Who Are Dispensed the Bulk Amount of Prescription Opioids?
Abstract
1. Introduction
2. Experimental Section
2.1. Prescription Opioid Dispensing in Australia
2.2. Dataset
2.3. Outcome and Exposure Variables for Regression Models
2.4. Analysis
2.5. Ethics Approval
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ghelardini, C.; Di Cesare Mannelli, L.; Bianchi, E. The pharmacological basis of opioids. Clin. Cases Miner. Bone Metab. Off. J. Ital. Soc. Osteoporos. Miner. Metab. Skelet. Dis. 2015, 12, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, B.L.; Gaveriaux-Ruff, C. Exploring the opioid system by gene knockout. Prog. Neurobiol. 2002, 66, 285–306. [Google Scholar] [CrossRef]
- Morgan, M.M.; Christie, M.J. Analysis of opioid efficacy, tolerance, addiction and dependence from cell culture to human. Br. J. Pharmacol. 2011, 164, 1322–1334. [Google Scholar] [CrossRef] [PubMed]
- Murthy, V.H. Ending the Opioid Epidemic—A Call to Action. N. Engl. J. Med. 2016, 375, 2413–2415. [Google Scholar] [CrossRef] [PubMed]
- Lisa, B.; Jessica, H. Evidence synthesis—The opioid crisis in Canada: A national perspective. Health Promot. Chronic Dis. Prev. Can. 2018, 38, 224–233. [Google Scholar]
- Berecki-Gisolf, J.; Hassani-Mahmooei, B.; Clapperton, A.; McClure, R. Prescription opioid dispensing and prescription opioid poisoning: Population data from Victoria, Australia 2006 to 2013. Aust. N. Z. J. Public Health 2017, 41, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Roxburgh, A.; Bruno, R.; Larance, B.; Burns, L. Prescription of opioid analgesics and related harms in Australia. Med. J. Aust. 2011, 195, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Blanch, B.; Pearson, S.A.; Haber, P.S. An overview of the patterns of prescription opioid use, costs and related harms in Australia. Br. J. Clin. Pharm. 2014, 78, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Turner, J.A.; Devine, E.B.; Hansen, R.N.; Sullivan, S.D.; Blazina, I.; Dana, T.; Bougatsos, C.; Deyo, R.A. The effectiveness and risks of long-term opioid therapy for chronic pain: A systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann. Intern. Med. 2015, 162, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Paulozzi, L.J.; Zhang, K.; Jones, C.M.; Mack, K.A. Risk of adverse health outcomes with increasing duration and regularity of opioid therapy. J. Am. Board Fam. Med. JABFM 2014, 27, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.; Jones, W.; Urbanoski, K.; Skinner, R.; Rehm, J. Correlations between prescription opioid analgesic dispensing levels and related mortality and morbidity in Ontario, Canada, 2005–2011. Drug Alcohol Rev. 2014, 33, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Monheit, B.; Pietrzak, D.; Hocking, S. Prescription drug abuse—A timely update. Aust. Fam. Physician 2016, 45, 862–866. [Google Scholar] [PubMed]
- Sun, E.C.; Jena, A.B. Distribution of Prescription Opioid Use Among Privately Insured Adults Without Cancer: United States, 2001 to 2013. Ann. Intern. Med. 2017, 167, 684–686. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; McRae, I.S.; Mazumdar, S.; Simpson, P.; Wollersheim, D.; Fatema, K.; Butler, T. Prescription opioid dispensing in New South Wales, Australia: Spatial and temporal variation. BMC Pharm. Toxicol. 2018, 19, 30. [Google Scholar] [CrossRef] [PubMed]
- The Australian National Audit Office. Medicare Australia’s Administration of the Pharmaceutical Benefits Scheme. The Auditor-General Audit Report No.39 2009–10. Performance Audit; The Australian National Audit Office: Canberra, Australia, 2010; ISBN 0-642-81131-8.
