The Benefice of Mobile Parts’ Exchange in the Management of Infected Total Joint Arthroplasties with Prosthesis Retention (DAIR Procedure)
Abstract
:1. Introduction
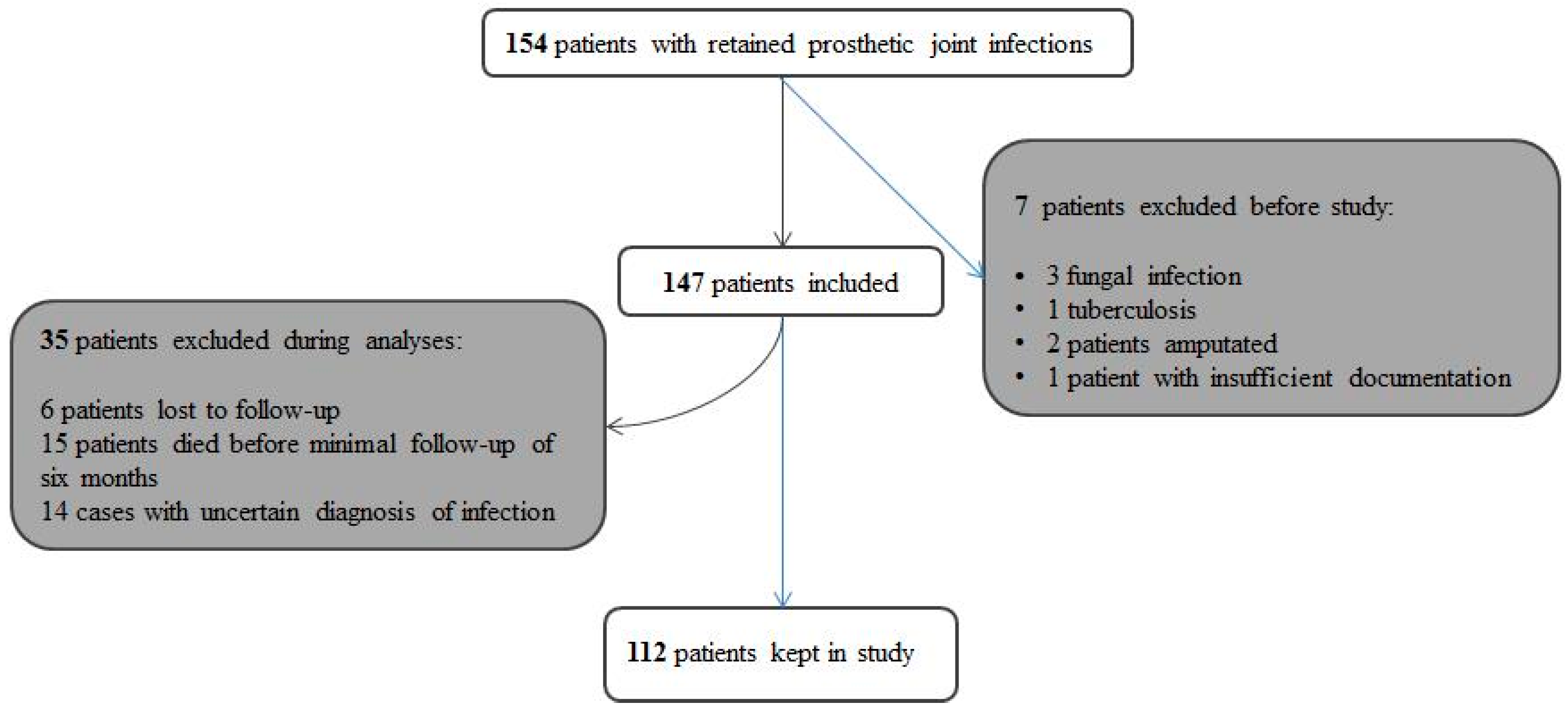
2. Methods
Statistical Analyses
3. Results
3.1. Patients and Pathogens
3.2. Treatment
3.3. Outcomes
4. Discussion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Chaussade, H.; Uçkay, I.; Vuagnat, A.; Druon, J.; Gras, G.; Rosset, P.; Lipsky, B.A.; Bernard, L. Antibiotic therapy duration for prosthetic joint infections treated by Debridement and Implant Retention (DAIR): Similar long-term remission for 6 weeks as compared to 12 weeks. Int. J. Infect. Dis. 2017, 63, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Tsang, S.J.; Ting, J.; Simpson, A.H.R.W.; Gaston, P. Outcomes following debridement, antibiotics and implant retention in the management of periprosthetic infections of the hip: A review of cohort studies. Bone Joint. J. 2017, 99, 1458–1466. [Google Scholar] [CrossRef]
- Moojen, D.J.; Zwiers, J.H.; Scholtes, V.A.; Verheyen, C.C.; Poolman, R.W. Similar success rates for single and multiple debridement surgery for acute hip arthroplasty infection. Acta. Orthop. 2014, 85, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Haasper, C.; Buttaro, M. Irrigation and Debridement. In Proceedings of the International Consensus Meeting on Prosthetic Joint Infection, Philadelphia, PA, USA, 31 July–1 August 2013; pp. 273–285. [Google Scholar]
- Uçkay, I.; Hoffmeyer, P.; Lew, D.; Pittet, D. Prevention of surgical site infections in orthopaedic surgery and bone trauma: State-of-the-art update. J. Hosp. Infect. 2013, 84, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Jugun, K.; Vaudaux, P.; Garbino, J.; Pagani, L.; Hoffmeyer, P.; Lew, D.; Uçkay, I. The safety and efficacy of high-dose daptomycin combined with rifampicin for the treatment of Gram-positive osteoarticular infections. Int. Orthop. 2013, 37, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Sendi, P.; Christensson, B.; Uçkay, I.; Trampuz, A.; Achermann, Y.; Boggian, K.; Svensson, D.; Widerström, M.; Zimmerlim, W.; GBS PJI Study Group. Group B streptococcus in prosthetic hip and knee joint-associated infections. J. Hosp. Infect. 2011, 79, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Bouvet, C.; Lübbeke, A.; Bandi, C.; Pagani, L.; Stern, R.; Hoffmeyer, P.; Uçkay, I. Is there any benefit in pre-operative urinary analysis before elective total joint replacement? Bone Joint. J. 2014, 96, 390–394. [Google Scholar] [CrossRef]
- Ferry, T.; Uçkay, I.; Vaudaux, P.; François, P.; Schrenzel, J.; Harbarth, S.; Laurent, F.; Bernard, L.; Vandenesch, F.; Etienne, J.; et al. Risk factors for treatment failure in orthopedic device-related methicillin-resistant Staphylococcus aureus infection. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 171–180. [Google Scholar] [CrossRef]
- Uçkay, I.; Lübbeke, A.; Harbarth, S.; Emonet, S.; Tovmirzaeva, L.; Agostinho, A.; Longtin, Y.; Peter, R.; Hoffmeyer, P.; Pittet, D. Low risk despite high endemicity of methicillin-resistant Staphylococcus aureus infections following elective total joint arthroplasty: A 12-year experience. Ann. Med. 2012, 44, 360–368. [Google Scholar] [CrossRef]
