Early Effects of Sacubitril/Valsartan on Exercise Tolerance in Patients with Heart Failure with Reduced Ejection Fraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection and Study Design
2.2. CPET Protocol
2.3. Statistical Analyses
3. Results
3.1. Patients Characteristics
3.2. CPET, NT-ProBNP, and Left Ventricular Function
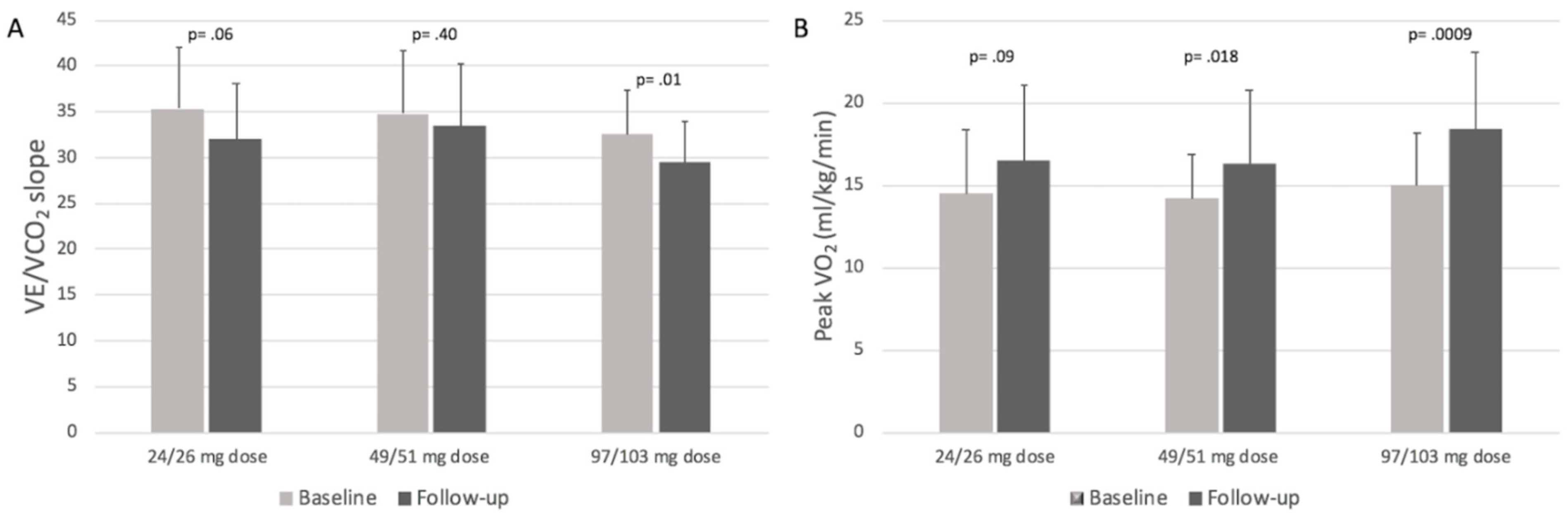
3.3. CPET Results Stratified by Sacubitril/Valsartan Dosages
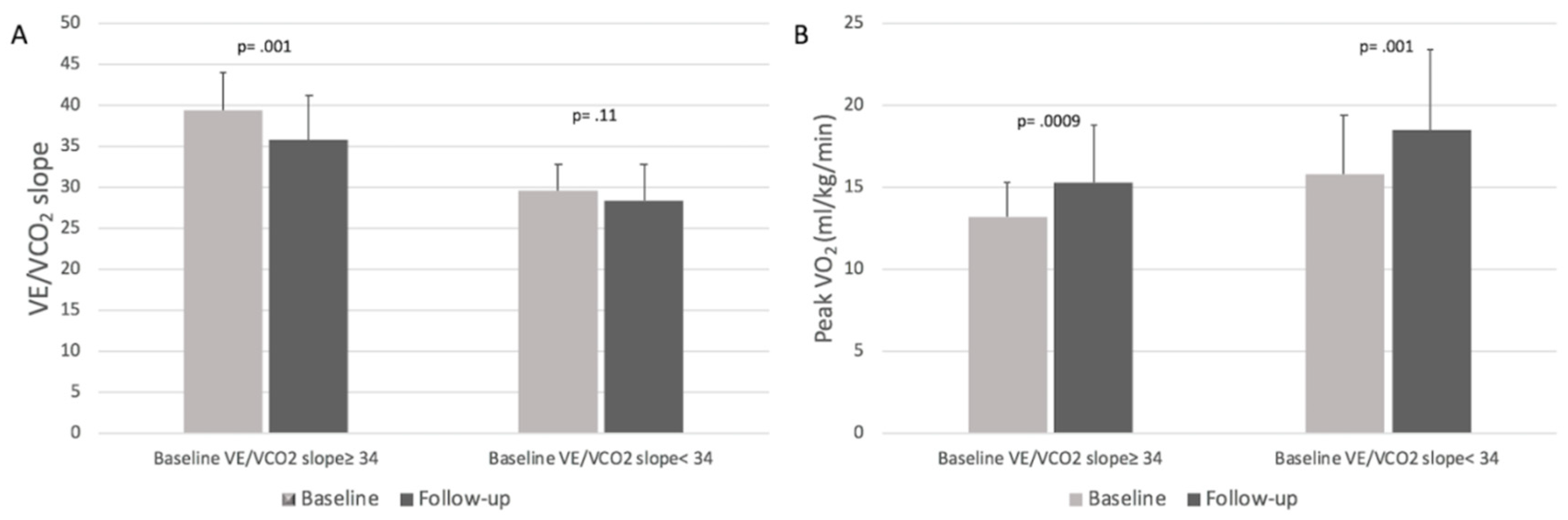
3.4. CPET Results Stratified by Baseline VE/VCO2 Slope Values
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Acknowledgements
Conflicts of Interest
Abbreviations List
| AT-VO2 | oxygen consumption at anaerobic threshold |
| CPET | cardiopulmonary exercise test |
| HF | heart failure |
| HFrEF | heart failure with reduced ejection fraction |
| LVEF | left ventricular ejection fraction |
| NYHA | New York Heart Association |
| NT-proBNP | N-terminal pro-brain natriuretic peptides |
| RER | respiratory exchange ratio |
| VE/VCO2 | ventilation/carbon dioxide production relationship |
| VO2 | oxygen consumption |
References
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.; Swedberg, K.; et al. PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Rodil Fraile, R.; Malafarina, V.; Lopez, G.T. Sacubitril/valsartan in heart failure and multimorbidity patients. ESC Heart Fail. 2018, 5, 957–960. [Google Scholar] [CrossRef]
- Sgorbini, L.; Rossetti, A.; Galati, A. Sacubitril/valsartan: Effect on walking test and physical capability. Cardiology 2017, 138, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, P.; Palau, P.; Domínguez, E.; Faraudo, M.; Núñez, E.; Guri, O.; Mollar, A.; Sanchis, J.; Bayés-Genís, A.; Núñez, J. Sacubitril/valsartan and short-term changes in the 6-min walk test: A pilot study. Int. J. Cardiol. 2018, 252, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Palau, P.; Mollar, A.; Dominguez, E.; Sanchis, J.; Bayés-Genís, A.; Núñez, J. Early Sacubitril/valsartan-driven benefit on exercise capacity in heart failure with reduced ejection fraction: A pilot study. Rev. Esp. Cardiol. 2019, 72, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Mancini, D.M.; Eisen, H.; Kussmaul, W.; Mull, R.; Edmunds, L.H., Jr.; Wilson, J.R. