Effects of Feeding-Related Peptides on Neuronal Oscillation in the Ventromedial Hypothalamus
Abstract
1. Introduction
2. Experimental Section
2.1. Animals, Preparation, and Solutions
2.2. Drugs
2.3. Electrophysiological Measurements of Neuronal Activity within the VMH
2.4. Statistics
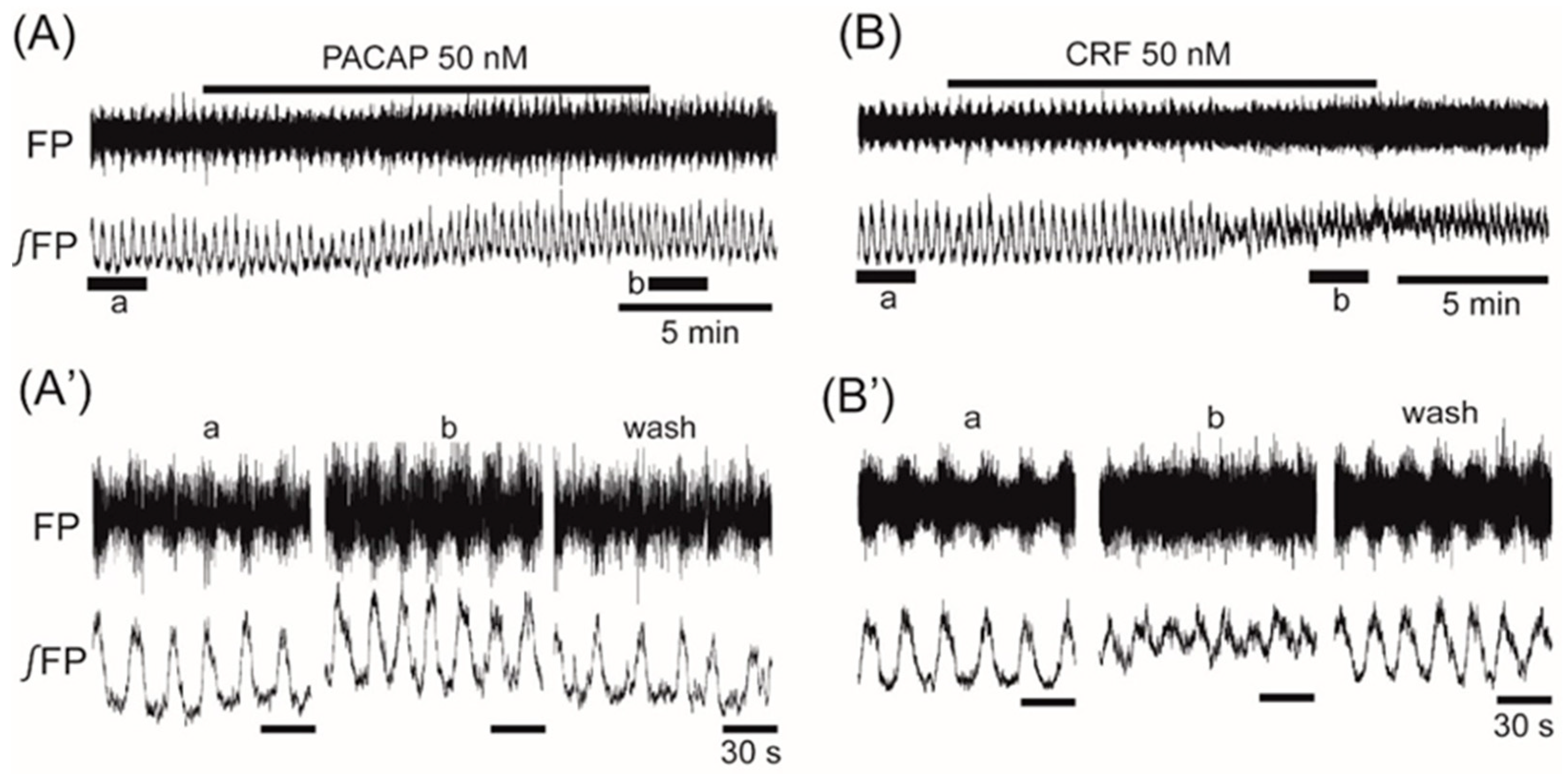
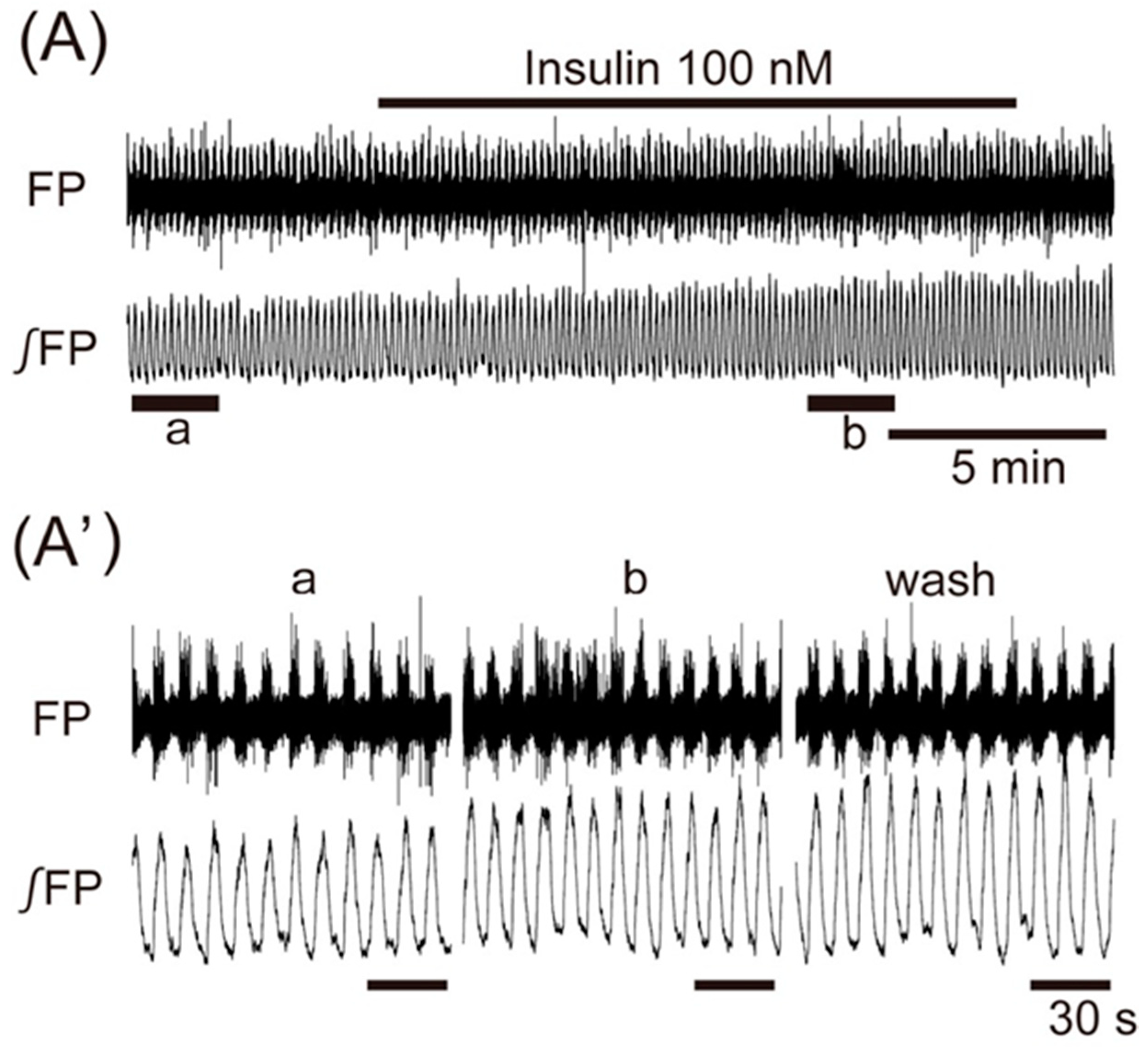
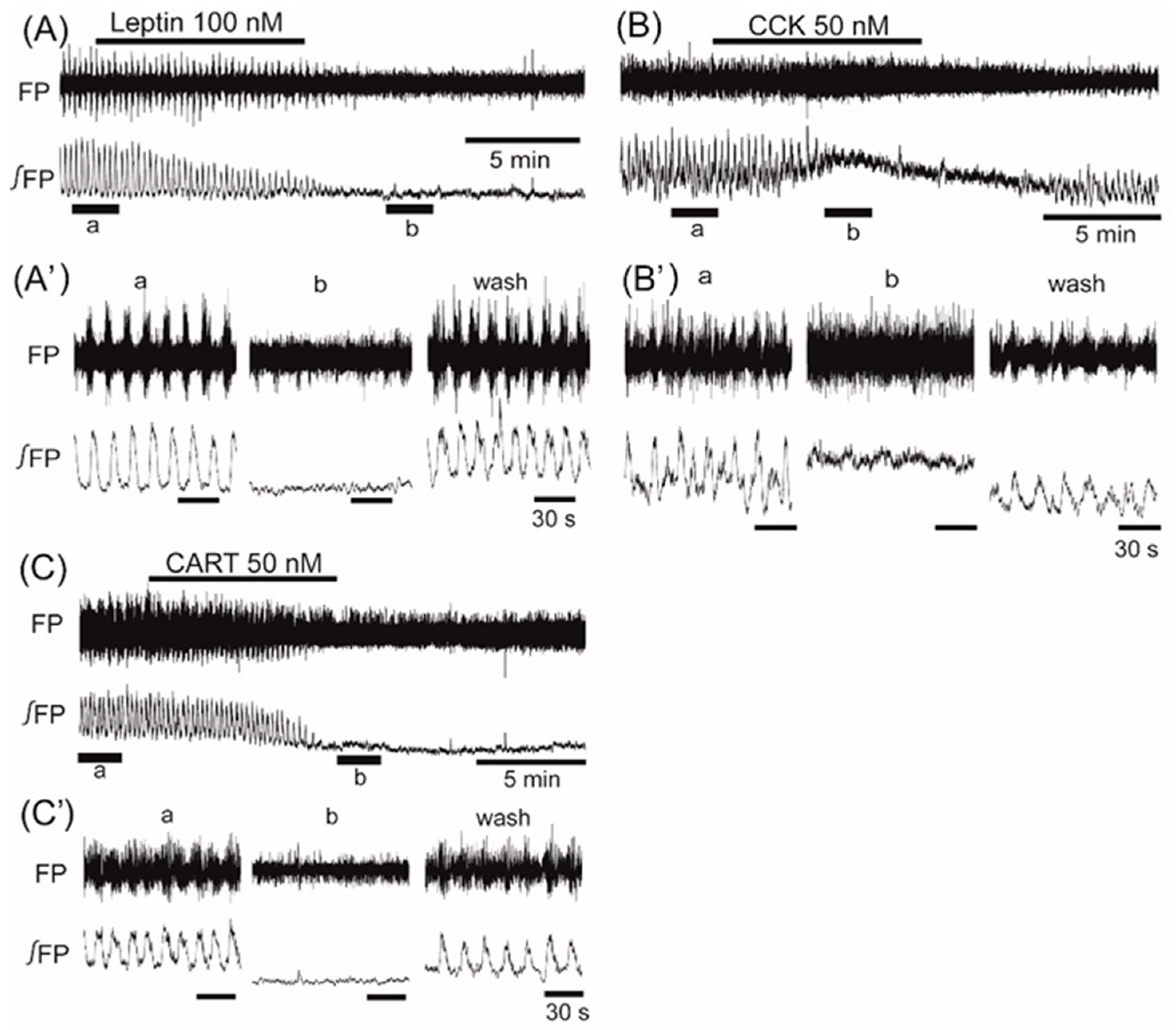
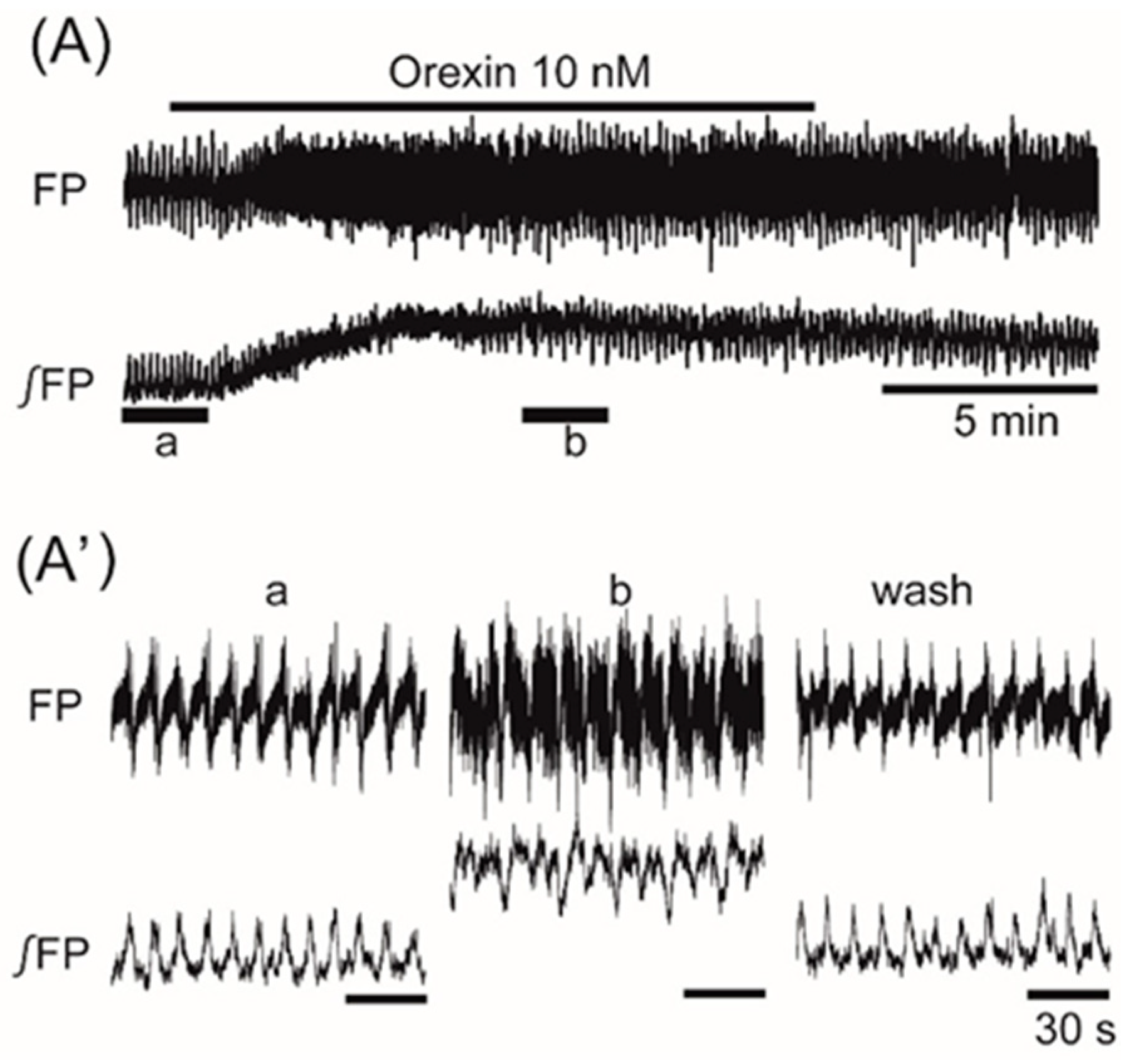
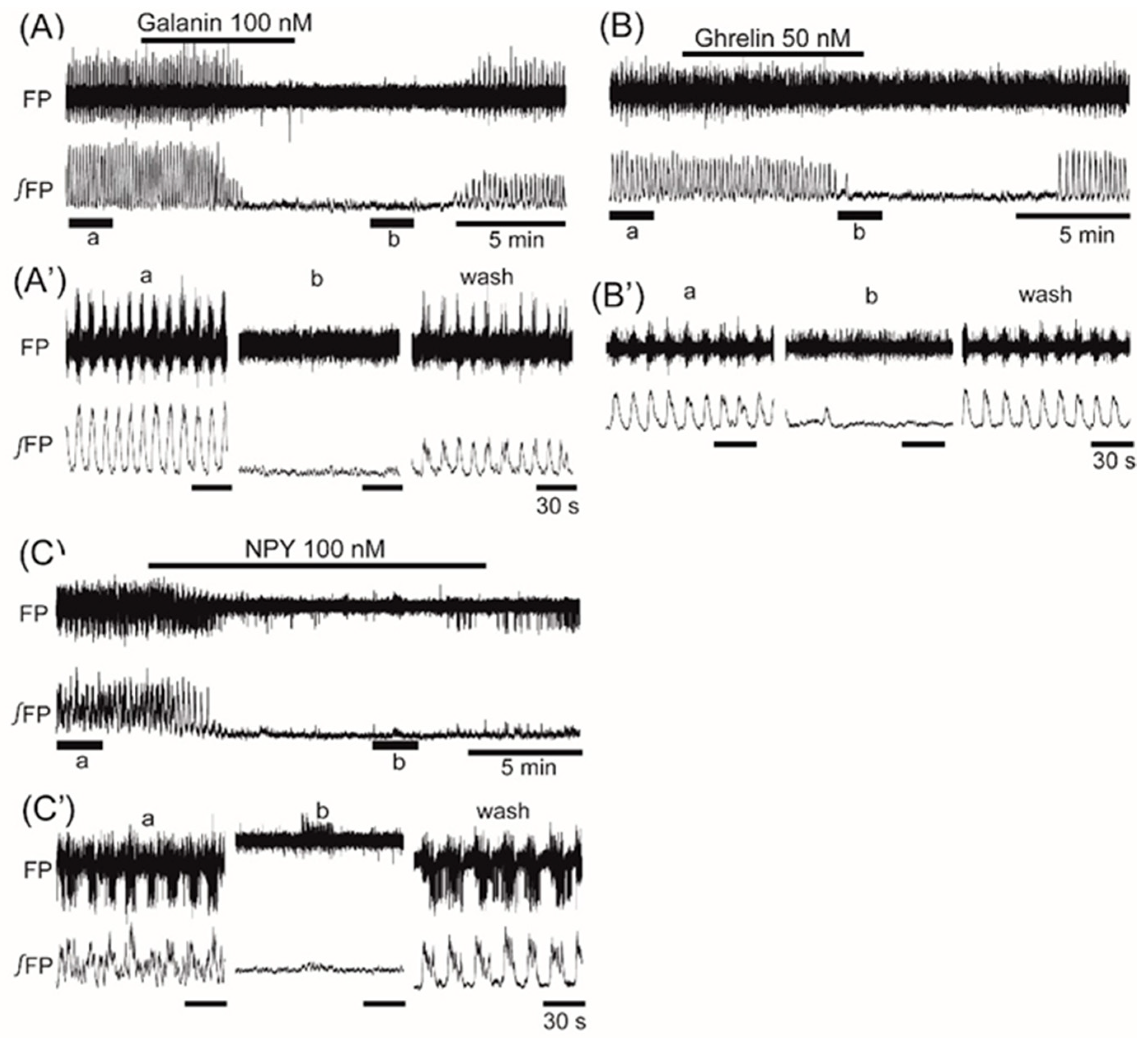
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, G.A. Reciprocal relation of food intake and sympathetic activity: Experimental observations and clinical implications. Int. J. Obes. 2000, 24 (Suppl. 2), S8–S17. [Google Scholar] [CrossRef]
- Song, Z.; Levin, B.E.; McArdle, J.J.; Bakhos, N.; Routh, V.H. Convergence of pre- and postsynaptic influences on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes 2001, 50, 2673–2681. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, T.; Minokoshi, Y. Systemic glucoregulation by glucose-sensing neurons in the ventromedial hypothalamic nucleus (VMH). J. Endocr. Soc. 2017, 1, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.A.; Bloom, S.R. Hypothalamic neuropeptides and the regulation of appetite. Neuropharmacology 2012, 63, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Paranjape, S.A.; Chan, O.; Zhu, W.; Horblitt, A.M.; Grillo, C.A.; Wilson, S.; Reagan, L.; Sherwin, R.S. Chronic reduction of insulin receptors in the ventromedial hypothalamus produces glucose intolerance and islet dysfunction in the absence of weight gain. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E978–E983. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.M.; Adam, C.L.; Mercer, J.G.; Moar, K.M.; Slater, D.; Hunter, L.; Findlay, P.A.; Hoggard, N. Leptin receptor and neuropeptide Y gene expression in the sheep brain. J. Neuroendocrinol. 