- Teterycz, D.; Ferry, T.; Lew, D.; Stern, R.; Assal, M.; Hoffmeyer, P.; Bernard, L.; Uçkay, I. Outcome of orthopedic implant infections due to different staphylococci. Int. J. Infect. Dis. 2010, 14, 913–918. [Google Scholar] [CrossRef]
- Schindler, M.; Bernard, L.; Belaieff, W.; Gamulin, A.; Racloz, G.; Emonet, S.; Lew, D.; Hoffmeyer, P.; Uçkay, I. Epidemiology of adverse events and Clostridium difficile-associated diarrhea during long-term antibiotic therapy for osteoarticular infections. J. Infect. 2013, 67, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Rao, N.; Hanssen, A.; Wilson, W.R.; Infectious Diseases Society of America. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Mont, M.A.; Waldman, B.; Banerjee, C.; Pacheco, I.H.; Hungerford, D.S. Multiple irrigation; debridement; and retention of components in infected total knee arthroplasty. J. Arthroplasty 1997, 12, 426–433. [Google Scholar] [CrossRef]
- Marculescu, C.E.; Berbari, E.F.; Hanssen, A.D.; Steckelberg, J.M.; Harmsen, S.W.; Mandrekar, J.N.; Osmon, D.R. Outcome of prosthetic joint infections treated with debridement and retention of components. Clin. Infect. Dis. 2006, 42, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Deirmengian, C.; Greenbaum, J.; Lotke, P.A.; Booth, R.E., Jr.; Lonner, J.H. Limited success with open debridement and retention of components in the treatment of acute Staphylococcus aureus infections after total knee arthroplasty. J. Arthroplasty 2003, 18, 22–26. [Google Scholar] [CrossRef]
- Theis, J.C.; Gambhir, S.; White, J. Factors affecting implant retention in infected joint replacements. ANZ J. Surg. 2007, 77, 877–879. [Google Scholar] [CrossRef] [PubMed]
- Tsumura, H.; Ikeda, S.; Ono, T.; Itonaga, I.; Taira, H.; Torisu, T. Synovectomy, debridement, and continuous irrigation for infected total knee arthroplasty. Int. Orthop. 2005, 29, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Grammatopoulos, G.; Kendrick, B.; McNally, M.; Athanasou, N.A.; Atkins, B.; McLardy-Smith, P.; Taylor, A.; Gundle, R. Outcome Following Debridement; Antibiotics; and Implant Retention in Hip Periprosthetic Joint Infection-An 18-Year Experience. J. Arthroplasty 2017, 32, 2248–2255. [Google Scholar] [CrossRef]
- Buller, L.T.; Sabry, F.Y.; Easton, R.W.; Klika, A.K.; Barsoum, W.K. The preoperative prediction of success following irrigation and debridement with polyethylene exchange for hip and knee prosthetic joint infections. J. Arthroplasty 2012, 27, 857–864. [Google Scholar] [CrossRef]
- Gardner, J.; Gioe, T.J.; Tatman, P. Can this prosthesis be saved? Implant salvage attempts in infected primary TKA. Clin. Orthop. Relat. Res. 2011, 469, 970–976. [Google Scholar] [CrossRef]
- Vilchez, F.; Martínez-Pastor, J.C.; García-Ramiro, S.; Bori, G.; Maculé, F.; Sierra, J.; Font, L.; Mensa, J.; Soriano, A. Outcome and predictors of treatment failure in early post-surgical prosthetic joint infections due to Staphylococcus aureus treated with debridement. Clin. Microbiol. Infect. 2011, 17, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Koyonos, L.; Zmistowski, B.; Della Valle, C.J.; Parvizi, J. Infection control rate of irrigation and debridement for periprosthetic joint infection. Clin. Orthop. Relat. Res. 2011, 469, 3043–3048. [Google Scholar] [CrossRef] [PubMed]
