The Scientific Response to Zika Virus
Abstract
:Author Contributions
Conflicts of Interest
References
- Azevedo, R.S.S.; Araujo, M.T.; Oliveira, C.S.; Filho, A.J.M.; Nunes, B.T.D.; Henriques, D.F.; Silva, E.V.P.; Carvalho, V.L.; Chiang, J.O.; Martins, L.C.; et al. Zika Virus Epidemic in Brazil. II. Post-Mortem Analyses of Neonates with Microcephaly, Stillbirths, and Miscarriage. J. Clin. Med. 2018, 7, 496. [Google Scholar] [CrossRef] [PubMed]
- Pierson, T.C.; Diamond, M.S. The emergence of Zika virus and its new clinical syndromes. Nature 2018, 560, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Saiz, J.C.; Martin-Acebes, M.A.; Bueno-Mari, R.; Salomon, O.D.; Villamil-Jimenez, L.C.; Heukelbach, J.; Alencar, C.H.; Armstrong, P.K.; Ortiga-Carvalho, T.M.; Mendez-Otero, R.; et al. Zika Virus: What Have We Learnt Since the Start of the Recent Epidemic? Front Microbiol. 2017, 8, 1554. [Google Scholar] [CrossRef] [PubMed]
- Kurscheidt, F.A.; Mesquita, C.S.S.; Damke, G.; Damke, E.; Carvalho, A.; Suehiro, T.T.; Teixeira, J.J.V.; da Silva, V.R.S.; Souza, R.P.; Consolaro, M.E.L. Persistence and clinical relevance of Zika virus in the male genital tract. Nat. Rev. Urol. 2018. [Google Scholar] [CrossRef] [PubMed]
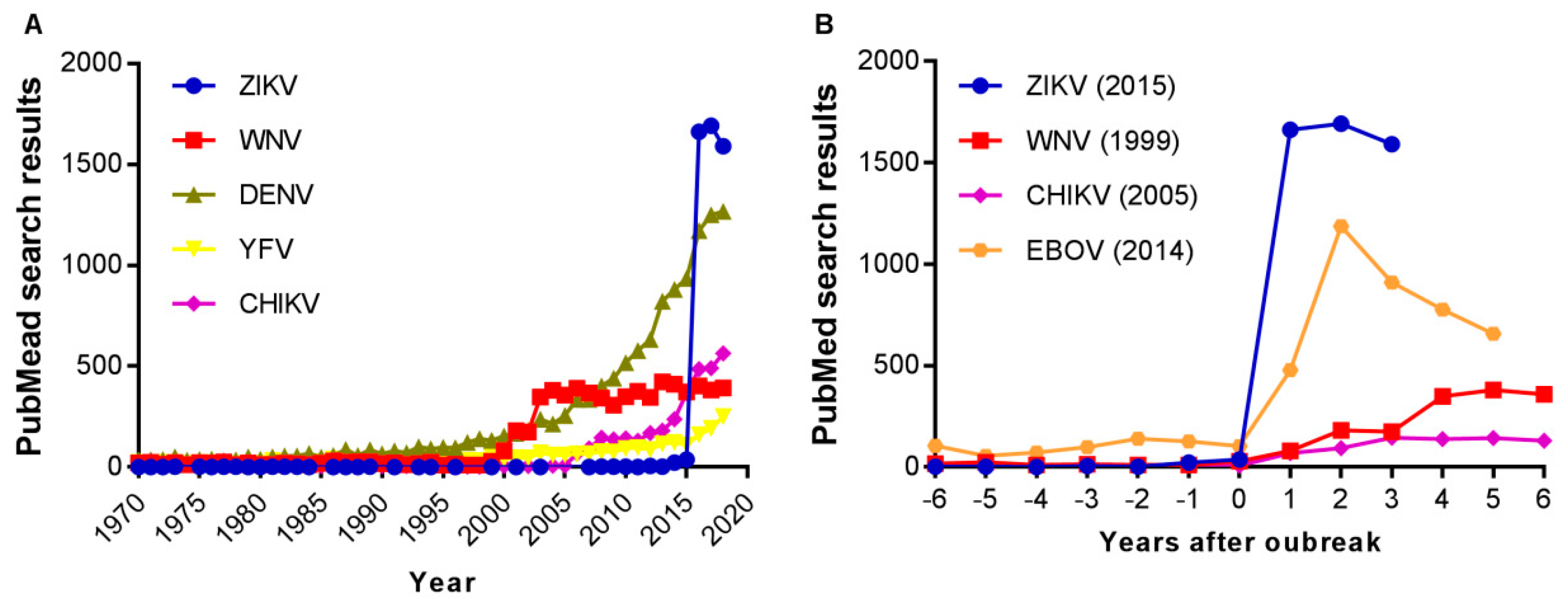
- Martinez-Pulgarin, D.F.; Acevedo-Mendoza, W.F.; Cardona-Ospina, J.A.; Rodriguez-Morales, A.J.; Paniz-Mondolfi, A.E. A bibliometric analysis of global Zika research. Travel Med. Infect. Dis. 2016, 14, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, P.C.; Castro, M.J.; Santos-Gandelman, J.; Oliveira, A.C.; Peralta, J.M.; Rodrigues, M.L. Bibliometric Indicators of the Zika Outbreak. PLoS Negl. Trop. Dis. 2017, 11, e0005132. [Google Scholar] [CrossRef]
- de Melo, M.C.; Dionalisio, M.R.; Ribas de Freitas, A.R. Zika virus, from discovery to the present days: bibliometric review study. Int. J. Curr. Res. 2017, 9, 13. [Google Scholar]
- Delwiche, F.A. Bibliometric Analysis of Scholarly Publications on the Zika Virus, 1952–2016. Sci. Tech. Libr. 2018, 37, 113–129. [Google Scholar] [CrossRef]
- Katzelnick, L.C.; Coloma, J.; Harris, E. Dengue: knowledge gaps, unmet needs, and research priorities. Lancet Infect. Dis. 2017, 17, e88–e100. [Google Scholar] [CrossRef]