1999, 11, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Shiuchi, T.; Haque, M.S.; Okamoto, S.; Inoue, T.; Kageyama, H.; Lee, S.; Toda, C.; Suzuki, A.; Bachman, E.S.; Kim, Y.B.; et al. Hypothalamic orexin stimulates feeding-associated glucose utilization in skeletal muscle via sympathetic nervous system. Cell Metab. 2009, 10, 466–480. [Google Scholar] [CrossRef] [PubMed]
- Crawley, J.N.; Austin, M.C.; Fiske, S.M.; Martin, B.; Consolo, S.; Berthold, M.; Langel, U.; Fisone, G.; Bartfai, T. Activity of centrally administered galanin fragments on stimulation of feeding behavior and on galanin receptor binding in the rat hypothalamus. J. Neurosci. 1990, 10, 3695–3700. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, H.; Morita, T.; Kim, J.; Yoshida, K.; Nakajima, K.; Oomura, Y.; Wayner, M.J.; Sasaki, K. Effects of ghrelin on neuronal activity in the ventromedial nucleus of the hypothalamus in infantile rats: An in vitro study. Peptides 2008, 29, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Roubos, E.W.; Lazar, G.; Calle, M.; Barendregt, H.P.; Gaszner, B.; Kozicz, T. Brain distribution and evidence for both central and neurohormonal actions of cocaine- and amphetamine-regulated transcript peptide in Xenopus laevis. J. Comp. Neurol. 2008, 507, 1622–1638. [Google Scholar] [CrossRef] [PubMed]
- Sorimachi, M.; Yamagami, K.; Uramura, K. Functional expression of cholecystokinin-A receptor on ventromedial hypothalamic neurons in the immature rat brain. Neurosci. Lett. 2001, 300, 91–94. [Google Scholar] [CrossRef]
