following anaesthesia. Expert Opin. Drug Saf. 2013, 12, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.J.; Meyer, T.; Apfel, C.C.; Chung, F.; Davis, P.J.; Eubanks, S.; Kovac, A.; Philip, B.K.; Sessler, D.I.; Temo, J.; et al. Consensus guidelines for managing postoperative nausea and vomiting. Anesth. Analg. 2003, 97, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.D. Miller’s Anesthesia; Elsevier Saunders: Philadelphia, PA, USA; Edinburgh, UK, 2009. [Google Scholar]
- Barends, C.R.; Absalom, A.; van Minnen, B.; Vissink, A.; Visser, A. Dexmedetomidine versus Midazolam in Procedural Sedation. A Systematic Review of Efficacy and Safety. PLoS ONE 2017, 12, e0169525. [Google Scholar] [CrossRef] [PubMed]
- Williamson, A.; Hoggart, B. Pain: A review of three commonly used pain rating scales. J. Clin. Nurs. 2005, 14, 798–804. [Google Scholar] [CrossRef]
| Variables | Placebo (n 321) | Ondansetron (n 316) | Dexamethasone (n 328) | Ondansetron + Dexamethasone (n 322) | p |
|---|---|---|---|---|---|
| Demographics | |||||
| Mean ± SD age (years) | 66 ± 7 | 66 ± 10 | 67 ± 8 | 66 ± 9 | 0.344 |
| Male sex, No. (%) | 160 (49.8) | 157 (49.6) | 164 (50.0) | 162 (50.3) | 0.999 |
| Systemic | |||||
| Hypertension, No. (%) | 140 (43.6) | 146 (45.5) | 142 (43.2) | 139 (43.1) | 0.850 |
| Cerebral Stroke, No. (%) | 7 (2.1) | 4 (1.2) | 6 (1.8) | 9 (2.7) | 0.575 |
| Cardiovascular diseases, No. (%) | 31 (9.6) | 26 (8.2) | 30 (9.1) | 23 (7.1) | 0.680 |
| Antiplatelet agents/anticoagulants No. (%) | 109 (33.9) | 99 (31.3) | 102 (31.0) | 114 (35.4) | 0.592 |
| Ophthalmic | |||||
| Mean ± SD axial length (mm) | 24.9 ± 1.6 | 25.1 ± 1.8 | 25.2 ± 1.8 | 25.0 ± 1.7 | 0.143 |
| Mean ± SD preoperative IOP (mmHg) | 14.5 ± 1.9 | 14.3 ± 1.3 | 14.6 ± 1.7 | 14.4 ± 1.5 | 0.105 |
| Preoperative pseudophakic/aphakic, No. (%) | 139 (43.3) | 141 (44.6) | 129 (39.3) | 144 (44.7) | 0.468 |
| Operative | |||||
| Rhegmatogenous retinal detachment, No. (%) | 181 (56.3) | 183 (57.9) | 187 (57.0) | 190 (59.0) | 0.916 |
| Macular hole, No. (%) | 26 (8.0) | 25 (7.9) | 28 (8.5) | 26 (8.0) | 0.619 |
| Epiretinal membrane, No. (%) | 90 (28.0) | 88 (27.8) | 90 (27.4) | 85 (26.3) | 0.967 |
| Dropped lens/IOL, No. (%) | 10 (3.1) | 8 (2.5) | 12 (3.6) | 11 (3.4) | 0.865 |
| Others, No. (%) | 14 (4.3) | 12 (3.7) | 11 (3.3) | 10 (3.1) | 0.841 |
| Combined vitrectomy and phaco, No. (%) | 118 (36.7) | 120 (37.9) | 122 (37.1) | 114 (35.4) | 0.924 |
| 23 gauge vitrectomy, No. (%) | 247 (76.9) | 239 (75.6) | 252 (76.8) | 254 (78.8) | 0.806 |
| 25 gauge vitrectomy, No. (%) | 69 (21.4) | 73 (23.1) | 70 (21.3) | 64 (19.8) | 0.804 |
| 27 gauge vitrectomy, No. (%) | 5 (1.5) | 4 (1.2) | 6 (1.8) | 4 (1.2) | 0.916 |
| No photocoagulation, No. (%) | 132 (41.1) | 126 (39.8) | 133 (40.5) | 126 (39.1) | 0.961 |
| Localized photocoagulation, No. (%) | 74 (23.0) | 71 (22.4) | 85 (25.9) | 76 (23.6) | 0.748 |
| Extensive photocoagulation, No. (%) | 115 (35.8) | 119 (37.6) | 110 (33.5) | 120 (37.2) | 0.690 |
| Buckling, No. (%) | 4 (1.2) | 4 (1.2) | 3 (0.9) | 3 (0.9) | 0.954 |