- Roehrig, J.T. West nile virus in the United States - a historical perspective. Viruses 2013, 5, 3088–3108. [Google Scholar] [CrossRef]
- Weaver, S.C.; Forrester, N.L. Chikungunya: Evolutionary history and recent epidemic spread. Antiviral Res. 2015, 120, 32–39. [Google Scholar] [CrossRef]
- Kaner, J.; Schaack, S. Understanding Ebola: the 2014 epidemic. Global Health 2016, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Herrada, C.A.; Kabir, M.A.; Altamirano, R.; Asghar, W. Advances in Diagnostic Methods for Zika Virus Infection. J. Med. Device 2018, 12, 0408021–4080211. [Google Scholar] [CrossRef] [PubMed]
- Nazerai, L.; Pravsgaard Christensen, J.; Randrup Thomsen, A. A ’Furry-Tale’ of Zika Virus Infection: What Have We Learned from Animal Models? Viruses 2019, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Sutarjono, B. Can We Better Understand How Zika Leads to Microcephaly? A Systematic Review of the Effects of the Zika Virus on Human Brain Organoids. J. Infect. Dis. 2019, 219, 734–745. [Google Scholar] [CrossRef]
- Wen, Z.; Song, H.; Ming, G.L. How does Zika virus cause microcephaly? Genes Dev. 2017, 31, 849–861. [Google Scholar] [CrossRef]
- Saiz, J.C.; Martin-Acebes, M.A. The Race To Find Antivirals for Zika Virus. Antimicrob. Agents Chemother. 2017, 61, e00411–e00417. [Google Scholar] [CrossRef]
- Hasan, S.S.; Sevvana, M.; Kuhn, R.J.; Rossmann, M.G. Structural biology of Zika virus and other flaviviruses. Nat. Struct. Mol. Biol. 2018, 25, 13–20. [Google Scholar] [CrossRef]
- Boldescu, V.; Behnam, M.A.M.; Vasilakis, N.; Klein, C.D. Broad-spectrum agents for flaviviral infections: dengue, Zika and beyond. Nat. Rev. Drug. Discov. 2017, 16, 565–586. [Google Scholar] [CrossRef]
- Salam, A.P.; Rojek, A.; Dunning, J.; Horby, P.W. Clinical Trials of Therapeutics for the Prevention of Congenital Zika Virus Disease: Challenges and Potential Solutions. Ann. Intern. Med. 2017, 166, 725–732. [Google Scholar] [CrossRef]
- Diamond, M.S.; Ledgerwood, J.E.; Pierson, T.C. Zika Virus Vaccine Development: Progress in the Face of New Challenges. Annu. Rev. Med. 2018, 70, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Wilder-Smith, A.; Ooi, E.E.; Horstick, O.; Wills, B. Dengue. Lancet 2019, 393, 350–363. [Google Scholar] [CrossRef]