- Cheng, H.; Zhou, L.; Zhu, W.; Wang, A.; Tang, C.; Chan, O.; Sherwin, R.S.; McCrimmon, R.J. Type 1 corticotropin-releasing factor receptors in the ventromedial hypothalamus promote hypoglycemia-induced hormonal counterregulation. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E705–E712. [Google Scholar] [CrossRef] [PubMed]
- Hawke, Z.; Ivanov, T.R.; Bechtold, D.A.; Dhillon, H.; Lowell, B.B.; Luckman, S.M. PACAP neurons in the hypothalamic ventromedial nucleus are targets of central leptin signaling. J. Neurosci. 2009, 29, 14828–14835. [Google Scholar] [CrossRef] [PubMed]
- Cassaglia, P.A.; Hermes, S.M.; Aicher, S.A.; Brooks, V.L. Insulin acts in the arcuate nucleus to increase lumbar sympathetic nerve activity and baroreflex function in rats. J. Physiol. 2011, 589, 1643–1662. [Google Scholar] [CrossRef] [PubMed]
- Tanida, M.; Hayata, A.; Shintani, N.; Yamamoto, N.; Kurata, Y.; Shibamoto, T.; Morgan, D.A.; Rahmouni, K.; Hashimoto, H. Central PACAP mediates the sympathetic effects of leptin in a tissue-specific manner. Neuroscience 2013, 238, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A. Peptides affect the intake of specific nutrients and the sympathetic nervous system. Am. J. Clin. Nutr. 1992, 55, 265S–271S. [Google Scholar] [CrossRef] [PubMed]
- Iigaya, K.; Okazaki, S.; Minoura, Y.; Onimaru, H. Interaction between novel oscillation within the ventromedial hypothalamus and the sympathetic nervous system. Neuroscience 2017, 343, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Silver, I.A.; Erecinska, M. Extracellular glucose concentration in mammalian brain: Continuous monitoring of changes during increased neuronal activity and upon limitation in oxygen supply in normo-, hypo-, and hyperglycemic animals. J. Neurosci. 1994, 14, 5068–5076. [Google Scholar] [CrossRef] [PubMed]
