Chimeric Antigen Receptor (CAR) T Cell Therapy in Acute Myeloid Leukemia (AML)
Abstract
:1. Introduction
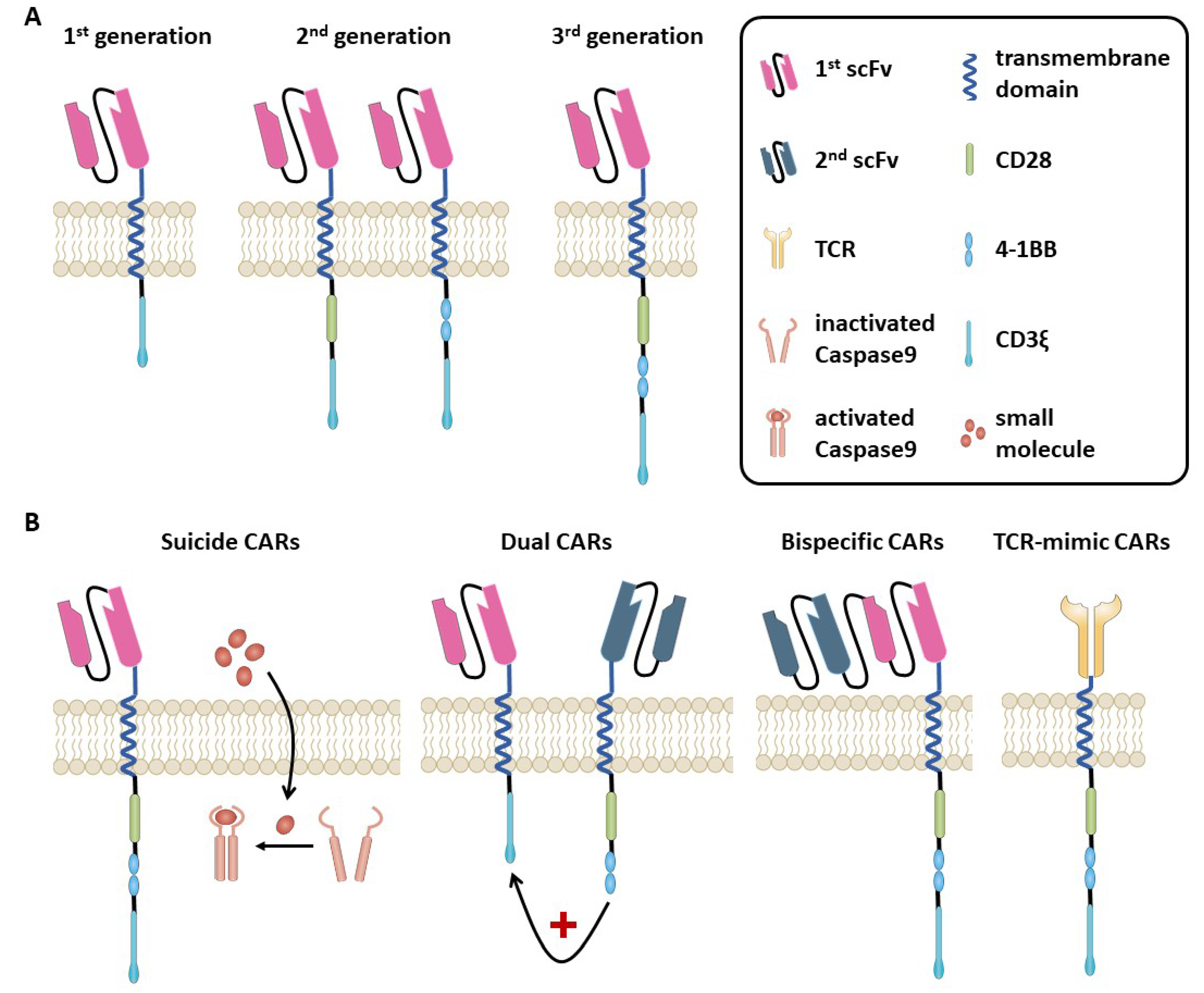
2. Adoptive Cellular Therapies
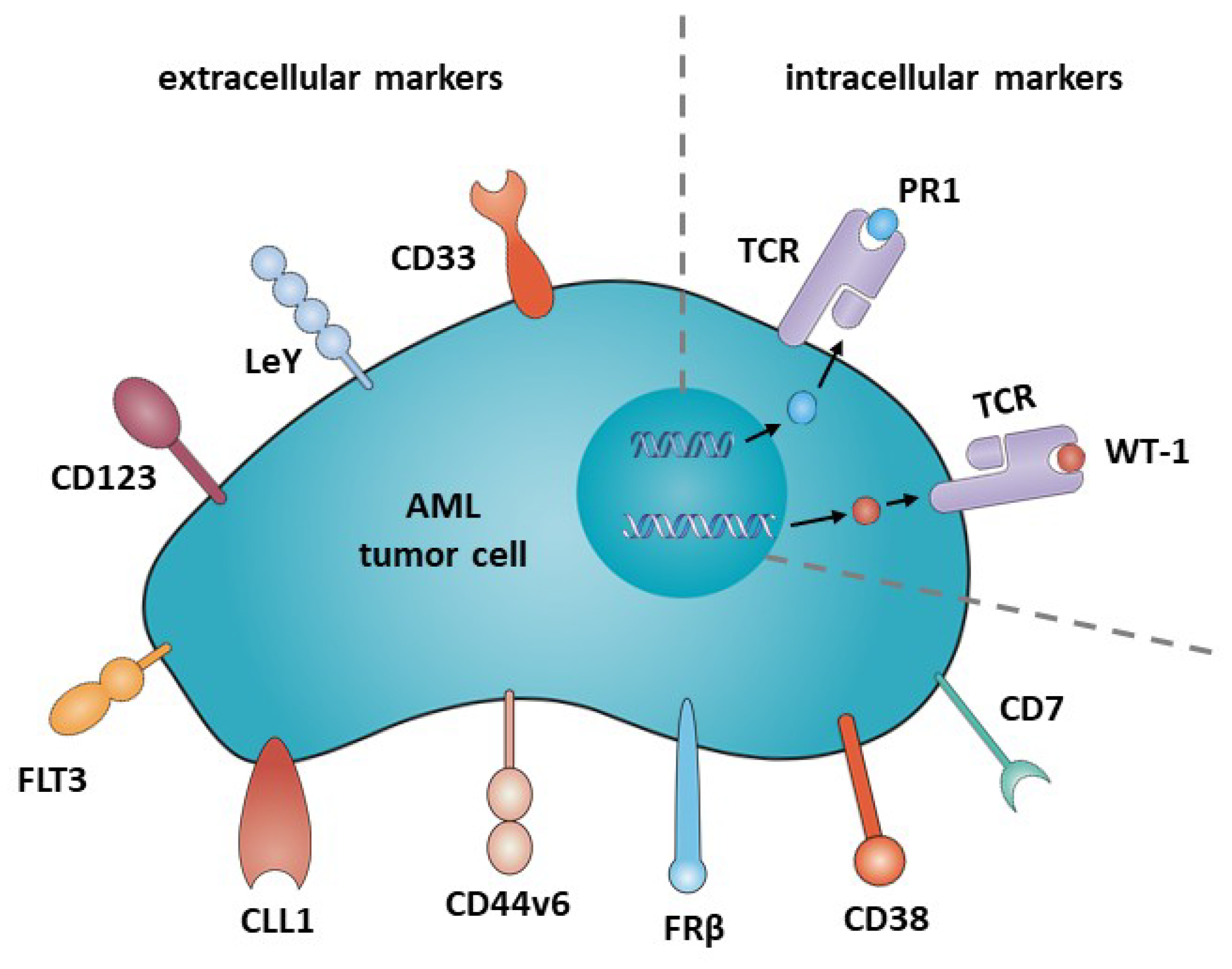
3. CD33
4. Lewis Y (LeY)
5. CD123
6. FLT3 (CD135)
7. CLL1
8. CD44v6
9. Folate Receptor ß (FRß)
10. CD38
11. CD7
12. Intracellular Targets: PR1/HLA-A2; WT1/HLA-A2
13. Safety Affairs
14. Conclusions
Acknowledgments
Conflicts of Interest
References
- Döhner, H.; Weisdorf, D.J.; Bloomfield, C.D. Acute myeloid leukemia. N. Engl. J. Med. 2015, 373, 1136–1152. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Estey, E.H.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Burnett, A.K.; Dombret, H.; Fenaux, P.; Grimwade, D.; Larson, R.A.; et al. Diagnosis and management of acute myeloid leukemia in adults: Recommendations from an international expert panel, on behalf of the european leukemianet. Blood 2010, 115, 453–474. [Google Scholar] [CrossRef] [PubMed]
- Eppert, K.; Takenaka, K.; Lechman, E.R.; Waldron, L.; Nilsson, B.; van Galen, P.; Metzeler, K.H.; Poeppl, A.; Ling, V.; Beyene, J.; et al. Stem cell gene expression programs influence clinical outcome in human leukemia. Nat. Med. 2011, 17, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, D.; Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef]
- Available online: https://seer.cancer.gov/statfacts/html/amyl.html (accessed on 5 February 2019).
- Cheever, M.A.; Allison, J.P.; Ferris, A.S.; Finn, O.J.; Hastings, B.M.; Hecht, T.T.; Mellman, I.; Prindiville, S.A.; Viner, J.L.; Weiner, L.M.; et al. The prioritization of cancer antigens: A national cancer institute pilot project for the acceleration of translational research. Clin. Cancer Res. 2009, 15, 5323–8337. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.J.; Mittermuller, J.; Clemm, C.; Holler, E.; Ledderose, G.; Brehm, G.; Heim, M.; Wilmanns, W. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood 1990, 76, 2462–2465. [Google Scholar] [PubMed]
- Kolb, H.J.; Schattenberg, A.; Goldman, J.M.; Hertenstein, B.; Jacobsen, N.; Arcese, W.; Ljungman, P.; Ferrant, A.; Verdonck, L.; Niederwieser, D.; et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood 1995, 86, 2041–2050. [Google Scholar]
- Kolb, H.J.; Schmid, C.; Barrett, A.J.; Schendel, D.J. Graft-versus-leukemia reactions in allogeneic chimeras. Blood 2004, 103, 767–776. [Google Scholar] [CrossRef]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric antigen receptor t cells for sustained remissions in leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef]