- Puhto, A.P.; Puhto, T.; Niinimäki, T.; Ohtonen, P.; Leppilahti, J.; Syrjälä, H.S. Predictors of treatment outcome in prosthetic joint infections treated with prosthesis retention. Int. Orthop. 2015, 39, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Peel, T.N.; Buising, K.L.; Dowsey, M.M.; Aboltins, C.A.; Daffy, J.R.; Stanley, P.A.; Coong, P.F. Outcome of Debridement and Retention in Prosthetic Joint Infections by Methicillin-Resistant Staphylococci; with Special Reference to Rifampin and Fusidic Acid Combination Therapy. Antimicrob. Agents. Chemother. 2013, 57, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Achermann, Y.; Stasch, P.; Preiss, S.; Lucke, K.; Vogt, M. Characteristics and treatment outcomes of 69 cases with early prosthetic joint infections of the hip and knee. Infection 2014, 42, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Sukeik, M.; Patel, S.; Haddad, F.S. Aggressive early debridement for treatment of acutely infected cemented total hip arthroplasty. Clin. Orthop. Relat. Res. 2012, 470, 3164–3170. [Google Scholar] [CrossRef] [PubMed]
- Westberg, M.; Grøgaard, B.; Snorrason, F. Early prosthetic joint infections treated with debridement and implant retention: 38 primary hip arthroplasties prospectively recorded and followed for median 4 years. Acta. Orthop. 2012, 83, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Geurts, J.A.; Janssen, D.M.; Kessels, A.G.; Walenkamp, G.H. Good results in postoperative and hematogenous deep infections of 89 stable total hip and knee replacements with retention of prosthesis and local antibiotics. Acta. Orthop. 2013, 84, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Tornero, E.; Morata, L.; Martínez-Pastor, J.C.; Bori, G.; Climent, C.; García-Velez, D.M.; Bosch, J.; Mensa, J.; Soriano, A. KLIC-score for predicting early failure in prosthetic joint infections treated with debridement, implant retention and antibiotics. Clin. Microbiol. Infect. 2015, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Konigsberg, B.S.; Della Valle, C.J.; Ting, N.T.; Qiu, F.; Sporer, S.M. Acute hematogenous infection following total hip and knee arthroplasty. J. Arthroplasty 2014, 29, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Duque, A.F.; Post, Z.D.; Lutz, R.W.; Orozco, F.R.; Pulido, S.H.; Ong, A.C. Is There Still a Role for Irrigation and Debridement With Liner Exchange in Acute Periprosthetic Total Knee Infection? J. Arthroplasty 2017, 32, 1280–1284. [Google Scholar] [CrossRef] [PubMed]
