Changing Spectrum of Opportunistic Illnesses among HIV-Infected Taiwanese Patients in Response to a 10-Year National Anti-TB Programme
Abstract
1. Introduction
2. Materials and Methods
2.1. Design, Setting, and Data Source
2.2. Participants
2.3. Definitions
2.4. AOI Diagnosis
2.5. Outcomes of Interest
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. AOI Incidence
3.3. AOI Spectrum
3.4. Factors Associated with MTB Disease at Presentation
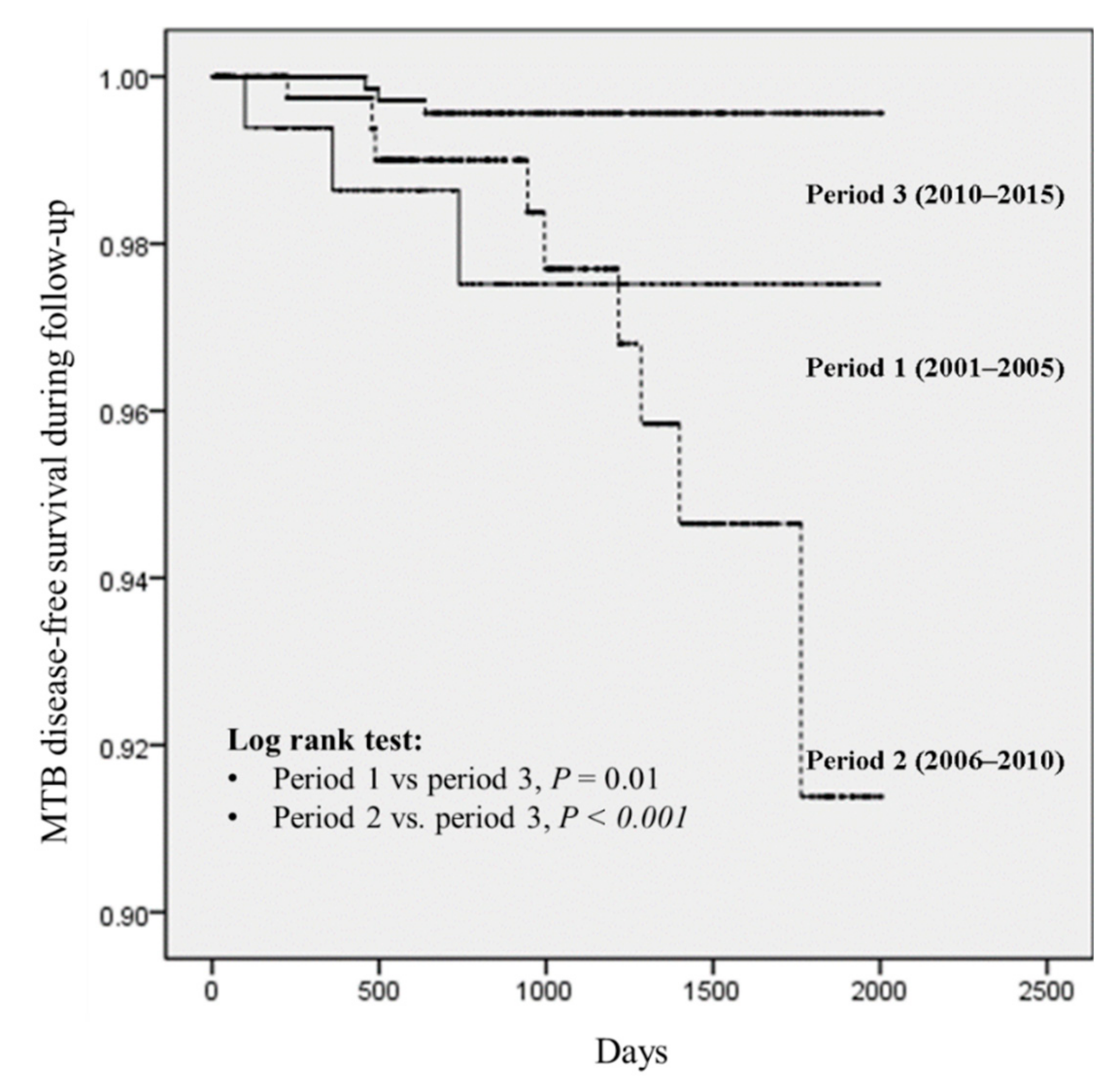
3.5. Factors Associated with MTB Disease During Follow-Up
3.6. Characteristics of 78 MTB Disease Cases
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Buchacz, K.; Baker, R.K.; Palella, F.J., Jr.; Chmiel, J.S.; Lichtenstein, K.A.; Novak, R.M.; Wood, K.C.; Brooks, J.T.; Investigators, H. AIDS-defining opportunistic illnesses in US patients, 1994–2007: A cohort study. Aids 2010, 24, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Kitahata, M.M.; Gange, S.J.; Abraham, A.G.; Merriman, B.; Saag, M.S.; Justice, A.C.; Hogg, R.S.; Deeks, S.G.; Eron, J.J.; Brooks, J.T. Effect of early versus deferred antiretroviral therapy for HIV on survival. N. Engl. J. Med. 2009, 360, 1815–1826. [Google Scholar] [CrossRef] [PubMed]
- Honge, B.L.; Jespersen, S.; Aunsborg, J.; Mendes, D.V.; Medina, C.; da Silva Te, D.; Laursen, A.L.; Erikstrup, C.; Wejse, C. High prevalence and excess mortality of late presenters among HIV-1, HIV-2 and HIV-1/2 dually infected patients in Guinea-Bissau—A cohort study from West Africa. Pan Afr. Med. J. 2016, 25, 40. [Google Scholar] [CrossRef] [PubMed]
- Drain, P.K.; Losina, E.; Parker, G.; Giddy, J.; Ross, D.; Katz, J.N.; Coleman, S.M.; Bogart, L.M.; Freedberg, K.A.; Walensky, R.P.; et al. Risk factors for late-stage HIV disease presentation at initial HIV diagnosis in Durban, South Africa. PLoS ONE 2013, 8, e55305. [Google Scholar] [CrossRef] [PubMed]
- Mocroft, A.; Lundgren, J.D.; Sabin, M.L.; Monforte, A.; Brockmeyer, N.; Casabona, J.; Castagna, A.; Costagliola, D.; Dabis, F.; De Wit, S.; et al. Risk factors and outcomes for late presentation for HIV-positive persons in Europe: Results from the Collaboration of Observational HIV Epidemiological Research Europe Study (COHERE). PLoS Med. 2013, 10, e1001510. [Google Scholar] [CrossRef]
- Brannstrom, J.; Svedhem Johansson, V.; Marrone, G.; Wendahl, S.; Yilmaz, A.; Blaxhult, A.; Sonnerborg, A. Deficiencies in the health care system contribute to a high rate of late HIV diagnosis in Sweden. HIV Med. 2016, 17, 425–435. [Google Scholar] [CrossRef]
- Lee, C.Y.; Jen, I.A.; Lan, Y.C.; Yen, Y.F.; Chuang, P.H.; Chen, M.; Lee, Y.; Chen, Y.A. AIDS incidence trends at presentation and during follow-up among HIV-at-risk populations: A 15-year nationwide cohort study in Taiwan. BMC Public Health 2018, 18, 589. [Google Scholar] [CrossRef]