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation 1991, 83, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.; Gullestad, L.; Vagelos, R.; Do, D.; Bellin, D.; Ross, H.; Fowler, M.B. Clinical, hemodynamic, and cardiopulmonary exercise test determinants of survival in patients referred for evaluation of heart failure. Ann. Intern. Med. 1998, 129, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.; Gullestad, L. The role of exercise testing and gas-exchange measurement in the prognostic assessment of patients with heart failure. Curr. Opin. Cardiol. 1998, 13, 145–155. [Google Scholar] [PubMed]
- Stelken, A.M.; Younis, L.T.; Jennison, S.H.; Miller, D.D.; Miller, L.W.; Shaw, L.J.; Kargl, D.; Chaitman, B.R. Prognostic value of cardiopulmonary exercisetesting using percent achieved of predicted peak oxygen uptake for patients with ischemic and dilated cardiomyopathy. J. Am. Coll. Cardiol. 1996, 27, 345–352. [Google Scholar] [CrossRef]
- Corrà, U.; Agostoni, P.G.; Anker, S.D.; Coats, A.J.S.; Crespo Leiro, M.G.; de Boer, R.A.; Harjola, V.P.; Hill, L.; Lainscak, M.; Lund, L.H. Role of cardiopulmonary exercise testing in clinical stratification in heart failure. A position paper from the Committee on Exercise Physiology and Training of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 3–15. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; Gonzalez-Juanately, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Corrà, U.; Adamopoulos, S.; Benzer, W.; Bjarnason-Wehrens, B.; Cupples, M.; Dendale, P.; Doherty, P.; Gaita, D.; Höfer, S.; et al. Secondary prevention in the clinical management of patients with cardiovascular diseases. Core components, standards and outcome measures for referral and delivery. Eur. J. Prev. Cardiol. 2014, 21, 664–681. [Google Scholar] [CrossRef]
- Wasserman, K.; Hansen, J.E.; Sue, D.Y.; Stringer, W.; Whipp, B.J. Normal Values. In Principles of Exercise Testing and Interpretation, 4th ed.; Weinberg, R., Ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2005; pp. 160–182. [Google Scholar]
- Guazzi, M.; Arena, R.; Halle, M.; Piepoli, M.F.; Myers, J.; Lavie, C.J. 2016 Focused Update: Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation 2016, 133, e694–711. [Google Scholar] [CrossRef]
- Contini, M. Cardiopulmonary test as a tool to choose therapy in heart failure. Ann. Am. Thorac. Soc. 2017, 14, S67–S73. [Google Scholar] [CrossRef]