- Turtle, C.J.; Hanafi, L.A.; Berger, C.; Gooley, T.A.; Cherian, S.; Hudecek, M.; Sommermeyer, D.; Melville, K.; Pender, B.; Budiarto, T.M.; et al. Cd19 car-t cells of defined cd4+:Cd8+ composition in adult b cell all patients. J. Clin. Investig. 2016, 126, 2123–2138. [Google Scholar] [CrossRef]
- Eshhar, Z.; Waks, T.; Gross, G.; Schindler, D.G. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and t-cell receptors. Proc. Natl. Acad. Sci. USA 1993, 90, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.C.; Popplewell, L.; Cooper, L.J.; DiGiusto, D.; Kalos, M.; Ostberg, J.R.; Forman, S.J. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred cd20/cd19-specific chimeric antigen receptor redirected t cells in humans. Biol. Blood Marrow Trans. 2010, 16, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Stancovski, I.; Schindler, D.G.; Waks, T.; Yarden, Y.; Sela, M.; Eshhar, Z. Targeting of t lymphocytes to neu/her2-expressing cells using chimeric single chain fv receptors. J. Immunol. 1993, 151, 6577–6582. [Google Scholar] [PubMed]
- Till, B.G.; Jensen, M.C.; Wang, J.; Qian, X.; Gopal, A.K.; Maloney, D.G.; Lindgren, C.G.; Lin, Y.; Pagel, J.M.; Budde, L.E.; et al. Cd20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both cd28 and 4-1bb domains: Pilot clinical trial results. Blood 2012, 119, 3940–3950. [Google Scholar] [CrossRef] [PubMed]
- Finney, H.M.; Lawson, A.D.G.; Bebbington, C.R.; Weir, A.N.C. Chimeric receptors providing both primary and costimulatory signaling in t cells from a single gene product. J. Immunol. 1998, 161, 2791. [Google Scholar] [PubMed]
- Wang, E.; Wang, L.C.; Tsai, C.Y.; Bhoj, V.; Gershenson, Z.; Moon, E.; Newick, K.; Sun, J.; Lo, A.; Baradet, T.; et al. Generation of potent t-cell immunotherapy for cancer using dap12-based, multichain, chimeric immunoreceptors. Cancer Immunol. Res. 2015, 3, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Hombach, A.A.; Chmielewski, M.; Rappl, G.; Abken, H. Adoptive immunotherapy with redirected t cells produces ccr7- cells that are trapped in the periphery and benefit from combined cd28-ox40 costimulation. Hum. Gene Ther. 2013, 24, 259–269. [Google Scholar] [CrossRef]
- Guedan, S.; Chen, X.; Madar, A.; Carpenito, C.; McGettigan, S.E.; Frigault, M.J.; Lee, J.; Posey, A.D., Jr.; Scholler, J.; Scholler, N.; et al. Icos-based chimeric antigen receptors program bipolar th17/th1 cells. Blood 2014, 124, 1070–1080. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Q.J.; Yang, S.; Kochenderfer, J.N.; Zheng, Z.; Zhong, X.; Sadelain, M.; Eshhar, Z.; Rosenberg, S.A.; Morgan, R.A. A herceptin-based chimeric antigen receptor with modified signaling domains leads to enhanced survival of transduced t lymphocytes and antitumor activity. J. Immunol. 2009, 183, 5563–5574. [Google Scholar] [CrossRef]
