Baroreceptor Sensitivity Predicts Functional Outcome and Complications after Acute Ischemic Stroke
Abstract
1. Introduction
2. Materials and Methods
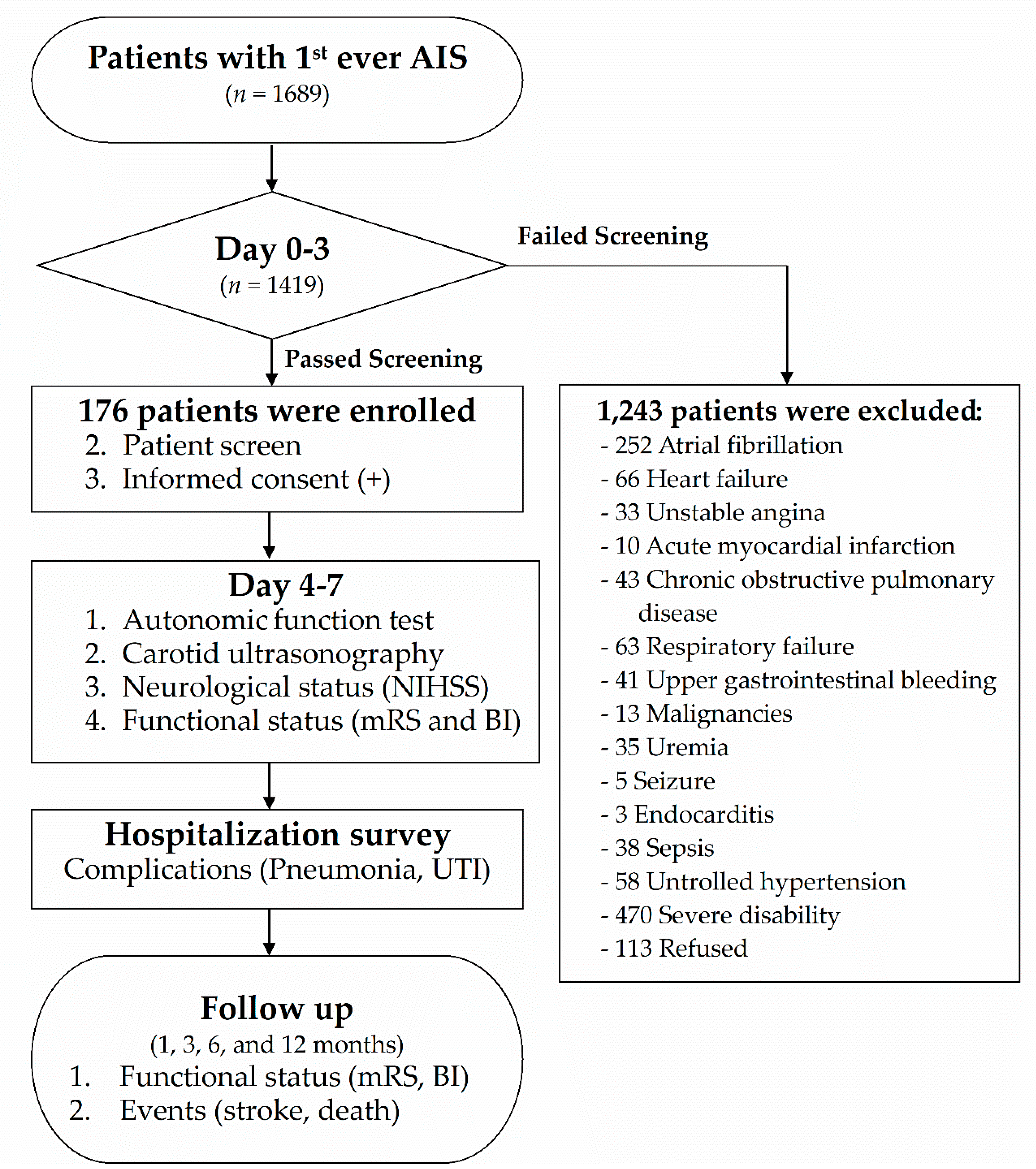
2.1. Subjects
2.2. Standard Protocol Approvals, Registrations, and Patient Consents
2.3. Protocol
2.4. Radiological Examinations
2.5. Carotid Duplex Ultrasonography
2.6. Clinical BRS Examinations
2.7. Neurological Functional Assessments
2.8. Complications During Hospitalization
2.9. Hematology and Serum Biochemical Analyses
2.10. Mortality/Recurrent Stroke
2.11. Data Availability
2.12. Statistics
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Robinson, T.G.; Dawson, S.L.; Eames, P.J.; Panerai, R.B.; Potter, J.F. Cardiac baroreceptor sensitivity predicts long-term outcome after acute ischemic stroke. Stroke 2003, 34, 705–712. [Google Scholar] [CrossRef] [PubMed]
- McLaren, A.; Kerr, S.; Allan, L.; Steen, I.N.; Ballard, C.; Allen, J.; Murray, A.; Kenny, R.A. Autonomic function is impaired in elderly stroke survivors. Stroke 2005, 36, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Diedler, J.; Sykora, M.; Rupp, A.; Poli, S.; Karpel-Massler, G.; Sakowitz, O.; Steiner, T. Impaired cerebral vasomotor activity in spontaneous intracerebral hemorrhage. Stroke 2009, 40, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Sare, G.M.; Ali, M.; Shuaib, A.; Bath, P.M. Relationship between hyperacute blood pressure and outcome after ischemic stroke: Data from the vista collaboration. Stroke 2009, 40, 2098–2103. [Google Scholar] [CrossRef] [PubMed]
- Tikhonoff, V.; Zhang, H.; Richart, T.; Staessen, J.A. Blood pressure as a prognostic factor after acute stroke. Lancet Neurol. 2009, 8, 938–948. [Google Scholar] [CrossRef]
- Yperzeele, L.; van Hooff, R.J.; Nagels, G.; de Smedt, A.; de Keyser, J.; Brouns, R. Heart rate variability and baroreceptor sensitivity in acute stroke: A systematic review. Int. J. Stroke Off. J. Int. Stroke Soc. 2015, 10, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Sykora, M.; Diedler, J.; Rupp, A.; Turcani, P.; Rocco, A.; Steiner, T. Impaired baroreflex sensitivity predicts outcome of acute intracerebral hemorrhage. Crit. Care Med. 2008, 36, 3074–3079. [Google Scholar] [CrossRef] [PubMed]