- Myers, J.; Arena, R.; Cahalin, L.P.; Labate, V.; Guazzi, M. Cardiopulmonary Exercise Testing in Heart Failure. Curr. Probl. Cardiol. 2015, 40, 322–372. [Google Scholar] [CrossRef]
- Swank, A.M.; Horton, J.; Fleg, J.L.; Fonarow, G.C.; Keteyian, S.; Goldberg, L.; Wolfel, G.; Handberg, E.M.; Bensimhon, D.; Illiou, M.C.; et al. HF-ACTION Investigators. Modest increase in peak VO2 is related to better clinical outcomes in chronic heart failure patients: Results from heart failure and a controlled trial to investigate outcomes of exercise training. Circ. Heart Fail. 2012, 5, 579–585. [Google Scholar] [CrossRef]
- Arena, R.; Myers, J.; Aslam, S.S.; Varughese, E.B.; Peberdy, M.A. Peak VO2 and VE/VCO2 slope in patients with heart failure: A prognostic comparison. Am. Heart J. 2004, 147, 354–360. [Google Scholar] [CrossRef]
- Francis, D.P.; Shamim, W.; Davies, L.C.; Piepoli, M.F.; Ponikowski, P.; Anker, S.D.; Coats, A.J. Cardiopulmonary exercise testing for prognosis in chronic heart failure: Continuous and independent prognostic value from VE/VCO(2)slope and peak VO(2). Eur. Heart J. 2000, 21, 154–161. [Google Scholar] [CrossRef]
- Robbins, M.; Francis, G.; Pashkow, F.J.; Snader, C.E.; Hoercher, K.; Young, J.B.; Lauer, M.S. Ventilatory and heart rate responses to exercise: Better predictors of heart failure mortality than peak oxygen consumption. Circulation 1999, 100, 2411–2417. [Google Scholar] [CrossRef]
- Corra, U.; Mezzani, A.; Bosimini, E.; Scapellato, F.; Imparato, A.; Giannuzzi, P. Ventilatory response to exercise improves risk stratification in patients with chronic heart failure and intermediate functional capacity. Am. Heart J. 2002, 143, 418–426. [Google Scholar] [CrossRef]
- Kleber, F.X.; Vietzke, G.; Wernecke, K.D.; Bauer, U.; Opitz, C.; Wensel, R.; Sperfeld, A.; Gläser, S. Impairment of ventilatory efficiency in heart failure: Prognostic impact. Circulation 2000, 101, 2803–2809. [Google Scholar] [CrossRef]
- Sarullo, F.M.; Fazio, G.; Brusca, I.; Fasullo, S.; Paterna, S.; Licata, P.; Novo, G.; Novo, S.; Di Pasquale, P. Cardiopulmonary exercise testing in patients with chronic heart failure: Prognostic comparison from peak VO2 and VE/VCO2 slope. Open Cardiovasc. Med. J. 2010, 4, 127–134. [Google Scholar] [CrossRef]