- Savoldo, B.; Ramos, C.A.; Liu, E.; Mims, M.P.; Keating, M.J.; Carrum, G.; Kamble, R.T.; Bollard, C.M.; Gee, A.P.; Mei, Z.; et al. Cd28 costimulation improves expansion and persistence of chimeric antigen receptor-modified t cells in lymphoma patients. J. Clin. Investig. 2011, 121, 1822–1826. [Google Scholar] [CrossRef]
- Han, E.Q.; Li, X.L.; Wang, C.R.; Li, T.F.; Han, S.Y. Chimeric antigen receptor-engineered t cells for cancer immunotherapy: Progress and challenges. J. Hematol. Oncol. 2013, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Long, A.H.; Haso, W.M.; Shern, J.F.; Wanhainen, K.M.; Murgai, M.; Ingaramo, M.; Smith, J.P.; Walker, A.J.; Kohler, M.E.; Venkateshwara, V.R.; et al. 4-1bb costimulation ameliorates t cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat. Med. 2015, 21, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Ruella, M.; Barrett, D.M.; Kenderian, S.S.; Shestova, O.; Hofmann, T.J.; Perazzelli, J.; Klichinsky, M.; Aikawa, V.; Nazimuddin, F.; Kozlowski, M.; et al. Dual cd19 and cd123 targeting prevents antigen-loss relapses after cd19-directed immunotherapies. J. Clin. Investig. 2016, 126, 3814–3826. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, S.; van Schalkwyk, M.C.; Hobbs, S.; Davies, D.M.; van der Stegen, S.J.; Pereira, A.C.; Burbridge, S.E.; Box, C.; Eccles, S.A.; Maher, J. Dual targeting of erbb2 and muc1 in breast cancer using chimeric antigen receptors engineered to provide complementary signaling. J. Clin. Immunol. 2012, 32, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Zah, E.; Lin, M.Y.; Silva-Benedict, A.; Jensen, M.C.; Chen, Y.Y. T cells expressing cd19/cd20 bispecific chimeric antigen receptors prevent antigen escape by malignant b cells. Cancer Immunol. Res. 2016, 4, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Perna, F.; Berman, S.H.; Soni, R.K.; Mansilla-Soto, J.; Eyquem, J.; Hamieh, M.; Hendrickson, R.C.; Brennan, C.W.; Sadelain, M. Integrating proteomics and transcriptomics for systematic combinatorial chimeric antigen receptor therapy of aml. Cancer Cell 2017, 32, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Dotti, G.; Gottschalk, S.; Savoldo, B.; Brenner, M.K. Design and development of therapies using chimeric antigen receptor-expressing t cells. Immunol. Rev. 2014, 257, 107–126. [Google Scholar] [CrossRef]
- Available online: https://clinicaltrials.gov/ct2/results?cond=aml&term=car&cntry=&state=&city=&dist= (accessed on 12 December 2018).