- Emsley, H.C.; Hopkins, S.J. Acute ischaemic stroke and infection: Recent and emerging concepts. Lancet Neurol. 2008, 7, 341–353. [Google Scholar] [CrossRef]
- Chamorro, A.; Urra, X.; Planas, A.M. Infection after acute ischemic stroke: A manifestation of brain-induced immunodepression. Stroke 2007, 38, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Dirnagl, U.; Klehmet, J.; Braun, J.S.; Harms, H.; Meisel, C.; Ziemssen, T.; Prass, K.; Meisel, A. Stroke-induced immunodepression: Experimental evidence and clinical relevance. Stroke 2007, 38, 770–773. [Google Scholar] [CrossRef] [PubMed]
- Sykora, M.; Diedler, J.; Poli, S.; Rizos, T.; Turcani, P.; Veltkamp, R.; Steiner, T. Autonomic shift and increased susceptibility to infections after acute intracerebral hemorrhage. Stroke 2011, 42, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Eames, P.J.; Blake, M.J.; Dawson, S.L.; Panerai, R.B.; Potter, J.F. Dynamic cerebral autoregulation and beat to beat blood pressure control are impaired in acute ischaemic stroke. J. Neurol. Neurosurg. Psychiatry 2002, 72, 467–472. [Google Scholar] [PubMed]
- Leonardi-Bee, J.; Bath, P.M.; Phillips, S.J.; Sandercock, P.A. Blood pressure and clinical outcomes in the international stroke trial. Stroke 2002, 33, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Prosser, J.; MacGregor, L.; Lees, K.R.; Diener, H.C.; Hacke, W.; Davis, S.; Investigators, V. Predictors of early cardiac morbidity and mortality after ischemic stroke. Stroke 2007, 38, 2295–2302. [Google Scholar] [CrossRef] [PubMed]
- La Rovere, M.T.; Pinna, G.D.; Maestri, R.; Sleight, P. Clinical value of baroreflex sensitivity. Neth. Heart J. Mon. J. Neth. Soc. Cardiol. Neth. Heart Found. 2013, 21, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control 2008, 36, 309–332. [Google Scholar] [CrossRef] [PubMed]
- La Rovere, M.T.; Bigger, J.T., Jr.; Marcus, F.I.; Mortara, A.; Schwartz, P.J. Baroreflex sensitivity and hear2.13rate variability in prediction of total cardiac mortality after myocardial infarction. Atrami (autonomic tone and reflexes after myocardial infarction) investigators. Lancet 1998, 351, 478–484. [Google Scholar] [CrossRef]