- MacGowan, G.A.; Janosko, K.; Cecchetti, A.; Murali, S. Exercise-related ventilatory abnormalities and survival in congestive heart failure. Am. J. Cardiol. 1997, 79, 1264–1266. [Google Scholar] [CrossRef]
- Vardeny, O.; Claggett, B.; Packer, M.; Zile, M.R.; Rouleau, J.; Swedberg, K.; Teerlink, J.R.; Desai, A.S.; Lefkowitz, M.; Shi, V.; et al. Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: The PARADIGM-HF trial. Eur. J. Heart Fail. 2016, 18, 1228–1234. [Google Scholar] [CrossRef]
- Bayés-Genís, A.; Barallat, J.; Richards, A.M. A test in context: Neprilysin: Function, inhibition, and biomarker. J. Am. Coll. Cardiol. 2016, 68, 639–653. [Google Scholar] [CrossRef]
- D’Elia, E.; Iacovoni, A.; Vaduganathan, M.; Lorini, F.L.; Perlini, S.; Senni, M. Neprilysin inhibition in heart failure: Mechanisms and substrates beyond modulating natriuretic peptides. Eur. J. Heart Fail. 2017, 19, 710–717. [Google Scholar] [CrossRef]
- Martens, P.; Beliën Dupont, M.; Vandervoort, P.; Mullens, W. The reverse remodeling response to Sacubitril/valsartan therapy in heart failure with reduced ejection fraction. Cardiovasc. Ther. 2018, 36, e12435. [Google Scholar] [CrossRef]
- Almufleh, A.; Marbach, J.; Chih, S.; Stadnick, E.; Davies, R.; Liu, P.; Mielniczuk, L. Ejection fraction improvement and reverse remodeling achieved with Sacubitril/valsartan in heart failure with reduced ejection fraction patients. Am. J. Cardiovasc. Dis. 2017, 7, 108–113. [Google Scholar]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017, 136, e137–e161. [Google Scholar] [CrossRef]

| Demographics | |
|---|---|
| Age, year, mean ± SD | 58.7 ± 9.3 |
| Female sex, no. (%) | 14 (14) |
| SBP, mmHg, mean ± SD | 117 ± 14 |
| DBP, mmHg, mean ± SD | 72 ± 10 |
| Heart rate, beats/min, mean ± SD | 67 ± 11 |
| Body mass index, kg/m2, mean ± SD | 28.1 ± 4.2 |
| Medical History | |
| Hypertension, no. (%) | 51 (51) |
| Diabetes, no. (%) | 34 (34) |
| Atrial fibrillation, no. (%) | 17 (17) |
| COPD, no. (%) | 10 (10) |
| eGFR, mL/min/1.73m2, mean ± SD | 67.8 ± 23.7 |
| Nt-pro-BNP, median (IQ range) | 1200 (446–2120) |
| LVEF (%), mean ± SD | 27 ± 6 |
| LVEDV, mL, mean ± SD | 218 ± 57 |
| LVESV, mL, mean ± SD | 153 ± 56 |
| Ischemic cardiomyopathy, no. (%) | 51 (51) |
| Non-ischemic cardiomyopathy, no. (%) | 48 (49) |