- An, J.H.; Su, Y.; Radman, T.; Bikson, M. Effects of glucose and glutamine concentration in the formulation of the artificial cerebrospinal fluid (ACSF). Brain Res. 2008, 1218, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Funahashi, H.; Yada, T.; Muroya, S.; Takigawa, M.; Ryushi, T.; Horie, S.; Nakai, Y.; Shioda, S. The effect of leptin on feeding-regulating neurons in the rat hypothalamus. Neurosci. Lett. 1999, 264, 117–120. [Google Scholar] [CrossRef]
- Azhdari-Zarmehri, H.; Semnanian, S.; Fathollahi, Y. Orexin-a modulates firing of rat rostral ventromedial medulla neurons: An in vitro study. Cell J. 2015, 17, 163–170. [Google Scholar] [PubMed]
- Takei, H.; Fujita, S.; Shirakawa, T.; Koshikawa, N.; Kobayashi, M. Insulin facilitates repetitive spike firing in rat insular cortex via phosphoinositide 3-kinase but not mitogen activated protein kinase cascade. Neuroscience 2010, 170, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Wang, Y.; Park, S.; Bajpayee, N.S.; Vi, D.; Nagaoka, Y.; Birnbaumer, L.; Jiang, M. Go2 G protein mediates galanin inhibitory effects on insulin release from pancreatic beta cells. Proc. Natl. Acad. Sci. USA 2012, 109, 2636–2641. [Google Scholar] [CrossRef] [PubMed]
- Michel, S.; Itri, J.; Han, J.H.; Gniotczynski, K.; Colwell, C.S. Regulation of glutamatergic signalling by PACAP in the mammalian suprachiasmatic nucleus. BMC Neurosci. 2006, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Rajamanickam, S.; Justice, N.J. Local corticotropin-releasing factor signaling in the hypothalamic paraventricular nucleus. J. Neurosci. 2018, 38, 1874–1890. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, S.; Kawasaki, K. Electrophysiological changes in rat hippocampal pyramidal neurons produced by cholecystokinin octapeptide. Neuroscience 1997, 78, 1005–1016. [Google Scholar] [CrossRef]