- Chapleau, M.W.; Li, Z.; Meyrelles, S.S.; Ma, X.; Abboud, F.M. Mechanisms determining sensitivity of baroreceptor afferents in health and disease. Ann. N. Y. Acad. Sci. 2001, 940, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sykora, M.; Steiner, T.; Rocco, A.; Turcani, P.; Hacke, W.; Diedler, J. Baroreflex sensitivity to predict malignant middle cerebral artery infarction. Stroke 2012, 43, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Sykora, M.; Diedler, J.; Poli, S.; Rizos, T.; Kellert, L.; Turcani, P.; Steiner, T. Association of non-diabetic hyperglycemia with autonomic shift in acute ischaemic stroke. Eur. J. Neurol. 2012, 19, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Weimar, C.; Roth, M.P.; Zillessen, G.; Glahn, J.; Wimmer, M.L.; Busse, O.; Haberl, R.L.; Diener, H.C. Complications following acute ischemic stroke. Eur. Neurol. 2002, 48, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Westendorp, W.F.; Nederkoorn, P.J.; Vermeij, J.D.; Dijkgraaf, M.G.; van de Beek, D. Post-stroke infection: A systematic review and meta-analysis. BMC Neurol. 2011, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Katzan, I.L.; Cebul, R.D.; Husak, S.H.; Dawson, N.V.; Baker, D.W. The effect of pneumonia on mortality among patients hospitalized for acute stroke. Neurology 2003, 60, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Martino, R.; Foley, N.; Bhogal, S.; Diamant, N.; Speechley, M.; Teasell, R. Dysphagia after stroke: Incidence, diagnosis, and pulmonary complications. Stroke 2005, 36, 2756–2763. [Google Scholar] [CrossRef] [PubMed]
- Rocco, A.; Pasquini, M.; Cecconi, E.; Sirimarco, G.; Ricciardi, M.C.; Vicenzini, E.; Altieri, M.; Di Piero, V.; Lenzi, G.L. Monitoring after the acute stage of stroke: A prospective study. Stroke 2007, 38, 1225–1228. [Google Scholar] [CrossRef] [PubMed]
- Poisson, S.N.; Johnston, S.C.; Josephson, S.A. Urinary tract infections complicating stroke: Mechanisms, consequences, and possible solutions. Stroke 2010, 41, e180–e184. [Google Scholar] [CrossRef] [PubMed]
- Van der Poll, T.; Lowry, S.F. Lipopolysaccharide-induced interleukin 8 production by human whole blood is enhanced by epinephrine and inhibited by hydrocortisone. Infect. Immun. 1997, 65, 2378–2381. [Google Scholar] [PubMed]