| NYHA functional class II, no. (%) | 62 (63) |
| NYHA functional class III, no. (%) | 37 (37) |
| NYHA functional class IV, no. (%) | 0 (0) |
| Medical Therapy | |
| Furosemide, no. (%) | 88 (89) |
| Furosemide dosage, mean ± SD | 102 ± 105 |
| Antialdosterone, no. (%) | 87 (88) |
| ACE-inhibitors, no. (%) | 62 (63) |
| ARBs, no. (%) | 25 (25) |
| Beta-blockers, no. (%) | 93 (94) |
| Ivabradine, no. (%) | 20 (20) |
| Digoxin, no. (%) | 7 (7) |
| Implantable cardioverter defibrillator, no. (%) | 76 (77) |
| Cardiac resynchronization therapy, no. (%) | 22 (22) |
| Sacubitril/Valsartan 24/26 mg 28 pts | Sacubitril/Valsartan 97/103 mg 34 pts | P value | |
|---|---|---|---|
| Baseline Characteristics | |||
| Age, year, mean ± SD | 57.8 ± 10.8 | 57.4 ± 8.6 | 0.87 |
| Female sex, no. (%) | 7 (25) | 2 (6) | 0.06 |
| Ischemic cardiomyopathy, no. (%) | 14 (50) | 18 (53) | 0.99 |
| NYHA II, no. (%) | 14 (50) | 27 (79) | 0.018 |
| NYHA III, no. (%) | 14 (50) | 7 (21) | 0.018 |
| Diabetes, no. (%) | 7 (25) | 10 (29) | 0.77 |
| Atrial fibrillation, no. (%) | 6 (21) | 2 (6) | 0.12 |
| eGFR (MDRD), ml/min/1.73m2, mean ± SD | 63.3 ± 21.6 | 72.6 ± 16.7 | 0.07 |
| Furosemide dose, mean ± SD | 108 ± 126 | 63 ± 95 | 0.03 |
| Implantable cardioverter defibrillator, no. (%) | 22 (78) | 24 (70) | 0.56 |
| Cardiac resynchronization therapy, no. (%) | 8 (28) | 8 (23) | 0.77 |
| SBP, NT-pro-BNP, EDV, ESV, and LVEF (Baseline and Follow-up Data) | |||
| SBP, mmHg, mean ± SD (Baseline) | 114.3 ± 12.1 | 120.5 ± 14.7 | 0.07 |
| SBP, mmHg, mean ± SD (Follow-up) | 96 ± 11 | 105 ± 12 | 0.004 |
| Nt-pro-BNP, median (IQ range) (Baseline) | 1623.5 (477–2947) | 815 (358–1929) | 0.013 |
| Nt-pro-BNP, median (IQ range) (Follow-up) | 1065 (376–1739) | 394.5 (195–952) | 0.01 |
| LVEDV, ml, mean±SD (Baseline) | 208 ± 54 | 222 ± 55 | 0.31 |
| LVEDV, ml, mean±SD (Follow-up) | 209 ± 56 | 209 ± 59 | 0.98 |
| LVESV, ml, mean±SD (Baseline) | 147 ± 57 | 161 ± 48 | 0.29 |
| LVESV, ml, mean±SD (Follow-up) | 146 ± 57 | 143 ± 50 | 0.89 |
| LVEF (%), mean ± SD (Baseline) | 28.1 ± 5.7 | 28.3 ± 5.1 | 0.88 |
| LVEF (%), mean ± SD (Follow-up) | 28.6 ± 6.3 | 32.3 ± 6.6 | 0.026 |
| Baseline | Follow-up | p value | |
|---|---|---|---|
| Peak VO2, mL/kg/min, mean ± SD | 14.6 ± 3.3 | 17.2 ± 4.7 | <0.0001 |
| Predicted peak VO2, %, mean ± SD | 53.8 ± 14.1 | 64.7 ± 17.8 | <0.0001 |