- World Health Organization. Guidelines for ATC Classification and DDD Assignment; WHO Collaborating Centre for Drug Statistics Methodology: Oslo, Norway, 2017; Available online: https://www.whocc.no/filearchive/publications/2017_guidelines_web.pdf (accessed on 8 October 2018).
- Department of Infrastructure and Regional Development (DIRD). Local Government National Report, 2013–14; DIRD: Canberra, Australia, 2015. Available online: http://regional.gov.au/local/publications/reports/2013_2014/INFRA2466_LGNR_2013-14.pdf (accessed on 8 June 2018).
- Sketris, I.S.; Metge, C.J.; Ross, J.L.; MacCara, M.E.; Comeau, D.G.; Kephart, G.C.; Blackburn, J.L. The Use of the World Health Organisation Anatomical Therapeutic Chemical/Defined Daily Dose Methodology in Canada. Drug Inf. J. 2004, 38, 15–27. [Google Scholar] [CrossRef]
- Naing, N.N. Easy way to learn standardization: Direct and indirect methods. Malays. J. Med. Sci. MJMS 2000, 7, 10–15. [Google Scholar] [PubMed]
- StataCorp. meglm—Multilevel Mixed-Effects Generalized Linear Mode. Stata Statistical Software: Release 14; StataCorp LP: College Station, TX, USA, 2013. [Google Scholar]
- The Stata Blog. Multilevel Random Effects in Xtmixed and Sem—The Long and Wide of it. Available online: https://blog.stata.com/2011/09/28/multilevel-random-effects-in-xtmixed-and-sem-the-long-and-wide-of-it/ (accessed on 7 September 2018).
- Wickham, H. tidyverse: Easily Install and Load the ‘Tidyverse’. R Package Version 1.2.1. Available online: https://CRAN.R-project.org/package=tidyverse (accessed on 7 September 2018).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 2 July 2018).
- Tennekes, M. tmap: Thematic Maps in R. J. Stat. Softw. 2018, 84, 1–39. [Google Scholar] [CrossRef]
- Ballantyne, J.C.; Mao, J. Opioid therapy for chronic pain. N. Engl. J. Med. 2003, 349, 1943–1953. [Google Scholar] [CrossRef] [PubMed]
- Braden, J.B.; Russo, J.; Fan, M.Y.; Edlund, M.J.; Martin, B.C.; DeVries, A.; Sullivan, M.D. Emergency department visits among recipients of chronic opioid therapy. Arch. Intern. Med. 2010, 170, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Dunn, K.M.; Saunders, K.W.; Rutter, C.M.; Banta-Green, C.J.; Merrill, J.O.; Sullivan, M.D.; Weisner, C.M.; Silverberg, M.J.; Campbell, C.I.; Psaty, B.M.; et al. Opioid prescriptions for chronic pain and overdose: A cohort study. Ann. Intern. Med. 2010, 152, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Hojsted, J.; Sjogren, P. Addiction to opioids in chronic pain patients: A literature review. Eur. J. Pain 2007, 11, 490–518. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Hayes, C.J.; Martin, B.C. Characteristics of Initial Prescription Episodes and Likelihood of Long-Term Opioid Use—United States, 2006–2015. Morb. Mortal Wkly. Rep. 2017, 66, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Ampon, R.D.; Reddel, H.K.; Correll, P.K.; Poulos, L.M.; Marks, G.B. Cost is a major barrier to the use of inhaled corticosteroids for obstructive lung disease. Med. J. Aust. 2009, 191, 319–323. [Google Scholar] [PubMed]