- Griffin, J.D.; Linch, D.; Sabbath, K.; Larcom, P.; Schlossman, S.F. A monoclonal antibody reactive with normal and leukemic human myeloid progenitor cells. Leuk. Res. 1984, 8, 521–534. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Ball, E.D.; Varki, A. Myeloid precursors and acute myeloid leukemia cells express multiple cd33-related siglecs. Exp. Hematol. 2006, 34, 728–735. [Google Scholar] [CrossRef]
- Hauswirth, A.W.; Florian, S.; Printz, D.; Sotlar, K.; Krauth, M.T.; Fritsch, G.; Schernthaner, G.H.; Wacheck, V.; Selzer, E.; Sperr, W.R.; et al. Expression of the target receptor cd33 in cd34+/cd38-/cd123+ aml stem cells. Eur. J. Clin. Investig. 2007, 37, 73–82. [Google Scholar] [CrossRef]
- Thol, F.; Schlenk, R.F. Gemtuzumab ozogamicin in acute myeloid leukemia revisited. Expert Opin. Biol. Ther. 2014, 14, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Giles, F.J.; Kantarjian, H.M.; Kornblau, S.M.; Thomas, D.A.; Garcia-Manero, G.; Waddelow, T.A.; David, C.L.; Phan, A.T.; Colburn, D.E.; Rashid, A.; et al. Mylotarg (gemtuzumab ozogamicin) therapy is associated with hepatic venoocclusive disease in patients who have not received stem cell transplantation. Cancer 2001, 92, 406–413. [Google Scholar] [CrossRef]
- Walter, R.B.; Appelbaum, F.R.; Estey, E.H.; Bernstein, I.D. Acute myeloid leukemia stem cells and cd33-targeted immunotherapy. Blood 2012, 119, 6198. [Google Scholar] [CrossRef] [PubMed]
- Hills, R.K.; Castaigne, S.; Appelbaum, F.R.; Delaunay, J.; Petersdorf, S.; Othus, M.; Estey, E.H.; Dombret, H.; Chevret, S.; Ifrah, N.; et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: A meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014, 15, 986–996. [Google Scholar] [CrossRef]
- Wang, Q.-S.; Wang, Y.; Lv, H.-Y.; Han, Q.-W.; Fan, H.; Guo, B.; Wang, L.-L.; Han, W.-D. Treatment of cd33-directed chimeric antigen receptor-modified t cells in one patient with relapsed and refractory acute myeloid leukemia. Mol. Ther. 2015, 23, 184–191. [Google Scholar] [CrossRef]
- Kenderian, S.S.; Ruella, M.; Shestova, O.; Klichinsky, M.; Aikawa, V.; Morrissette, J.J.; Scholler, J.; Song, D.; Porter, D.L.; Carroll, M.; et al. Cd33-specific chimeric antigen receptor t cells exhibit potent preclinical activity against human acute myeloid leukemia. Leukemia 2015, 29, 1637–1647. [Google Scholar] [CrossRef]
- Kim, M.Y.; Yu, K.-R.; Kenderian, S.S.; Ruella, M.; Chen, S.; Shin, T.-H.; Aljanahi, A.A.; Schreeder, D.; Klichinsky, M.; Shestova, O.; et al. Genetic inactivation of cd33 in hematopoietic stem cells to enable car t cell immunotherapy for acute myeloid leukemia. Cell 2018, 173, 1439–1453. [Google Scholar] [CrossRef]
- Westwood, J.A.; Murray, W.K.; Trivett, M.; Haynes, N.M.; Solomon, B.; Mileshkin, L.; Ball, D.; Michael, M.; Burman, A.; Mayura-Guru, P.; et al. The lewis-y carbohydrate antigen is expressed by many human tumors and can serve as a target for genetically redirected t cells despite the presence of soluble antigen in serum. J. Immunother. 2009, 32, 292–301. [Google Scholar] [CrossRef]
- Sakamoto, J.; Furukawa, K.; Cordon-Cardo, C.; Yin, B.W.T.; Rettig, W.J.; Oettgen, H.F.; Old, L.J.; Lloyd, K.O. Expression of Lewisa, X, and Y blood group antigens in human colonic tumors and normal tissue and in human tumor-derived cell lines. Cancer Res. 1986, 46, 1553. [Google Scholar]
- Peinert, S.; Prince, H.M.; Guru, P.M.; Kershaw, M.H.; Smyth, M.J.; Trapani, J.A.; Gambell, P.; Harrison, S.; Scott, A.M.; Smyth, F.E.; et al. Gene-modified t cells as immunotherapy for multiple myeloma and acute myeloid leukemia expressing the lewis y antigen. Gene Ther. 2010, 17, 678. [Google Scholar] [CrossRef]
- Kobayashi, K.; Sakamoto, J.; Kito, T.; Yamamura, Y.; Koshikawa, T.; Fujita, M.; Watanabe, T.; Nakazato, H. Lewis blood group-related antigen expression in normal gastric epithelium, intestinal metaplasia, gastric adenoma, and gastric carcinoma. Am. J. Gastroenterol. 1993, 88, 919–924. [Google Scholar] [PubMed]
- Neeson, P.; Shin, A.; Tainton, K.M.; Guru, P.; Prince, H.M.; Harrison, S.J.; Peinert, S.; Smyth, M.J.; Trapani, J.A.; Kershaw, M.H.; et al. Ex vivo culture of chimeric antigen receptor t cells generates functional cd8+ t cells with effector and central memory-like phenotype. Gene Ther. 2010, 17, 1105. [Google Scholar] [CrossRef]