- Prass, K.; Meisel, C.; Hoflich, C.; Braun, J.; Halle, E.; Wolf, T.; Ruscher, K.; Victorov, I.V.; Priller, J.; Dirnagl, U.; et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke t helper cell type 1-like immunostimulation. J. Exp. Med. 2003, 198, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Odemuyiwa, O.; Farrell, T.; Staunton, A.; Sneddon, J.; Poloniecki, J.; Bennett, D.; Malik, M.; Camm, J. Influence of thrombolytic therapy on the evolution of baroreflex sensitivity after myocardial infarction. Am. Heart J. 1993, 125, 285–291. [Google Scholar] [CrossRef]
- Camm, A.J.; Malik, M.; Bigger, J.T.; Breithardt, G.; Cerutti, S.; Cohen, R.; Coumel, P.; Fallen, E.; Kennedy, H.; Kleiger, R.E.; et al. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task force of the european society of cardiology and the north american society of pacing and electrophysiology. Circulation 1996, 93, 1043–1065. [Google Scholar]
- Bernardi, L.; Porta, C.; Spicuzza, L.; Bellwon, J.; Spadacini, G.; Frey, A.W.; Yeung, L.Y.; Sanderson, J.E.; Pedretti, R.; Tramarin, R. Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation 2002, 105, 143–145. [Google Scholar] [CrossRef] [PubMed]
| Demographic Variables | Total (n = 176) | Low-BRS (n = 99) | High-BRS (n = 77) | p-Value |
|---|---|---|---|---|
| Age (years, mean) | 62.9 ± 12.3 | 64.4 ± 11.2 | 61.1 ± 13.3 | 0.08 |
| Sex (male, %) | 135 (76.7%) | 74 (74.7%) | 61 (79.2%) | 0.49 |
| BMI (mean ± SD) | 24.8 ± 3.5 | 24.6 ± 3.7 | 25.0 ± 3.2 | 0.51 |
| Hypertension (n, %) | 149 (84.7%) | 90 (90.9%) | 59 (76.6%) | 0.09 |
| Diabetes (n, %) | 74 (42.0%) | 51 (51.5%) | 23 (29.9%) | 0.004 |
| Family history of stroke | 63 (39.4%) | 38 (42.2%) | 25 (35.7%) | 0.403 |
| Smoking (n, %) | 87 (49.4%) | 42 (42.4%) | 45 (58.4%) | 0.035 |
| Alcohol (n, %) | 34 (19.3%) | 18 (18.2%) | 16 (20.8%) | 0.665 |
| Hypercholesterolemia (n, %) | 141 (80.6%) | 81 (81.8%) | 60 (78.9%) | 0.634 |
| Hypertriglyceridemia (n, %) | 84 (47.4%) | 44 (44.9%) | 37 (50.7%) | 0.453 |
| CCA-IMT (mm) | 6.2 ± 1.2 | 6.4 ± 1.2 | 5.9 ± 1.2 | 0.026 |
| TOAST (n, %) | 0.012 | |||
| Atherothrombotic | 66 (37.5%) | 39 (39.4%) | 27 (35.1%) | |
| Lacunar | 91 (51.7%) | 55 (55.6%) | 36 (46.8%) | |