- Katz, W.A. Musculoskeletal pain and its socioeconomic implications. Clin. Rheumatol. 2002, 21 (Suppl. 1), S2–S4. [Google Scholar] [CrossRef]
- Mazumdar, S.; McRae, I.S.; Islam, M.M. How Can Geographical Information Systems and Spatial Analysis Inform a Response to Prescription Opioid Misuse? A Discussion in the Context of Existing Literature. Curr. Drug Abus Rev. 2015, 8, 104–110. [Google Scholar] [CrossRef]
- Dasgupta, N.; Kramer, E.D.; Zalman, M.A.; Carino, S., Jr.; Smith, M.Y.; Haddox, J.D.; Wright, C.T. Association between non-medical and prescriptive usage of opioids. Drug Alcohol. Depend. 2006, 82, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Piantadosi, S.; Byar, D.P.; Green, S.B. The ecological fallacy. Am. J. Epidemiol. 1988, 127, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, C.A.; Carrie, A.G.; Grymonpre, R.E.; Metge, C.J.; St John, P. Access and intensity of use of prescription analgesics among older Manitobans. Can. J. Clin. Pharmacol. 2009, 16, e322–e330. [Google Scholar] [PubMed]
- Blyth, F.M.; March, L.M.; Brnabic, A.J.; Jorm, L.R.; Williamson, M.; Cousins, M.J. Chronic pain in Australia: A prevalence study. Pain 2001, 89, 127–134. [Google Scholar] [CrossRef]
- Islam, M.M.; Wollersheim, D. Variation in Prescription Opioid Dispensing across Neighborhoods of Diverse Socioeconomic Disadvantages in Victoria, Australia. Pharmaceuticals 2018, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Fillingim, R.B.; King, C.D.; Ribeiro-Dasilva, M.C.; Rahim-Williams, B.; Riley, J.L., 3rd. Sex, gender, and pain: A review of recent clinical and experimental findings. J. Pain 2009, 10, 447–485. [Google Scholar] [CrossRef] [PubMed]
- Back, S.E.; Payne, R.L.; Wahlquist, A.H.; Carter, R.E.; Stroud, Z.; Haynes, L.; Hillhouse, M.; Brady, K.T.; Ling, W. Comparative profiles of men and women with opioid dependence: Results from a national multisite effectiveness trial. Am. J. Drug Alcohol Abus. 2011, 37, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Back, S.E.; Lawson, K.M.; Singleton, L.M.; Brady, K.T. Characteristics and correlates of men and women with prescription opioid dependence. Addict. Behav. 2011, 36, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Gibson, S.J.; Lussier, D. Prevalence and relevance of pain in older persons. Pain Med. 2012, 13 (Suppl. 2), S23–S26. [Google Scholar] [CrossRef] [PubMed]
- Adler, J.A.; Mallick-Searle, T. An overview of abuse-deterrent opioids and recommendations for practical patient care. J. Multidiscip. Healthc. 2018, 11, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Victoria State Government. SafeScript. Available online: https://www2.health.vic.gov.au/public-health/drugs-and-poisons/safescript/about-safescript (accessed on 30 October 2018).
- Hayes, P. NSW Push for Real-Time Monitoring to Reduce Prescription Drug Misuse. Available online: https://www.racgp.org.au/newsGP/Professional/NSW-push-for-real-time-monitoring-to-reduce-prescr (accessed on 30 October 2018).