| Cryopexy, No. (%) | 3 (0.9) | 1 (0.3) | 4 (1.2) | 3 (0.9) | 0.650 |
| No tamponade, No. (%) | 79 (24.6) | 74 (23.4) | 86 (26.2) | 87 (27.0) | 0.724 |
| Air tamponade, No. (%) | 88 (27.4) | 81 (25.6) | 84 (25.6) | 79 (24.5) | 0.869 |
| SF6 tamponade, No. (%) | 97 (30.2) | 98 (31.0) | 102 (31.0) | 94 (29.1) | 0.949 |
| C3F8 tamponade, No. (%) | 8 (2.4) | 7 (2.2) | 8 (2.4) | 9 (2.7) | 0.973 |
| Silicone oil, No. (%) | 49 (15.2) | 56 (17.7) | 48 (14.6) | 53 (16.4) | 0.722 |
| Length of surgery (minutes) | 82 ± 27 (35–135) | 81 ± 25 (40–140) | 79 ± 23 (40–130) | 80 ± 24 (35–140) | 0.515 |
| Treatment Group | Number of Patients | Number of Patients with Complete Response | Percentage of Patients with Complete Response |
|---|---|---|---|
| Placebo, No. (%) | 321 | 231 | 71.96 (%) |
| Ondansetron, No. (%) | 316 | 254 a | 80.38 (%) |
| Dexamethasone, No. (%) | 328 | 265 b, d | 80.79 (%) |
| Ondansetron+Dexamethasone, No. (%) | 322 | 309 c, e, f | 95.96 (%) |
| Treatment Group | Number of Patients (%) | Number of Patients with PONV (%) |
|---|---|---|
| Placebo, No. (%) | 321 160 males–161 females (49.8–50.2) | 90 41 males–49 females (45.2–54.8) a |
| Ondansetron, No. (%) | 316 157 males–159 females (49.7–50.3) | 62 28 males–34 females (44.9–55.1) a |
| Dexamethasone, No. (%) | 328 164 males–164 females (50.0–50.0) | 63 27 males–36 females (43.3–56.7) a |
| Ondansetron + Dexamethasone, No. (%) | 322 162 males–160 females (50.3–49.7) | 13 6 males–7 females (44.2–55.8) |
| Treatment Group | Number of Patients with PONV | Cases of PONV in Patients with Hypertension (%) | Cases of PONV in Patients without Hypertension (%) |
|---|---|---|---|
| Placebo, No. (%) | 90 | 57 (63.5) | 33 (36.5) a |
| Ondansetron, No. (%) | 62 | 38 (61.3) | 24 (38.7) b |
| Dexamethasone, No. (%) | 63 | 36 (57.1) | 27 (42.7) c |
| Ondansetron + Dexamethasone, No. (%) | 13 | 8 (61.5) | 5 (38.5) |
| PONV Score | Placebo (n = 321) | Ondansetron (n = 316) | Dexamethasone (n = 328) | Ondansetron + Dexamethasone (n = 322) |
|---|---|---|---|---|
| No PONV, No. (%) | 231 (71.96%) | 254 (80.38%) | 265 (80.79%) | 309 (95.96%) |
| Mild, No. (%) | 62 (19.31%) | 46 (14.56%) | 45 (13.72%) | 11 (3.42%) |
| Moderate, No. (%) | 23 (7.17%) | 16 (5.06%) | 18 (5.49%) | 2 (0.62%) |
| Severe, No. (%) | 5 (1.56%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Pain score | ||||
| No pain, No. (%) | 221 (68.8) | 214 (67.7) | 242 (73.7) | 243 (75.4) |
| Mild pain, No. (%) | 75 (23.3) | 79 (25.0) | 69 (21.0) | 62 (19.2) |
| Moderate pain, No. (%) | 23 (7.1) | 21 (6.6) | 16 (4.8) | 17 (5.2) |
| Severe pain, No. (%) | 2 (0.6) | 2 (0.6) | 1 (0.3) | 0 (0%) |
| Non-Ocular Adverse Events | Placebo (n = 321) | Ondansetron (n = 316) | Dexamethasone (n = 328) | Ondansetron + Dexamethasone (n = 322) | KRUSKALL-WALLIS |
|---|---|---|---|---|---|
| Akathisia/Restlessness, No. (%) | 0 (0%) | 0 (0%) | 1 (0.3%) | 0 (0%) | - |
| Death, No. (%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Dizziness, No. (%) | 6 (1.9%) | 4 (1.3%) | 2 (0.6%) | 2 (0.6%) | p = 0.353 |