| VE/VCO2 slope, mean ± SD | 34.1 ± 6.3 | 31.7 ± 6.1 | 0.006 |
| VE/VCO2 slope≥ 34, no. (%) | 46 (46) | 33 (33) | 0.08 |
| Peak RER, mean ± SD | 1.12 ± 0.09 | 1.13 ± 0.09 | 0.45 |
| Watt (Peak), mean ± SD | 70 ± 22 | 88 ± 29 | <0.0001 |
| AT VO2, mL/kg/min, mean ± SD | 11.3 ± 2.6 | 12.6 ± 3.5 | 0.007 |
| Predicted AT VO2, %, mean ± SD | 42.3 ± 11.5 | 47.2 ± 12.5 | 0.009 |
| AT undetectable, no. (%), mean ± SD | 16 (16) | 9 (9) | 0.19 |
| O2pulse (ml/beat) | 11.5 ± 3.0 | 13.4 ± 4.3 | 0.0007 |
| ∆VO2/∆work, mL/min/watt, mean ± SD | 9.2 ± 1.5 | 10.1 ± 1.8 | 0.0002 |
| Peak ventilation, L/min, mean ± SD | 48.7 ± 12.7 | 59.3 ± 18.9 | <0.0001 |
| Peak tidal volume, L, mean ± SD | 1.57 ± 0.43 | 1.75 ± 0.53 | 0.009 |
| Peak Respiratory rate, b/m, mean ± SD | 30.5 ± 6.7 | 33.3 ± 7.2 | 0.006 |
| Ventilatory Oscillation, no. (%) | 31 (31) | 19 (19) | 0.07 |
| Baseline | Follow-up | p value | |
|---|---|---|---|
| Peak VO2, mL/kg/min, mean ± SD | |||
| 24/26 mg dose (28 pts) | 14.5 ± 3.9 | 16.5 ± 4.6 | 0.09 |
| 49/51 mg dose (37 pts) | 14.2 ± 2.7 | 16.3 ± 4.5 | 0.018 |
| 97/103 mg dose (34 pts) | 15 ± 3.2 | 18.4 ± 4.7 | 0.0009 |
| Predicted peak VO2, %, mean ± SD | |||
| 24/26 mg dose (28 pts) | 54 ± 12.9 | 62.1 ± 14.1 | 0.029 |
| 49/51 mg dose (37 pts) | 53.8 ± 13.9 | 61.9 ± 16.6 | 0.02 |
| 97/103 mg dose (34 pts) | 53.6 ± 15.6 | 68.6 ± 20.6 | 0.001 |
| VE/VCO2 slope, mean ± SD | |||
| 24/26 mg dose (28 pts) | 35.3 ± 6.8 | 32 ± 6.1 | 0.06 |
| 49/51 mg dose (37 pts) | 34.8 ± 6.9 | 33.4 ± 6.9 | 0.4 |
| 97/103 mg dose (34 pts) | 32.5 ± 4.9 | 29.5 ± 4.5 | 0.01 |
| O2 pulse, ml/beat, mean ± SD | |||
| 24/26 mg dose (28 pts) | 11.4 ± 3.1 | 12.8 ± 4.3 | 0.016 |
| 49/51 mg dose (37 pts) | 11 ± 3.1 | 12.3 ± 3.9 | 0.12 |
| 97/103 mg dose (34 pts) | 12.2 ± 2.8 | 14.9 ± 4.4 | 0.003 |
| ∆VO2/∆work, mL/min/watt, mean ± SD | |||
| 24/26 mg dose (28 pts) | 9.1 ± 1.3 | 9.7 ± 2.2 | 0.24 |
| 49/51 mg dose (37 pts) | 9 ± 1.6 | 9.9 ± 1.8 | 0.028 |
| 97/103 mg dose (34 pts) | 9.3 ± 1.5 | 10.5 ± 1.4 | 0.001 |
| Baseline | Follow-up | p value | |
|---|---|---|---|
| Peak VO2, mL/kg/min, mean ± SD | |||
| 3 months (24 pts) | 15.3 ± 3 | 16.9 ± 4.1 | 0.12 |
| 6 months (40 pts) | 14.8 ± 3.6 | 17.1 ± 5 | 0.02 |
| 12 months (35 pts) | 13.8 ± 3 | 17.3 ± 4.6 | 0.0006 |
| Predicted peak VO2, %, mean ± SD | |||