- Islam, M.M.; McRae, I.S. An inevitable wave of prescription drug monitoring programs in the context of prescription opioids: Pros, cons and tensions. BMC Pharm. Toxicol. 2014, 15, 46. [Google Scholar] [CrossRef] [PubMed]
- Gisev, N.; Pearson, S.A.; Karanges, E.A.; Larance, B.; Buckley, N.A.; Larney, S.; Dobbins, T.; Blanch, B.; Degenhardt, L. To what extent do data from pharmaceutical claims under-estimate opioid analgesic utilisation in Australia? Pharm. Drug Saf. 2018, 27, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, D.; Von Korff, M.; Rutter, C.M.; Saunders, K.; Ray, G.T.; Sullivan, M.D.; Campbell, C.I.; Merrill, J.O.; Silverberg, M.J.; Banta-Green, C.; et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharm. Drug Saf. 2009, 18, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Raebel, M.A.; Newcomer, S.R.; Reifler, L.M.; Boudreau, D.; Elliott, T.E.; DeBar, L.; Ahmed, A.; Pawloski, P.A.; Fisher, D.; Donahoo, W.T.; et al. Chronic use of opioid medications before and after bariatric surgery. JAMA 2013, 310, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Hadlandsmyth, K.; Mosher, H.; Vander Weg, M.W.; Lund, B.C. Decline in Prescription Opioids Attributable to Decreases in Long-Term Use: A Retrospective Study in the Veterans Health Administration 2010–2016. J. Gen. Intern. Med. 2018, 33, 818–824. [Google Scholar] [CrossRef] [PubMed]