- True, C.; Verma, S.; Grove, K.L.; Smith, M.S. Cocaine- and amphetamine-regulated transcript is a potent stimulator of GnRH and kisspeptin cells and may contribute to negative energy balance-induced reproductive inhibition in females. Endocrinology 2013, 154, 2821–2832. [Google Scholar] [CrossRef] [PubMed]
- Hassouna, R.; Zizzari, P.; Viltart, O.; Yang, S.K.; Gardette, R.; Videau, C.; Badoer, E.; Epelbaum, J.; Tolle, V. A natural variant of obestatin, Q90L, inhibits ghrelin’s action on food intake and GH secretion and targets NPY and GHRH neurons in mice. PLoS ONE 2012, 7, e51135. [Google Scholar] [CrossRef] [PubMed]
- Giesbrecht, C.J.; Mackay, J.P.; Silveira, H.B.; Urban, J.H.; Colmers, W.F. Countervailing modulation of Ih by neuropeptide Y and corticotrophin-releasing factor in basolateral amygdala as a possible mechanism for their effects on stress-related behaviors. J. Neurosci. 2010, 30, 16970–16982. [Google Scholar] [CrossRef] [PubMed]
- Antunes, V.R.; Brailoiu, G.C.; Kwok, E.H.; Scruggs, P.; Dun, N.J. Orexins/hypocretins excite rat sympathetic preganglionic neurons in vivo and in vitro. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, R1801–R1807. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.; Hauger, R.; Brown, M. Central corticotropin-releasing hormone activates the sympathetic nervous system and reduces immune function: Increased responsivity of the aged rat. Endocrinology 1992, 131, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Satoh, N.; Ogawa, Y.; Katsuura, G.; Numata, Y.; Tsuji, T.; Hayase, M.; Ebihara, K.; Masuzaki, H.; Hosoda, K.; Yoshimasa, Y.; et al. Sympathetic activation of leptin via the ventromedial hypothalamus: Leptin-induced increase in catecholamine secretion. Diabetes 1999, 48, 1787–1793. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, K.; Tsuchihashi, T.; Abe, I. Central human cocaine- and amphetamine-regulated transcript peptide 55-102 increases arterial pressure in conscious rabbits. Hypertension 2001, 38, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Yoshimatsu, H.; Egawa, M.; Bray, G.A. Effects of cholecystokinin on sympathetic activity to interscapular brown adipose tissue. Brain Res. 1992, 597, 298–303. [Google Scholar] [CrossRef]
- Jokiaho, A.J.; Donovan, C.M.; Watts, A.G. The rate of fall of blood glucose determines the necessity of forebrain-projecting catecholaminergic neurons for male rat sympathoadrenal responses. Diabetes 2014, 63, 2854–2865. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, K.; Tsuchihashi, T.; Fujii, K.; Abe, I.; Iida, M. Central ghrelin modulates sympathetic activity in conscious rabbits. Hypertension 2002, 40, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Masaki, T.; Kakuma, T.; Yoshimatsu, H. Centrally administered ghrelin suppresses sympathetic nerve activity in brown adipose tissue of rats. Neurosci. Lett. 2003, 349, 75–78. [Google Scholar] [CrossRef]
- Sabatier, N.; Leng, G. Responses to cholecystokinin in the ventromedial nucleus of the rat hypothalamus in vivo. Eur. J. Neurosci. 2010, 31, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Sohn, J.W.; Oh, Y.; Kim, K.W.; Lee, S.; Williams, K.W.; Elmquist, J.K. Leptin and insulin engage specific PI3K subunits in hypothalamic SF1 neurons. Mol. Metab. 2016, 5, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, H.; Zigman, J.M.; Ye, C.; Lee, C.E.; McGovern, R.A.; Tang, V.; Kenny, C.D.; Christiansen, L.M.; White, R.D.; Edelstein, E.A.; et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 2006, 49, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.H.; Chua, S.C., Jr. The brain-liver connection between BDNF and glucose control. Diabetes 2013, 62, 1367–1368. [Google Scholar] [CrossRef] [PubMed]