| Drowsiness, No. (%) | 18 (5.6%) | 14 (4.4%) | 11 (3.3%) | 21 (6.5%) | p = 0.270 |
| Extrapyramidal symptoms, No. (%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Headache, No. (%) | 64 (19.9%) | 77 (24.4%) | 59 (18.0%) | 73 (22.7%) | p = 0.209 |
| Hypertension, No. (%) | 14 (4.4%) | 12 (3.8%) | 16 (4.9%) | 11 (3.4%) | p = 0.739 |
| Myocardial infarction, No. (%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Nasopharyngitis, No. (%) | 9 (2.8%) | 10 (3.2%) | 8 (2.4%) | 13 (4%) | p = 0.773 |
| Stroke, No. (%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Ocular adverse events | |||||
| Choroidal detachment, No. (%) | 4 (1.2%) | 3 (0.9%) | 3 (0.9%) | 2 (0.6%) | p = 0.878 |
| Corneal abrasion, No. (%) | 7 (2.2%) | 11 (3.5%) | 6 (1.8%) | 10 (3.1%) | p = 0.524 |
| Corneal edema, No. (%) | 6 (1.9%) | 9 (2.8) | 13 (4%) | 12 (3.7%) | p = 0.406 |
| Endophthalmitis, No. (%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | - |
| IOP ≥ 30 mmHG, No. (%) | 12 (3.7%) | 8 (2.5%) | 9 (2.7%) | 8 (2.5%) | p = 0.757 |
| Hypotony, No. (%) | 4 (1.2%) | 6 (1.9%) | 3 (0.9%) | 7 (2.2%) | p = 0.546 |
| Retinal detachment, No. (%) | 1 (0.3%) | 0 (0%) | 1 (0.3%) | 0 (0%) | p = 0.974 |
| Suprachoroidal hemorrhage, No. (%) | 2 (0.6%) | 1 (0.3%) | 1 (0.3%) | 0 (0%) | p = 0.931 |
| Vitreous hemorrhage, No. (%) | 2 (0.6%) | 2 (0.6%) | 5 (1.5%) | 3 (0.9%) | p = 0.461 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reibaldi, M.; Fallico, M.; Longo, A.; Avitabile, T.; Astuto, M.; Murabito, P.; Minardi, C.; Bonfiglio, V.; Boscia, F.; Furino, C.; et al. Efficacy of Three Different Prophylactic Treatments for Postoperative Nausea and Vomiting after Vitrectomy: A Randomized Clinical Trial. J. Clin. Med. 2019, 8, 391. https://doi.org/10.3390/jcm8030391
Reibaldi M, Fallico M, Longo A, Avitabile T, Astuto M, Murabito P, Minardi C, Bonfiglio V, Boscia F, Furino C, et al. Efficacy of Three Different Prophylactic Treatments for Postoperative Nausea and Vomiting after Vitrectomy: A Randomized Clinical Trial. Journal of Clinical Medicine. 2019; 8(3):391. https://doi.org/10.3390/jcm8030391
Chicago/Turabian StyleReibaldi, Michele, Matteo Fallico, Antonio Longo, Teresio Avitabile, Marinella Astuto, Paolo Murabito, Carmelo Minardi, Vincenza Bonfiglio, Francesco Boscia, Claudio Furino, and et al. 2019. "Efficacy of Three Different Prophylactic Treatments for Postoperative Nausea and Vomiting after Vitrectomy: A Randomized Clinical Trial" Journal of Clinical Medicine 8, no. 3: 391. https://doi.org/10.3390/jcm8030391
APA StyleReibaldi, M., Fallico, M., Longo, A., Avitabile, T., Astuto, M., Murabito, P., Minardi, C., Bonfiglio, V., Boscia, F., Furino, C., Rejdak, R., Nowomiejska, K., Toro, M., Cennamo, G., Cillino, S., Rinaldi, M., Fiore, T., Cagini, C., & Russo, A. (2019). Efficacy of Three Different Prophylactic Treatments for Postoperative Nausea and Vomiting after Vitrectomy: A Randomized Clinical Trial. Journal of Clinical Medicine, 8(3), 391. https://doi.org/10.3390/jcm8030391