| Cardiac embolism | 7 (4.0%) | 0 (0%) | 7 (9.1%) | |
| Other determined | 0 (0%) | 0 (0%) | 0 (0%) | |
| Undetermined | 12 (6.8%) | 5 (5.1%) | 7 (9.1%) | |
| Ant/post circulation (n, %) | 0.08 | |||
| Anterior | 124 (70.5%) | 75 (75.8%) | 49 (63.6%) | |
| Posterior | 52 (29.5) | 24 (24.2%) | 28 (36.4%) | |
| CRP | 1.1 ± 2.2 | 1.5 ± 2.8 | 0.5 ± 0.8 | 0.028 |
| HbA1c (%) | 7.8 ± 2.4 | 8.4 ± 2.6 | 7.0 ± 1.8 | 0.004 |
| rtPA infusion (n, %) | 28 (15.9%) | 17 (17.2%) | 11 (14.3%) | 0.604 |
| Demographic Variables | Total (n = 176) | Low-BRS (n = 99) | High-BRS (n = 77) | p-Value |
|---|---|---|---|---|
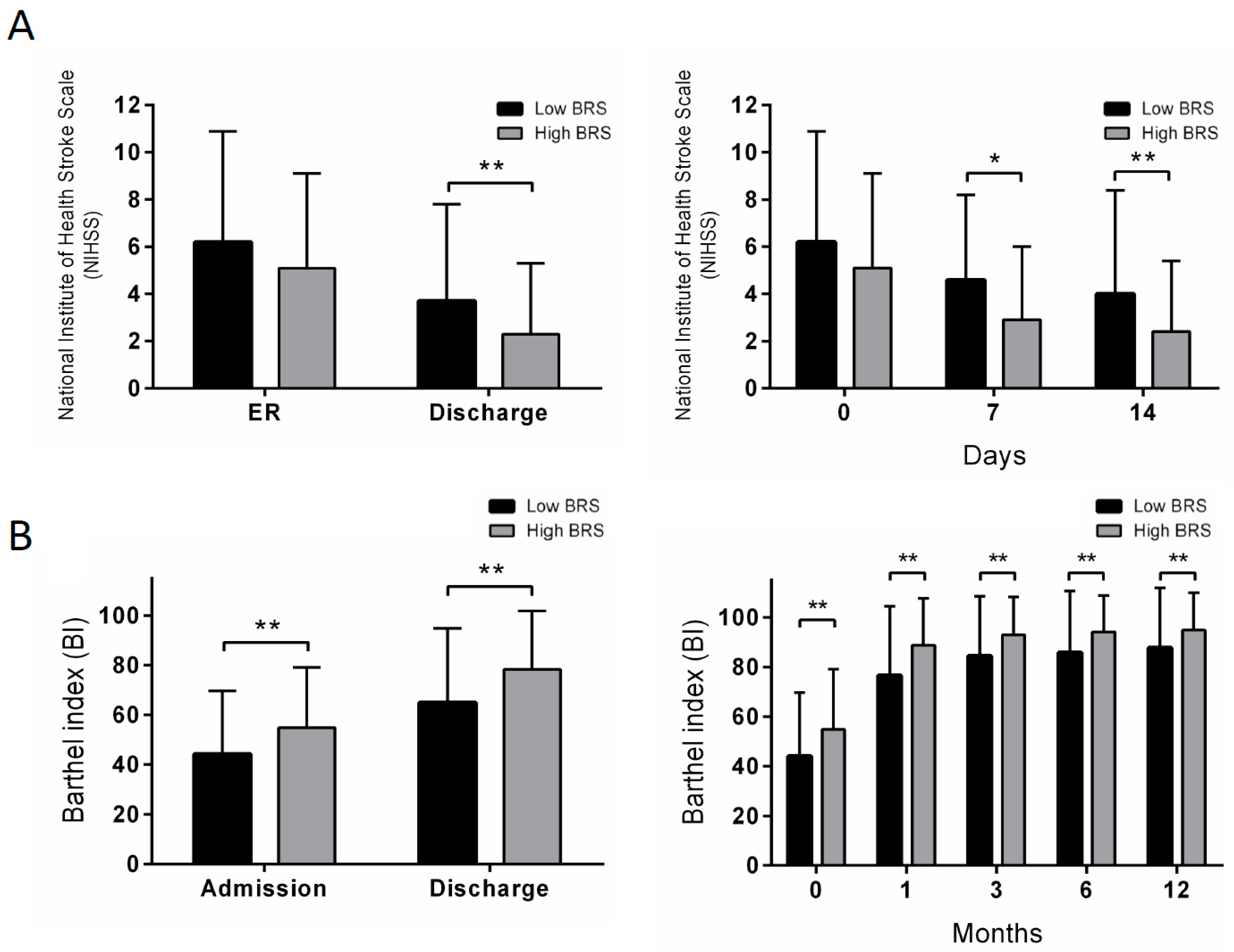
| NIHSS, in ER | 5.7 ± 4.4 | 6.2 ± 4.7 | 5.1 ± 4.0 | 0.089 |
| NIHSS, 7 days | 3.8 ± 3.5 | 4.6 ± 3.6 | 2.9 ± 3.1 | 0.044 |
| NIHSS, 14 days | 3.3 ± 3.9 | 4.0 ± 4.4 | 2.4 ± 3.0 | 0.004 |
| NIHSS, discharge | 3.1 ± 3.7 | 3.7 ± 4.1 | 2.3 ± 3.0 | 0.008 |
| mRS, 1 month | 2.7 ± 1.1 | 2.9 ± 1.2 | 2.3 ± 1.0 | 0.001 |
| mRS, 3 months | 2.1 ± 1.3 | 2.3 ± 1.4 | 1.8 ± 1.2 | 0.006 |
| mRS, 6 months | 1.7 ± 1.5 | 2.0 ± 1.5 | 1.4 ± 1.3 | 0.003 |
| mRS, 12 months | 1.3 ± 1.5 | 1.6 ± 1.6 | 1.0 ± 1.4 | 0.005 |
| BI, admission | 48.8 ± 25.5 | 44.1 ± 25.6 | 54.9 ± 24.2 | 0.005 |
| BI, discharge | 70.7 ± 28.1 | 64.8 ± 30.0 | 78.3 ± 23.5 | 0.001 |
| BI, 1 month | 81.9 ± 25.1 | 76.6 ± 27.9 | 88.8 ± 18.9 | 0.001 |
| BI, 3 months | 88.2 ± 21.0 | 84.5 ± 24.0 | 92.9 ± 15.4 | 0.006 |
| BI, 6 months | 89.5 ± 21.4 | 85.8 ± 24.9 | 94.2 ± 14.6 | 0.006 |
| BI, 12 months | 90.8 ± 21.0 | 87.7 ± 24.2 | 94.9 ± 15.1 | 0.017 |
| Complications | 20 (11.4%) | 18 (18.2%) | 2 (2.6%) | 0.001 |
| Pneumonia | 9 (5.1%) | 9 (9.1%) | 0 (0%) | 0.007 |
| UTI | 9 (5.1%) | 9 (9.1%) | 0 (0%) | 0.001 |
| Event | 8 (4.5%) | 8 (8.1%) | 0 (0.0%) | 0.01 |
| Recurrent stroke | 6 (3.4%) | 6 (6.1%) | 0 (0.0%) | 0.036 |
| Death | 2 (1.1%) | 2 (2%) | 0 (0.0%) | 0.210 |
| Hemorrhagic transformation | 5 (2.8%) | 4 (4.0%) | 1 (1.3%) | 0.388 |
| Demographic Variables | mRS 0–2 (n = 77) | mRS 3–6 (n = 99) | p-Value |
|---|---|---|---|
| Age (years, mean) | 59 ± 12.2 | 66 ± 11.5 | <0.001 |
| Sex (male, %) | 61 (79.2%) | 74 (74.7%) | 0.590 |
| Hypertension | 60 (77.9%) | 89 (89.9%) | 0.035 |
| Diabetes | 24 (31.2%) | 50 (50.5%) | 0.014 |