- Sendi, P.; Lötscher, P.O.; Kessler, B.; Graber, P.; Zimmerli, W.; Clauss, M. Debridement and implant retention in the management of hip periprosthetic joint infection: Outcomes following guided and rapid treatment at a single centre. Bone Joint. J. 2017, 99, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Lora-Tamayo, J.; Senneville, E.; Ribera, A.; Bernard, L.; Dupon, M.; Zeller, V.; Li, H.K.; Arvieux, C.; Clauss, M.; Uçkay, I.; et al. The Not-So-Good Prognosis of Streptococcal Periprosthetic Joint Infection Managed by Implant Retention: The Results of a Large Multicenter Study. Clin. Infect. Dis. 2017, 64, 1742–1752. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pardo, D.; Pigrau, C.; Lora-Tamayo, J.; Soriano, A.; Del Toro, M.D.; Cobo, J.; alomino, J.; Euba, G.; Riera, M.; Sánchez-Somolinos, M.; et al. Gram-negative prosthetic joint infection: outcome of a debridement; antibiotics and implant retention approach. A large multicentre study. Clin. Microbiol. Infect. 2014, 20, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.R.; von Knoch, F.; Kandil, A.O.; Zurakowski, D.; Moore, S.; Malchau, H. Retention treatment after periprosthetic total hip arthroplasty infection. Int. Orthop. 2012, 36, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.R.; von Knoch, F.; Zurakowski, D.; Nelson, S.B.; Malchau, H. Can implant retention be recommended for treatment of infected TKA? Clin. Orthop. Relat. Res. 2011, 469, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Bernard, L.; Legout, L.; Zürcher-Pfund, L.; Stern, R.; Rohner, P.; Peter, R.; Assal, M.; Lew, D.; Hoffmeyer, P.; Uçkay, I. Six weeks of antibiotic treatment is sufficient following surgery for septic arthroplasty. J. Infect. 2010, 61, 125–132. [Google Scholar] [CrossRef]
- Uçkay, I.; Pires, D.; Agostinho, A.; Guanziroli, N.; Öztürk, M.; Bartolone, P.; Tscholl, P.; Betz, M.; Pittet, D. Enterococci in orthopaedic infections: Who is at risk getting infected? J. Infect. 2017, 75, 309–314. [Google Scholar] [CrossRef]
- Fehring, T.K.; Odum, S.M.; Berend, K.R.; Jiranek, W.A.; Parvizi, J.; Bozic, K.J.; Della Valle, C.J.; Gioe, T.J. Failure of irrigation and debridement for early postoperative periprosthetic infection. Clin. Orthop. Relat. Res. 2013, 471, 250–257. [Google Scholar] [CrossRef]

| Remission | Clinical Failure | ||
|---|---|---|---|
| Total n = 112 | n = 94 | p value | n = 18 |
| Female sex | 46 (49%) | 0.224 | 6 (33%) |
| Age (median) | 75 years | 0.601 | 72 years |
| American Society of Anesthesiologists’ score (median) | 3 points | 0.991 | 3 points |
| Pain score on admission (median) | 5 points | 0.610 | 4 points |
| Total hip prostheses | 58 (62%) | 0.962 | 11 (61%) |
| Prior revision arthroplasty | 26 (28%) | 0.338 | 7 (39%) |
| Bacteremic infection | 18 (19%) | 0.406 | 5 (28%) |
| Infection due to Staphylococcus aureus | 26 (28%) | 0.329 | 3 (17%) |
| -Infection due to MRSA | 8 (9%) | 0.199 | 0 (0%) |
| -Infection due to streptococci | 21 (22%) | 0.101 | 1 (6%) |
| -Infection due to skin commensals | 26 (28%) | 0.625 | 6 (33%) |
| -Infection due to enterococci | 4 (4%) | 0.237 | 2 (11%) |
| -Infection due to gram-negative pathogens | 11 (12%) | 0.440 | 1 (6%) |
| Immune suppression + | 27 (29%) | 0.572 | 4 (22%) |
| Number of surgical interventions (median) | 2 | 0.973 | 2 |
| -more than 1 intervention | 49 (52%) | 0.869 | 9 (50%) |
| -exchange of mobile parts | 40 (43%) | 0.882 | 8 (44%) |