- Jordan, C.T.; Upchurch, D.; Szilvassy, S.J.; Guzman, M.L.; Howard, D.S.; Pettigrew, A.L.; Meyerrose, T.; Rossi, R.; Grimes, B.; Rizzieri, D.A.; et al. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia 2000, 14, 1777–1784. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Lee, E.M.; Ramshaw, H.S.; Busfield, S.J.; Peoppl, A.G.; Wilkinson, L.; Guthridge, M.A.; Thomas, D.; Barry, E.F.; Boyd, A.; et al. Monoclonal antibody-mediated targeting of cd123, il-3 receptor α chain, eliminates human acute myeloid leukemic stem cells. Cell Stem Cell 2009, 5, 31–42. [Google Scholar] [CrossRef]
- Thokala, R.; Olivares, S.; Mi, T.; Maiti, S.; Deniger, D.; Huls, H.; Torikai, H.; Singh, H.; Champlin, R.E.; Laskowski, T.; et al. Redirecting specificity of t cells using the sleeping beauty system to express chimeric antigen receptors by mix-and-matching of vl and vh domains targeting cd123+ tumors. PLoS ONE 2016, 11, e0159477. [Google Scholar] [CrossRef] [PubMed]
- Levis, M.; Small, D. Flt3: Itdoes matter in leukemia. Leukemia 2003, 17, 1738–1752. [Google Scholar] [CrossRef]
- Chien, C.D.; Sauter, C.T.; Ishii, K.; Nguyen, S.M.; Shen, F.; Tasian, S.K.; Chen, W.; Dimitrov, D.S.; Fry, T.J. Preclinical development of flt3-redirected chimeric antigen receptor t cell immunotherapy for acute myeloid leukemia. Blood 2016, 128, 1072. [Google Scholar]
- Wang, Y.; Xu, Y.; Li, S.; Liu, J.; Xing, Y.; Xing, H.; Tian, Z.; Tang, K.; Rao, Q.; Wang, M.; et al. Targeting flt3 in acute myeloid leukemia using ligand-based chimeric antigen receptor-engineered t cells. J. Hematol. Oncol. 2018, 11, 60. [Google Scholar] [CrossRef]
- Bakker, A.B.H.; van den Oudenrijn, S.; Bakker, A.Q.; Feller, N.; van Meijer, M.; Bia, J.A.; Jongeneelen, M.A.C.; Visser, T.J.; Bijl, N.; Geuijen, C.A.W.; et al. C-type lectin-like molecule-1. Cancer Res. 2004, 64, 8443. [Google Scholar] [CrossRef]
- Laborda, E.; Mazagova, M.; Shao, S.; Wang, X.; Quirino, H.; Woods, A.K.; Hampton, E.N.; Rodgers, D.T.; Kim, C.H.; Schultz, P.G.; et al. Development of a chimeric antigen receptor targeting c-type lectin-like molecule-1 for human acute myeloid leukemia. Int. J. Mol. Sci. 2017, 18, 2259. [Google Scholar] [CrossRef]
- Kenderian, S.S.; Habermann, T.M.; Macon, W.R.; Ristow, K.M.; Ansell, S.M.; Colgan, J.P.; Johnston, P.B.; Inwards, D.J.; Markovic, S.N.; Micallef, I.N.; et al. Large b-cell transformation in nodular lymphocyte-predominant hodgkin lymphoma: 40-year experience from a single institution. Blood 2016, 127, 1960–1966. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, H.; Sauer, T.; Shum, T.; Parikh, K.; Mamonkin, M.; Omer, B.; Rouce, R.H.; Lulla, P.; Rooney, C.M.; Gottschalk, S.; et al. Treatment of acute myeloid leukemia with t cells expressing chimeric antigen receptors directed to c-type lectin-like molecule 1. Mol. Ther. J. Am. Soc. Gene Ther. 2017, 25, 2202–2213. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, S.; Xiao, W.; Li, W.; Wang, L.; Yang, S.; Wang, W.; Xu, L.; Liao, S.; Liu, W.; et al. Car-t cells targeting cll-1 as an approach to treat acute myeloid leukemia. J. Hematol. Oncol. 2018, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Legras, S.; Gunthert, U.; Stauder, R.; Curt, F.; Oliferenko, S.; Kluin-Nelemans, H.C.; Marie, J.P.; Proctor, S.; Jasmin, C.; Smadja-Joffe, F. A strong expression of cd44-6v correlates with shorter survival of patients with acute myeloid leukemia. Blood 1998, 91, 3401–3413. [Google Scholar] [PubMed]
- Günthert, U.; Hofmann, M.; Rudy, W.; Reber, S.; Zöller, M.; Hauβmann, I.; Matzku, S.; Wenzel, A.; Ponta, H.; Herrlich, P. A new variant of glycoprotein cd44 confers metastatic potential to rat carcinoma cells. Cell 1991, 65, 13–24. [Google Scholar] [CrossRef]
- Neu, S.; Geiselhart, A.; Sproll, M.; Hahn, D.; Kuçi, S.; Niethammer, D.; Handgretinger, R. Expression of cd44 isoforms by highly enriched cd34-positive cells in cord blood, bone marrow and leukaphereses. Bone Marrow Trans. 1997, 20, 593. [Google Scholar] [CrossRef] [PubMed]
- Casucci, M.; Nicolis di Robilant, B.; Falcone, L.; Camisa, B.; Norelli, M.; Genovese, P.; Gentner, B.; Gullotta, F.; Ponzoni, M.; Bernardi, M.; et al. Cd44v6-targeted t cells mediate potent antitumor effects against acute myeloid leukemia and multiple myeloma. Blood 2013, 122, 3461–3472. [Google Scholar] [CrossRef] [PubMed]