| Heart disease | 7 (9.1%) | 10 (10.1%) | 1.000 |
| Family history of stroke | 27 (35.1%) | 36 (36.4%) | 0.745 |
| Smoking | 45 (58.4%) | 42 (42.4%) | 0.048 |
| Alcohol | 18 (23.4%) | 16 (16.2%) | 0.665 |
| Hypercholesterolemia | 62 (80.5%) | 79 (79.8%) | 0.848 |
| Hypertriglyceridemia | 36 (46.8%) | 45 (45.5%) | 0.877 |
| BRS within 1 week ≥ 9 | 45 (58.4%) | 32 (32.3%) | 0.001 |
| rtPA infusion | 10 (13.0%) | 18 (18.2%) | 0.410 |
| Variables | mRS | Complications | ||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |||
| BRS | 2.11 | 1.02 | 4.36 | 0.044 | 7.60 | 1.53 | 37.85 | 0.013 |
| NIHSS (ER) | 1.36 | 1.18 | 1.56 | <0.001 | 1.23 | 1.10 | 1.38 | <0.001 |
| Age | 1.05 | 1.02 | 1.09 | 0.004 | 1.03 | 0.98 | 1.08 | 0.273 |
| HTN | 1.81 | 0.64 | 5.11 | 0.260 | 1.15 | 0.17 | 7.65 | 0.888 |
| Diabetes | 1.98 | 0.93 | 4.18 | 0.075 | 0.79 | 0.26 | 2.38 | 0.673 |
| Smoking | 0.70 | 0.34 | 1.44 | 0.330 | 0.98 | 0.32 | 2.94 | 0.964 |
| IV tPA | 0.38 | 0.12 | 1.19 | 0.095 | 1.02 | 0.27 | 3.79 | 0.977 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-H.; Yen, C.-C.; Hsu, Y.-T.; Chen, H.-H.; Cheng, P.-W.; Tseng, C.-J.; Lo, Y.-K.; Chan, J.Y.H. Baroreceptor Sensitivity Predicts Functional Outcome and Complications after Acute Ischemic Stroke. J. Clin. Med. 2019, 8, 300. https://doi.org/10.3390/jcm8030300
Lin C-H, Yen C-C, Hsu Y-T, Chen H-H, Cheng P-W, Tseng C-J, Lo Y-K, Chan JYH. Baroreceptor Sensitivity Predicts Functional Outcome and Complications after Acute Ischemic Stroke. Journal of Clinical Medicine. 2019; 8(3):300. https://doi.org/10.3390/jcm8030300
Chicago/Turabian StyleLin, Ching-Huang, Cheng-Chung Yen, Yi-Ting Hsu, Hsin-Hung Chen, Pei-Wen Cheng, Ching-Jiunn Tseng, Yuk-Keung Lo, and Julie Y.H. Chan. 2019. "Baroreceptor Sensitivity Predicts Functional Outcome and Complications after Acute Ischemic Stroke" Journal of Clinical Medicine 8, no. 3: 300. https://doi.org/10.3390/jcm8030300
APA StyleLin, C.-H., Yen, C.-C., Hsu, Y.-T., Chen, H.-H., Cheng, P.-W., Tseng, C.-J., Lo, Y.-K., & Chan, J. Y. H. (2019). Baroreceptor Sensitivity Predicts Functional Outcome and Complications after Acute Ischemic Stroke. Journal of Clinical Medicine, 8(3), 300. https://doi.org/10.3390/jcm8030300