| Duration of antibiotic treatment (median) | 90 days | 0.224 | 98 days |
| -100 days compared to ≤ 100 days | 30 (32%) | 0.304 | 8 (44%) |
| Duration of intravenous treatment (median) | 10 days | 0.416 | 14 days |
| -7 days compared to ≤ 7 days | 61 (65%) | 0.125 | 15 (83%) |
| Use of rifampicin-ciprofloxacin combination | 46 (49%) | 0.934 | 9 (50%) |
| Use of clindamycin | 24 (26%) | 0.420 | 3 (17%) |
| Use of amoxicillin/clavulanate | 17 (18%) | 0.886 | 3 (17%) |
| Use of vancomycin | 38 (40%) | 0.451 | 9 (50%) |
| Remission | Clinical Failure | ||
|---|---|---|---|
| Total n = 85 | n = 72 (85%) | p value | n = 13 (15%) |
| Female sex | 35 (49%) | 0.500 | 5 (38%) |
| Age (median) | 73 years | 0.840 | 73 years |
| Immune suppression + | 25 (35%) | 0.411 | 3 (23%) |
| Number of surgical interventions (median) | 2 | 0.598 | 2 |
| Exchange of mobile parts | 26 (36%) | 0.492 | 6 (46%) |
| Total n= 112 | Univariate Analysis | Multivariate Analysis |
|---|---|---|
| Female sex | 0.8, 0.5–1.3 | n.d. |
| Age (median) | 1.0, 1.0–1.0 | n.d. |
| American Society of Anesthesiologists’ score (median) | 0.9, 0.7–1,2 | n.d. |
| -ASA score 2 compared to 1 | 1.0, 0.8–1.3 | n.d. |
| -ASA score 3 compared to 1 | 1.4, 0.5–4.0 | n.d. |
| -ASA score 4 compared to 1 | 1.2, 0.4–3.5 | n.d. |
| Pain score on admission (median) | 1.1, 0.3–3.5 | n.d. |
| Total hip prostheses | 0.8, 0.5–1.2 | n.d. |
| Revision arthroplasty | 0.8, 0.5–1.3 | n.d. |
| Bacteremic infection | 1.3, 0.7–2.1 | n.d. |
| Infection due to Staphylococcus aureus | 1.0, 0.6–1.5 | n.d. |
| -Infection due to MRSA | 1.0, 0.5–2.1 | 1.1, 0.5–2.3 |
| Infection due to streptococci | 1.3, 0.8–2.1 | n.d. |
| Infection due to enterococci | 1.2, 0.4–3.4 | n.d. |
| Number of surgical interventions (median) | 0.8, 0.6–1.0 | 0.7, 0.5–1.1 |
| -more than 1 intervention | 0.7, 0.5–1.1 | n.d. |
| -exchange of mobile parts | 2.0, 1.3–3.0 | 1.9, 1.2–2.9 |
| Duration of antibiotic treatment (median) | 1.0, 1.0–1.0 | 1.0, 1.0–1.0 |
| -100 days compared to ≤ 100 days | 1.6, 0.9–2.5 | n.d. |
| Duration of intravenous treatment (median) | 1.0, 1.0–1.0 | n.d. |
| -7 days compared to ≤ 7 days | 0.8, 0.5–1.2 | n.d. |
| Use of rifampicin-ciprofloxacin combination | 1.2, 0.8–1.8 | n.d. |
| Author | Number PJI | Main Pathogen | Identified Key Variables for Success | Exchange Mobile Parts | Remission Incidence and Remarks |
|---|---|---|---|---|---|
| Mont et al. [14] | 24 knees | S. aureus | Early PJI | 24 (100%) | 83% |
| Marculescu et al. [15] | 99 | S. aureus | Early PJI (< 8 days), absence fistula | 48 (48%) | 46% |
| Deirmengian et al. [16] | 31 | S. aureus | Lack of S. aureus | 10 (32%) | 35%, exchange no benefice |
| Theis et al. [17] | 73 | S. aureus | Early PJI (< 4 weeks) | not reported | 69% |
| Tsumura et al. [18] | 10 | S. aureus | none | 0 (0%) | 80% |
| Grammatopoulos [19] | 122 hips | S. aureus | Mobile parts’ exchange, (< 6 weeks) | 65 (53%) | 68%, four-fold benefice of exchanging |
| Buller et al. [20] | 309 | Gram-positives | Early PJI (< 3 weeks) | 309 (100%) | 52% |
| Gardner et al. [21] | 44 knees | S. aureus | none | 44 (100%) | 43% |