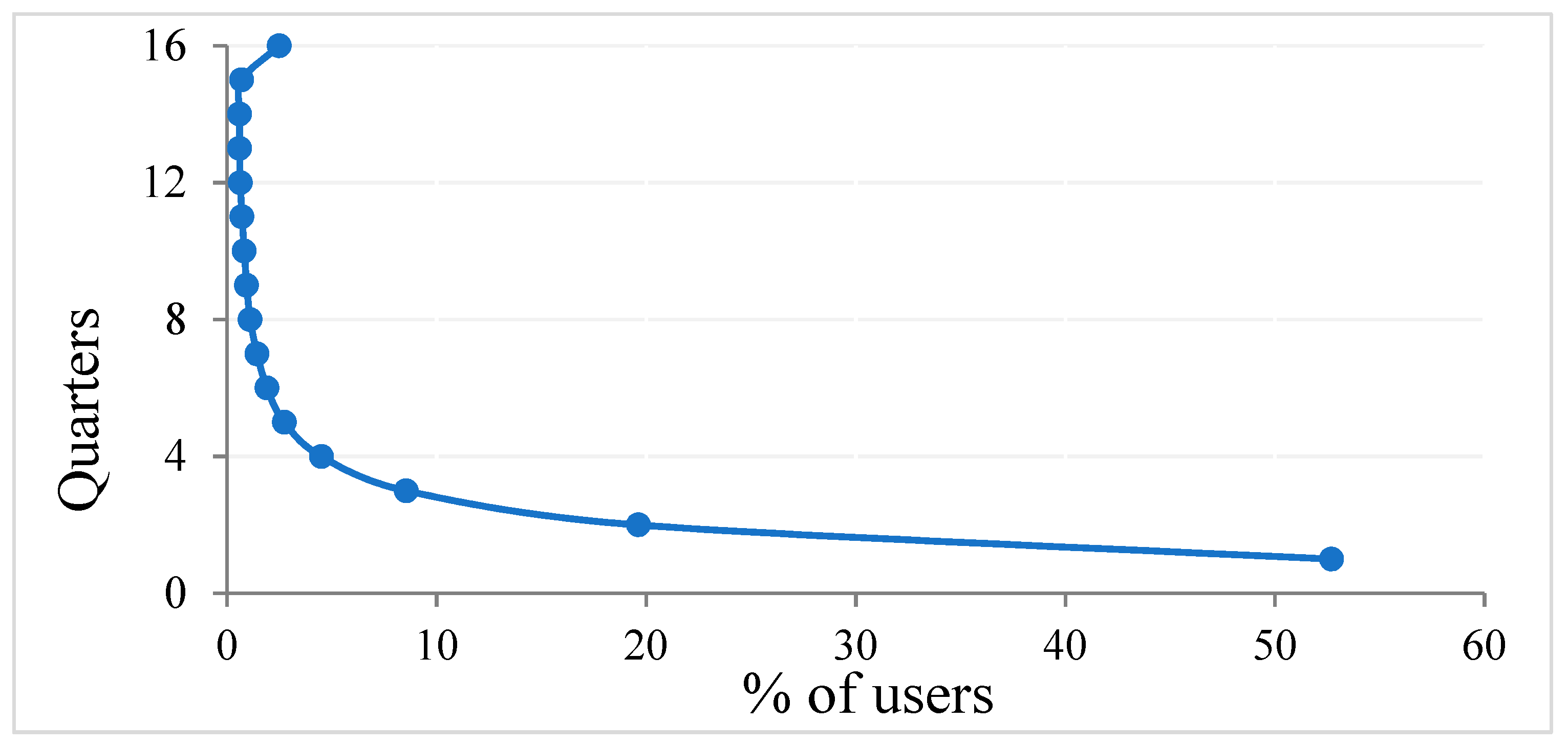
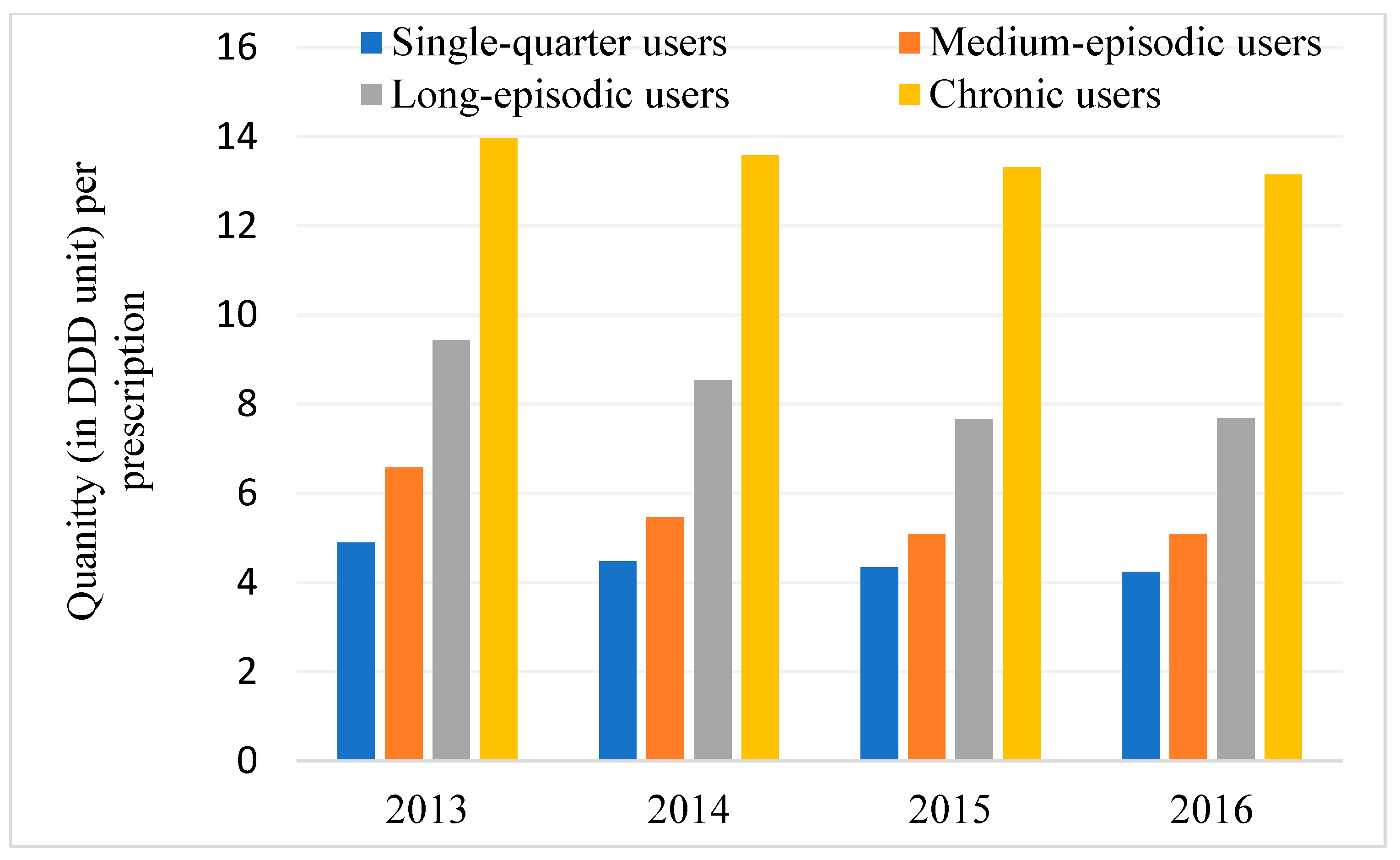

| Variable | IRR | p | 95% CI |
|---|---|---|---|
| Sex | |||
| Male | 1.00 | - | - |
| Female | 1.13 | <0.01 | 1.09–1.18 |
| Age | |||
| 0–19 | 1.00 | - | - |
| 20–44 | 1.44 | <0.01 | 1.29–1.60 |
| 45–64 | 1.93 | <0.01 | 1.74–2.15 |
| 65+ | 2.69 | <0.01 | 2.43–2.99 |
| Year | |||
| 2013 | 1.00 | - | - |
| 2014 | 0.74 | <0.01 | 0.71–0.78 |
| 2015 | 0.63 | <0.01 | 0.60–0.66 |
| 2016 | 0.50 | <0.01 | 0.47–0.53 |
| SEIFA | |||
| Very high | 1.00 | - | - |
| High | 1.17 | <0.01 | 1.10–1.26 |
| Moderate | 1.19 | <0.01 | 1.10–1.28 |
| Least | 1.22 | <0.01 | 1.13–1.32 |
| State | |||
| New South Wales | 1.00 | - | - |
| Victoria | 1.01 | 0.76 | 0.96–1.06 |
| Urbanization | |||
| Urban | 1.00 | - | - |
| Rural | 1.09 | 0.02 | 1.01–1.18 |
| Variance (cov.) of random effect | <0.01 | ||
| Constant | 1.57 | <0.01 | 1.39–1.76 |
| lnalpha | −0.88 | - | −0.93 to −0.84 |
| Level 2 (States) | 3.03 × 10−35 | - | - |
| Level 3 (LGA) | 0.01 | - | 0.01–0.02 |
| Variable | Coefficient | p | 95% CI |
|---|---|---|---|
| Cancer prevalence | −0.0000243 | 0.01 | −0.001 to −4.5 × 10−6 |
| Year | |||
| 2013 (reference) | |||
| 2014 | 0.03 | 0.15 | −0.01 to 0.07 |