- Ciceri, F.; Bonini, C.; Stanghellini, M.T.; Bondanza, A.; Traversari, C.; Salomoni, M.; Turchetto, L.; Colombi, S.; Bernardi, M.; Peccatori, J.; et al. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the tk007 trial): A non-randomised phase i-ii study. Lancet Oncol. 2009, 10, 489–500. [Google Scholar] [CrossRef]
- Di Stasi, A.; Tey, S.K.; Dotti, G.; Fujita, Y.; Kennedy-Nasser, A.; Martinez, C.; Straathof, K.; Liu, E.; Durett, A.G.; Grilley, B.; et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 2011, 365, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Jiang, M.; Hao, W.; Tao, L.; Salazar, M.; Fong, H.K. A human opsin-related gene that encodes a retinaldehyde-binding protein. Biochemistry 1994, 33, 13117–13125. [Google Scholar] [CrossRef]
- Ross, J.F.; Wang, H.; Behm, F.G.; Mathew, P.; Wu, M.; Booth, R.; Ratnam, M. Folate receptor type beta is a neutrophilic lineage marker and is differentially expressed in myeloid leukemia. Cancer 1999, 85, 348–357. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, X.; Behm, F.G.; Ratnam, M. Differentiation-independent retinoid induction of folate receptor type beta, a potential tumor target in myeloid leukemia. Blood 2000, 96, 3529–3536. [Google Scholar] [PubMed]
- Pan, X.Q.; Zheng, X.; Shi, G.; Wang, H.; Ratnam, M.; Lee, R.J. Strategy for the treatment of acute myelogenous leukemia based on folate receptor beta-targeted liposomal doxorubicin combined with receptor induction using all-trans retinoic acid. Blood 2002, 100, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Lynn, R.C.; Poussin, M.; Kalota, A.; Feng, Y.; Low, P.S.; Dimitrov, D.S.; Powell, D.J., Jr. Targeting of folate receptor beta on acute myeloid leukemia blasts with chimeric antigen receptor-expressing t cells. Blood 2015, 125, 3466–3476. [Google Scholar] [CrossRef] [PubMed]
- Lynn, R.C.; Feng, Y.; Schutsky, K.; Poussin, M.; Kalota, A.; Dimitrov, D.S.; Powell, D.J., Jr. High-affinity frbeta-specific car t cells eradicate aml and normal myeloid lineage without hsc toxicity. Leukemia 2016, 30, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Terstappen, L.W.; Safford, M.; Unterhalt, M.; Konemann, S.; Zurlutter, K.; Piechotka, K.; Drescher, M.; Aul, C.; Buchner, T.; Hiddemann, W.; et al. Flow cytometric characterization of acute myeloid leukemia: Iv. Comparison to the differentiation pathway of normal hematopoietic progenitor cells. Leukemia 1992, 6, 993–1000. [Google Scholar] [PubMed]
- Konopleva, M.; Rissling, I.; Andreeff, M. Cd38 in hematopoietic malignancies. Chem. Immunol. 2000, 75, 189–206. [Google Scholar] [PubMed]
- Yoshida, T.; Mihara, K.; Takei, Y.; Yanagihara, K.; Kubo, T.; Bhattacharyya, J.; Imai, C.; Mino, T.; Takihara, Y.; Ichinohe, T. All-trans retinoic acid enhances cytotoxic effect of t cells with an anti-cd38 chimeric antigen receptor in acute myeloid leukemia. Clin. Transl. Immunol. 2016, 5, e116. [Google Scholar] [CrossRef]
- Rabinowich, H.; Vitolo, D.; Altarac, S.; Herberman, R.B.; Whiteside, T.L. Role of cytokines in the adoptive immunotherapy of an experimental model of human head and neck cancer by human il-2-activated natural killer cells. J. Immunol. 1992, 149, 340–349. [Google Scholar]
- Campana, D.; van Dongen, J.J.; Mehta, A.; Coustan-Smith, E.; Wolvers-Tettero, I.L.; Ganeshaguru, K.; Janossy, G. Stages of t-cell receptor protein expression in t-cell acute lymphoblastic leukemia. Blood 1991, 77, 1546–1554. [Google Scholar]
- Campana, D.; Behm, F.G. Immunophenotyping of leukemia. J. Immunol. Methods 2000, 243, 59–75. [Google Scholar] [CrossRef]
- Tiftik, N.; Bolaman, Z.; Batun, S.; Ayyildiz, O.; Isikdogan, A.; Kadikoylu, G.; Muftuoglu, E. The importance of cd7 and cd56 antigens in acute leukaemias. Int. J. Clin. Pract. 2004, 58, 149–152. [Google Scholar] [CrossRef]
- Miwa, H.; Nakase, K.; Kita, K. Biological characteristics of cd7(+) acute leukemia. Leuk Lymphoma 1996, 21, 239–244. [Google Scholar] [PubMed]
- Gomes-Silva, D.; Atilla, E.; Atilla, P.A.; Mo, F.; Tashiro, H.; Srinivasan, M.; Lulla, P.; Rouce, R.H.; Cabral, J.M.S.; Ramos, C.A.; et al. Cd7 car t cells for the therapy of acute myeloid leukemia. Mol. Ther. 2018, 27, 272–280. [Google Scholar] [CrossRef]