- Klöckener, T.; Hess, S.; Belgardt, B.F.; Paeger, L.; Verhagen, L.A.; Husch, A.; Sohn, J.W.; Hampel, B.; Dhillon, H.; Zigman, J.M.; et al. High-fat feeding promotes obesity via insulin receptor/PI3K-dependent inhibition of SF-1 VMH neurons. Nat. Neurosci. 2011, 14, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, D.; Chen, P.; Li, C. Conditional viral tracing reveals that steroidogenic factor 1-positive neurons of the dorsomedial subdivision of the ventromedial hypothalamus project to autonomic centers of the hypothalamus and hindbrain. J. Comp. Neurol. 2013, 521, 3167–3190. [Google Scholar] [CrossRef] [PubMed]
- Marsh, A.J.; Fontes, M.A.; Killinger, S.; Pawlak, D.B.; Polson, J.W.; Dampney, R.A. Cardiovascular responses evoked by leptin acting on neurons in the ventromedial and dorsomedial hypothalamus. Hypertension 2003, 42, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Yoshimatsu, H.; Egawa, M.; Bray, G.A. Sympathetic nerve activity after discrete hypothalamic injections of L-glutamate. Brain Res. 1993, 601, 121–128. [Google Scholar] [CrossRef]
- Takahashi, A.; Ishimaru, H.; Ikarashi, Y.; Kishi, E.; Maruyama, Y. Effects of ventromedial hypothalamus stimulation on glycogenolysis in rat liver using in vivo microdialysis. Metabolism 1997, 46, 897–901. [Google Scholar] [CrossRef]
- Fioramonti, X.; Song, Z.; Vazirani, R.P.; Beuve, A.; Routh, V.H. Hypothalamic nitric oxide in hypoglycemia detection and counterregulation: A two-edged sword. Antioxid. Redox Signal. 2010, 14, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Garfield, A.S.; Shah, B.P.; Madara, J.C.; Burke, L.K.; Patterson, C.M.; Flak, J.; Neve, R.L.; Evans, M.L.; Lowell, B.B.; Myers, M.G., Jr.; et al. A parabrachial-hypothalamic cholecystokinin neurocircuit controls counterregulatory responses to hypoglycemia. Cell Metab. 