| 2015 | 0.01 | 0.80 | −0.04 to 0.05 |
| 2016 | −0.09 | <0.01 | −0.14 to −0.05 |
| SEIFA | |||
| Very high (reference) | |||
| High | 0.83 | <0.01 | 0.51 to 1.15 |
| Moderate | 1.42 | <0.01 | 1.10 to 1.75 |
| Least | 1.45 | <0.01 | 1.11 to 1.78 |
| Urbanization | |||
| Urban (reference) | |||
| Rural | 0.18 | 0.21 | −0.10 to 0.46 |
| Constant | 3.34 | <0.01 | 3.06 to 3.62 |
| Random-effects parameters | |||
| States (SD, Constant) | 5.58 × 10−13 | - | 2.06 × 10−26 to 15.19 |
| LGA (SD, Constant) | 0.82 | - | 0.74 to 0.91 |
| SD (Residual) | 0.23 | - | 0.22 to 0.25 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.M.; Wollersheim, D. Who Are Dispensed the Bulk Amount of Prescription Opioids? J. Clin. Med. 2019, 8, 293. https://doi.org/10.3390/jcm8030293
Islam MM, Wollersheim D. Who Are Dispensed the Bulk Amount of Prescription Opioids? Journal of Clinical Medicine. 2019; 8(3):293. https://doi.org/10.3390/jcm8030293
Chicago/Turabian StyleIslam, M. Mofizul, and Dennis Wollersheim. 2019. "Who Are Dispensed the Bulk Amount of Prescription Opioids?" Journal of Clinical Medicine 8, no. 3: 293. https://doi.org/10.3390/jcm8030293
APA StyleIslam, M. M., & Wollersheim, D. (2019). Who Are Dispensed the Bulk Amount of Prescription Opioids? Journal of Clinical Medicine, 8(3), 293. https://doi.org/10.3390/jcm8030293