- Gomes-Silva, D.; Srinivasan, M.; Sharma, S.; Lee, C.M.; Wagner, D.L.; Davis, T.H.; Rouce, R.H.; Bao, G.; Brenner, M.K.; Mamonkin, M. Cd7-edited t cells expressing a cd7-specific car for the therapy of t-cell malignancies. Blood 2017, 130, 285–296. [Google Scholar] [CrossRef]
- Silva, D.; Tashiro, H.; Srinivasan, M.; Brenner, M.K.; Mamonkin, M. Cd7 car for the treatment of acute myeloid and lymphoid leukemia. Blood 2016, 128, 4555. [Google Scholar]
- Molldrem, J.; Dermime, S.; Parker, K.; Jiang, Y.Z.; Mavroudis, D.; Hensel, N.; Fukushima, P.; Barrett, A.J. Targeted t-cell therapy for human leukemia: Cytotoxic t lymphocytes specific for a peptide derived from proteinase 3 preferentially lyse human myeloid leukemia cells. Blood 1996, 88, 2450–2457. [Google Scholar]
- Molldrem, J.J.; Clave, E.; Jiang, Y.Z.; Mavroudis, D.; Raptis, A.; Hensel, N.; Agarwala, V.; Barrett, A.J. Cytotoxic t lymphocytes specific for a nonpolymorphic proteinase 3 peptide preferentially inhibit chronic myeloid leukemia colony-forming units. Blood 1997, 90, 2529–2534. [Google Scholar] [PubMed]
- Ma, Q.; Garber, H.R.; Lu, S.; He, H.; Tallis, E.; Ding, X.; Sergeeva, A.; Wood, M.S.; Dotti, G.; Salvado, B.; et al. A novel tcr-like car with specificity for pr1/hla-a2 effectively targets myeloid leukemia in vitro when expressed in human adult peripheral blood and cord blood t cells. Cytotherapy 2016, 18, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, S.; Purdon, T.J.; Daniyan, A.F.; Koneru, M.; Dao, T.; Liu, C.; Scheinberg, D.A.; Brentjens, R.J. Optimized t-cell receptor-mimic chimeric antigen receptor t cells directed toward the intracellular wilms tumor 1 antigen. Leukemia 2017, 31, 1788–1797. [Google Scholar] [CrossRef]
- Rezvani, K.; Grube, M.; Brenchley, J.M.; Sconocchia, G.; Fujiwara, H.; Price, D.A.; Gostick, E.; Yamada, K.; Melenhorst, J.; Childs, R.; et al. Functional leukemia-associated antigen-specific memory cd8+ t cells exist in healthy individuals and in patients with chronic myelogenous leukemia before and after stem cell transplantation. Blood 2003, 102, 2892–2900. [Google Scholar] [CrossRef] [PubMed]
- Krug, L.M.; Dao, T.; Brown, A.B.; Maslak, P.; Travis, W.; Bekele, S.; Korontsvit, T.; Zakhaleva, V.; Wolchok, J.; Yuan, J.; et al. Wt1 peptide vaccinations induce cd4 and cd8 t cell immune responses in patients with mesothelioma and non-small cell lung cancer. Cancer Immunol. Immunother. 2010, 59, 1467–1479. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Bellantuono, I.; Elsässer, A.; Marley, S.B.; Gordon, M.Y.; Goldman, J.M.; Stauss, H.J. Selective elimination of leukemic CD34+ progenitor cells by cytotoxic t lymphocytes specific for wt1. Blood 2000, 95, 2198. [Google Scholar] [PubMed]
- Chang, A.Y.; Dao, T.; Gejman, R.S.; Jarvis, C.A.; Scott, A.; Dubrovsky, L.; Mathias, M.D.; Korontsvit, T.; Zakhaleva, V.; Curcio, M.; et al. A therapeutic t cell receptor mimic antibody targets tumor-associated prame peptide/hla-i antigens. J. Clin. Investig. 2017, 127, 2705–2718. [Google Scholar] [CrossRef] [PubMed]
- Press, M.F.; Cordon-Cardo, C.; Slamon, D.J. Expression of the her-2/neu proto-oncogene in normal human adult and fetal tissues. Oncogene 1990, 5, 953–962. [Google Scholar] [PubMed]
- Morgan, R.A.; Yang, J.C.; Kitano, M.; Dudley, M.E.; Laurencot, C.M.; Rosenberg, S.A. Case report of a serious adverse event following the administration of t cells transduced with a chimeric antigen receptor recognizing erbb2. Mol. Ther. 2010, 18, 843–851. [Google Scholar] [CrossRef]
- Bonini, C.; Ferrari, G.; Verzeletti, S.; Servida, P.; Zappone, E.; Ruggieri, L.; Ponzoni, M.; Rossini, S.; Mavilio, F.; Traversari, C.; et al. Hsv-tk gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science 1997, 276, 1719–1724. [Google Scholar] [CrossRef] [PubMed]
- Straathof, K.C.; Pulè, M.A.; Yotnda, P.; Dotti, G.; Vanin, E.F.; Brenner, M.K.; Heslop, H.E.; Spencer, D.M.; Rooney, C.M. An inducible caspase 9 safety switch for t-cell therapy. Blood 2005, 105, 4247. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, S.; Fang, C.; Yang, S.; Olalere, D.; Pequignot, E.C.; Cogdill, A.P.; Li, N.; Ramones, M.; Granda, B.; et al. Affinity-tuned erbb2 or egfr chimeric antigen receptor t cells exhibit an increased therapeutic index against tumors in mice. Cancer Res. 2015, 75, 3596–3607. [Google Scholar] [CrossRef]
| Trial ID | Status | Phase | Target | Indication | Institution |