2014, 20, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Flak, J.N.; Patterson, C.M.; Garfield, A.S.; D’Agostino, G.; Goforth, P.B.; Sutton, A.K.; Malec, P.A.; Wong, J.T.; Germani, M.; Jones, J.C.; et al. Leptin-inhibited PBN neurons enhance responses to hypoglycemia in negative energy balance. Nat. Neurosci. 2014, 17, 1744–1750. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, E.A.; Okamoto, S.; Ishikawa, A.W.; Yokota, S.; Wada, N.; Hirabayashi, T.; Saito, K.; Sato, T.; Takagi, K.; Wang, C.C.; et al. Activation of SF1 neurons in the ventromedial hypothalamus by DREADD technology increases insulin sensitivity in peripheral tissues. Diabetes 2017, 66, 2372–2386. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iigaya, K.; Minoura, Y.; Onimaru, H.; Kotani, S.; Izumizaki, M. Effects of Feeding-Related Peptides on Neuronal Oscillation in the Ventromedial Hypothalamus. J. Clin. Med. 2019, 8, 292. https://doi.org/10.3390/jcm8030292
Iigaya K, Minoura Y, Onimaru H, Kotani S, Izumizaki M. Effects of Feeding-Related Peptides on Neuronal Oscillation in the Ventromedial Hypothalamus. Journal of Clinical Medicine. 2019; 8(3):292. https://doi.org/10.3390/jcm8030292
Chicago/Turabian StyleIigaya, Kamon, Yoshino Minoura, Hiroshi Onimaru, Sayumi Kotani, and Masahiko Izumizaki. 2019. "Effects of Feeding-Related Peptides on Neuronal Oscillation in the Ventromedial Hypothalamus" Journal of Clinical Medicine 8, no. 3: 292. https://doi.org/10.3390/jcm8030292
APA StyleIigaya, K., Minoura, Y., Onimaru, H., Kotani, S., & Izumizaki, M. (2019). Effects of Feeding-Related Peptides on Neuronal Oscillation in the Ventromedial Hypothalamus. Journal of Clinical Medicine, 8(3), 292. https://doi.org/10.3390/jcm8030292