| Vilchez et al. [22] | 53 | S. aureus | Serum CRP < 22 mg/L, 1 debridement | not reported | 76% |
| Koyonos et al. [23] | 138 | S. aureus | S. aureus | not reported | 35% |
| Puhto et al. [24] | 113 | staphylococci | Leukocyte count < 10 G/L | not reported | 62% |
| Peel et al. [25] | 43 | MRSA | >1 debridement, antibiotics <3 months | 18 (42%) | 86%, exchange no benefice |
| Achermann et al. [26] | 50 | staphylococci | Early PJI (< 3 weeks) | 26 (52%) | 92%, exchange no benefice |
| Sukeik et al. [27] | 26 hips | staphylococci | Early PJI (< 5 days) | 26 (100%) | 77% |
| Westberg et al. [28] | 38 | S. aureus | Serum CRP < 10 mg/L | not reported | 71% |
| Geurts et al. [29] | 89 | S. aureus | Early PJI (< 4 weeks) | 0 (0%) | 83% |
| Kuiper et al. [30] | 91 | staphylococci | Coagulase-negative staphylococci | not reported | 66% |
| Fehring et al. [17] | 86 | S. aureus | none | not reported | 37% |
| Moojen et al. [3] | 68 hips | S. aureus | none | not reported | 79% |
| Konigsberg et al. [31] | 42 | staphylococci | Lack of S. aureus | 42 (100%) | 76% |
| Duque et al. [32] | 67 | S. aureus | not MRSA and not P. aeruginosa | 67 (100%) | 69% |
| Sendi et al. [33] | 30 hips | staphylococci | none | 14 (47%) | 90% |
| Lora-Tamayo et al. [34] | 444 | streptococci | Mobile parts’ exchange | 220 (50%) | 58%, two-fold benefice of exchanging |
| Chaussade et al. [1] | 87 | S. aureus | Lack of MRSA | 87 (100%) | 69% |
| Rodriguez-Pardo [35] | 174 | Gram-negatives | Ciprofloxacin treatment | 96 (55%) | 68%, exchange no benefice |
| Choi et al. [36] | 28 hips | S. aureus | Lack of S. aureus | 19 (68%) | 50%, exchange no benefice |
| Choi et al. [37] | 32 knees | S. aureus | Mobile parts’ exchange | 19 (59%) | 31%, three-fold benefice of exchanging |
| Present study | 112 | S. aureus | Mobile parts’ exchange | 48 (43%) | 84%, two-fold benefice of exchanging |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirsiger, S.; Betz, M.; Stafylakis, D.; Götschi, T.; Lew, D.; Uçkay, I. The Benefice of Mobile Parts’ Exchange in the Management of Infected Total Joint Arthroplasties with Prosthesis Retention (DAIR Procedure). J. Clin. Med. 2019, 8, 226. https://doi.org/10.3390/jcm8020226
Hirsiger S, Betz M, Stafylakis D, Götschi T, Lew D, Uçkay I. The Benefice of Mobile Parts’ Exchange in the Management of Infected Total Joint Arthroplasties with Prosthesis Retention (DAIR Procedure). Journal of Clinical Medicine. 2019; 8(2):226. https://doi.org/10.3390/jcm8020226
Chicago/Turabian StyleHirsiger, Stefanie, Michael Betz, Dimitrios Stafylakis, Tobias Götschi, Daniel Lew, and Ilker Uçkay. 2019. "The Benefice of Mobile Parts’ Exchange in the Management of Infected Total Joint Arthroplasties with Prosthesis Retention (DAIR Procedure)" Journal of Clinical Medicine 8, no. 2: 226. https://doi.org/10.3390/jcm8020226
APA StyleHirsiger, S., Betz, M., Stafylakis, D., Götschi, T., Lew, D., & Uçkay, I. (2019). The Benefice of Mobile Parts’ Exchange in the Management of Infected Total Joint Arthroplasties with Prosthesis Retention (DAIR Procedure). Journal of Clinical Medicine, 8(2), 226. https://doi.org/10.3390/jcm8020226