| 3 months (24 pts) | 54.9 ± 9.5 | 61.3 ± 13.1 | 0.06 |
| 6 months (40 pts) | 56.7 ± 14.5 | 66.9 ± 17.2 | 0.0005 |
| 12 months (35 pts) | 49.7 ± 15.6 | 63.4 ± 20.4 | 0.002 |
| VE/VCO2 slope, mean ± SD | |||
| 3 months (24 pts) | 33.7 ± 6.2 | 33.4 ± 7.8 | 0.9 |
| 6 months (40 pts) | 33.3 ± 6.6 | 31.4 ± 6 | 0.19 |
| 12 months (35 pts) | 35.4 ± 6 | 30.7 ± 4.8 | 0.0006 |
| O2 pulse, ml/beat, mean ± SD | |||
| 3 months (24 pts) | 12.3 ± 3.3 | 13.0 ± 4.2 | 0.52 |
| 6 months (40 pts) | 11.3 ± 3.2 | 13.3 ± 4.3 | 0.023 |
| 12 months (35 pts) | 11.2 ± 2.5 | 13.6 ± 4.5 | 0.007 |
| ∆VO2/∆work, mL/min/watt, mean ± SD | |||
| 3 months (24 pts) | 9.4 ± 1.4 | 10.3 ± 1.7 | 0.09 |
| 6 months (40 pts) | 9.3 ± 1.2 | 10.1 ± 2.1 | 0.042 |
| 12 months (35 pts) | 8.8 ± 1.7 | 9.9 ± 1.7 | 0.007 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitale, G.; Romano, G.; Di Franco, A.; Caccamo, G.; Nugara, C.; Ajello, L.; Storniolo, S.; Sarullo, S.; Agnese, V.; Giallauria, F.; et al. Early Effects of Sacubitril/Valsartan on Exercise Tolerance in Patients with Heart Failure with Reduced Ejection Fraction. J. Clin. Med. 2019, 8, 262. https://doi.org/10.3390/jcm8020262
Vitale G, Romano G, Di Franco A, Caccamo G, Nugara C, Ajello L, Storniolo S, Sarullo S, Agnese V, Giallauria F, et al. Early Effects of Sacubitril/Valsartan on Exercise Tolerance in Patients with Heart Failure with Reduced Ejection Fraction. Journal of Clinical Medicine. 2019; 8(2):262. https://doi.org/10.3390/jcm8020262
Chicago/Turabian StyleVitale, Giuseppe, Giuseppe Romano, Antonino Di Franco, Giuseppa Caccamo, Cinzia Nugara, Laura Ajello, Salvo Storniolo, Silvia Sarullo, Valentina Agnese, Francesco Giallauria, and et al. 2019. "Early Effects of Sacubitril/Valsartan on Exercise Tolerance in Patients with Heart Failure with Reduced Ejection Fraction" Journal of Clinical Medicine 8, no. 2: 262. https://doi.org/10.3390/jcm8020262
APA StyleVitale, G., Romano, G., Di Franco, A., Caccamo, G., Nugara, C., Ajello, L., Storniolo, S., Sarullo, S., Agnese, V., Giallauria, F., Novo, G., Clemenza, F., & Sarullo, F. M. (2019). Early Effects of Sacubitril/Valsartan on Exercise Tolerance in Patients with Heart Failure with Reduced Ejection Fraction. Journal of Clinical Medicine, 8(2), 262. https://doi.org/10.3390/jcm8020262