|---|---|---|---|---|---|
| NCT03585517 | R | I | CD123 | CD123+ AML | Xian Lu, Beijing, China |
| NCT03114670 | R | I | CD123 | recurred AML after allo | Fengtai District, Beijing Shi, China |
| NCT03556982 | R | I/II | CD123 | R/R AML | 307 Hospital of PLA, Beijing, Beijing, China |
| NCT02623582 | terminated | I | CD123 | R/R AML | Abramson Cancer Center of the University of Pennsylvania, Philadelphia, Pennsylvania, United States |
| NCT02159495 | R | I | CD123 | R/R AML, Persistent/Recurrent Blastic Plasmacytoid Dendritic Cell Neoplasm | City of Hope Medical Center, Duarte, California, United States |
| NCT03672851 | R | I | CD123 | R/R AML | Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, China |
| NCT03766126 | R | I | CD123 | R/R AML | University of Pennsylvania, Philadelphia, Pennsylvania, United States |
| NCT01864902 | R | I | UCART 123 | R/R AML, newly diagnosed high-risk AML | Weill Cornell Medical College, New York, New York, United States MD Anderson Cancer Center, Houston, Texas, United States |
| NCT03631576 | R | II/III | CD123/CLL1 | R/R AML | Fujian Medical University Union Hospital, Fuzhou, Fujian, China |
| NCT03126864 | R | I | CD33 | R/R CD33+ AML | University of Texas MD Anderson Cancer Center, Houston, Texas, United States |
| NCT02799680 | unknown | I | CD33 | R/R AML | Affiliated Hospital of Academy of Military Medical Sciences, Beijing, Beijing, China| Chinese PLA General Hospital, Beijing, Beijing, China |
| NCT01864902 | unknown | I/II | CD33 | R/R AML | Biotherapeutic Department and Pediatrics Department of Chinese PLA General Hospital, Hematological Department, Affiliated Hospital of Changzhi Medical College, Beijing, Beijing, China |
| NCT02944162 | unknown | I/II | anti-CD33 NK CAR | R/R CD33+ AML | PersonGen BioTherapeutics (Suzhou) Co., Ltd., Suzhou, Jiangsu, China |
| NCT03291444 | R | I | CD33, CD38 CD56, CD117, CD123, CD34, and Muc1 for AML and MDS | R/R AML, MDS; ALL | Zhujiang Hospital, Southern Medical University, Guangzhou, Guangdong, China |
| NCT03473457 | R | n.a. | single CAR-T or double CAR-T cells with CD33, CD38, CD56, CD123, CD117, CD133, CD34, or Mucl | R/R AML | Southern Medical University Zhujiang Hospital, Guangdong, Guangdong, China |
| NCT03222674 | R | I/II | Muc1/CLL1/CD33/CD38/CD56/CD123 | AML | Zhujiang Hospital of Southern Medical University, Guangzhou, Guangdong, China| Shenzhen Geno-immune Medical Institute, Shenzhen, Guangdong, China| Yunnan Cancer Hospital & The Third Affiliated Hospital of Kunming Medical University & Yunnan Cancer Center, KunMing, Yunnan, China |
| NCT02203825 | completed | I | NKG2D | AML, MDS-RAEB, and Multiple Myeloma. | Dana-Farber Cancer Institute, Boston, Massachusetts, United States |
| NCT03018405 | R | I/II | NKR2 (NKG2D) | R/R AML, AML, Myeloma | Tampa, Florida, United States| Buffalo, New York, United States| Brussels, Belgium|Brussels, Belgium| Ghent, Belgium |
| NCT03018405 | unknown | I/II | CD7/NK92 cell | CD7+ R/R Leukemia and Lymphoma | PersonGen BioTherapeutics (Suzhou) Co., Ltd., Suzhou, Jiangsu, China |
| NCT01716364 | unknown | I | Lewis Y | Myeloma, AML, MDS | Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hofmann, S.; Schubert, M.-L.; Wang, L.; He, B.; Neuber, B.; Dreger, P.; Müller-Tidow, C.; Schmitt, M. Chimeric Antigen Receptor (CAR) T Cell Therapy in Acute Myeloid Leukemia (AML). J. Clin. Med. 2019, 8, 200. https://doi.org/10.3390/jcm8020200
Hofmann S, Schubert M-L, Wang L, He B, Neuber B, Dreger P, Müller-Tidow C, Schmitt M. Chimeric Antigen Receptor (CAR) T Cell Therapy in Acute Myeloid Leukemia (AML). Journal of Clinical Medicine. 2019; 8(2):200. https://doi.org/10.3390/jcm8020200
Chicago/Turabian StyleHofmann, Susanne, Maria-Luisa Schubert, Lei Wang, Bailin He, Brigitte Neuber, Peter Dreger, Carsten Müller-Tidow, and Michael Schmitt. 2019. "Chimeric Antigen Receptor (CAR) T Cell Therapy in Acute Myeloid Leukemia (AML)" Journal of Clinical Medicine 8, no. 2: 200. https://doi.org/10.3390/jcm8020200
APA StyleHofmann, S., Schubert, M.-L., Wang, L., He, B., Neuber, B., Dreger, P., Müller-Tidow, C., & Schmitt, M. (2019). Chimeric Antigen Receptor (CAR) T Cell Therapy in Acute Myeloid Leukemia (AML). Journal of Clinical Medicine, 8(2), 200. https://doi.org/10.3390/jcm8020200