- George, J.; Valiant, W.G.; Mattapallil, M.J.; Walker, M.; Huang, Y.S.; Vanlandingham, D.L.; Misamore, J.; Greenhouse, J.; Weiss, D.E.; Verthelyi, D.; et al. Prior Exposure to Zika Virus Significantly Enhances Peak Dengue-2 Viremia in Rhesus Macaques. Sci. Rep. 2017, 7, 10498. [Google Scholar] [CrossRef] [PubMed]
- Fowler, A.M.; Tang, W.W.; Young, M.P.; Mamidi, A.; Viramontes, K.M.; McCauley, M.D.; Carlin, A.F.; Schooley, R.T.; Swanstrom, J.; Baric, R.S.; et al. Maternally Acquired Zika Antibodies Enhance Dengue Disease Severity in Mice. Cell Host Microbe 2018, 24, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Richner, J.M.; Himansu, S.; Dowd, K.A.; Butler, S.L.; Salazar, V.; Fox, J.M.; Julander, J.G.; Tang, W.W.; Shresta, S.; Pierson, T.C.; et al. Modified mRNA Vaccines Protect against Zika Virus Infection. Cell 2017, 168, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Valiant, W.G.; Lalani, T.; Yun, H.C.; Kunz, A.; Burgess, T.H.; Mattapallil, J.J. Human Serum with High Neutralizing Antibody Titers Against Both Zika and Dengue Virus Shows Delayed In Vitro Antibody-Dependent Enhancement of Dengue Virus Infection. Open Forum Infect. Dis. 2018, 5, ofy151. [Google Scholar] [CrossRef]
- Valiant, W.G.; Huang, Y.S.; Vanlandingham, D.L.; Higgs, S.; Lewis, M.G.; Mattapallil, J.J. Zika convalescent macaques display delayed induction of anamnestic cross-neutralizing antibody responses after dengue infection. Emerg. Microbes Infect. 2018, 7, 130. [Google Scholar] [CrossRef]
- Martin-Acebes, M.A.; Saiz, J.C.; Jimenez de Oya, N. Antibody-Dependent Enhancement and Zika: Real Threat or Phantom Menace? Front Cell Infect. Microbiol. 2018, 8, 44. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Acebes, M.A.; Saiz, J.-C. The Scientific Response to Zika Virus. J. Clin. Med. 2019, 8, 369. https://doi.org/10.3390/jcm8030369
Martín-Acebes MA, Saiz J-C. The Scientific Response to Zika Virus. Journal of Clinical Medicine. 2019; 8(3):369. https://doi.org/10.3390/jcm8030369
Chicago/Turabian StyleMartín-Acebes, Miguel A., and Juan-Carlos Saiz. 2019. "The Scientific Response to Zika Virus" Journal of Clinical Medicine 8, no. 3: 369. https://doi.org/10.3390/jcm8030369
APA StyleMartín-Acebes, M. A., & Saiz, J.-C. (2019). The Scientific Response to Zika Virus. Journal of Clinical Medicine, 8(3), 369. https://doi.org/10.3390/jcm8030369