- Xiao, J.; Gao, G.; Li, Y.; Zhang, W.; Tian, Y.; Huang, Y.; Su, W.; Han, N.; Yang, D.; Zhao, H. Spectrums of opportunistic infections and malignancies in HIV-infected patients in tertiary care hospital, China. PLoS ONE 2013, 8, e75915. [Google Scholar] [CrossRef]
- Gebo, K.A.; Fleishman, J.A.; Moore, R.D. Hospitalizations for metabolic conditions, opportunistic infections, and injection drug use among HIV patients: Trends between 1996 and 2000 in 12 states. JAIDS J. Acquir. Immune Defic. Syndr. 2005, 40, 609–616. [Google Scholar] [CrossRef]
- Palella, F.J., Jr.; Baker, R.K.; Moorman, A.C.; Chmiel, J.S.; Wood, K.C.; Brooks, J.T.; Holmberg, S.D.; Investigators, H.O.S. Mortality in the highly active antiretroviral therapy era: Changing causes of death and disease in the HIV outpatient study. JAIDS J. Acquir. Immune Defic. Syndr. 2006, 43, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Mirani, G.; Williams, P.L.; Chernoff, M.; Abzug, M.J.; Levin, M.J.; Seage, G.R.; Oleske, J.M.; Purswani, M.U.; Hazra, R.; Traite, S. Changing trends in complications and mortality rates among US youth and young adults with HIV infection in the era of combination antiretroviral therapy. Clin. Infect. Dis. 2015, 61, 1850–1861. [Google Scholar] [CrossRef] [PubMed]
- National Center for Infectious Diseases Division of HIV/AIDS. 1993 Revised Classification System for HIV Infection and Expanded Surveillance Case Definition for AIDS Among Adolescents and Adults. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/00018871.htm (accessed on 10 December 2018).
- Gangcuangco, L.M.A.; Sawada, I.; Tsuchiya, N.; Do, C.D.; Pham, T.T.T.; Rojanawiwat, A.; Alejandria, M.; Leyritana, K.; Yokomaku, Y.; Pathipvanich, P.; et al. Regional Differences in the Prevalence of Major Opportunistic Infections among Antiretroviral-Naive Human Immunodeficiency Virus Patients in Japan, Northern Thailand, Northern Vietnam, and the Philippines. Am. J. Trop. Med. Hyg. 2017, 97, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Kadhiravan, T.; Banga, A.; Goyal, T.; Bhatia, I.; Saha, P.K. Spectrum of clinical disease in a series of 135 hospitalised HIV-infected patients from north India. BMC Infect. Dis. 2004, 4, 52. [Google Scholar] [CrossRef] [PubMed]
- Dore, G.J.; Li, Y.; McDonald, A.; Kaldor, J.M. Spectrum of AIDS-defining illnesses in Australia, 1992 to 1998: Influence of country/region of birth. JAIDS J. Acquir. Immune Defic. Syndr. 2001, 26, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Podlasin, R.B.; Wiercinska-Drapalo, A.; Olczak, A.; Beniowski, M.; Smiatacz, T.; Malolepsza, E.; Juszczyk, J.; Leszczyszyn-Pynka, M.; Mach, T.; Mian, M.; et al. Opportunistic infections and other AIDS-defining illnesses in Poland in 2000–2002. Infection 2006, 34, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Naba, M.R.; Kanafani, Z.A.; Awar, G.N.; Kanj, S.S. Profile of opportunistic infections in HIV-infected patients at a tertiary care center in Lebanon. J. Infect. Public Health 2010, 3, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Fenner, L.; Reid, S.E.; Fox, M.P.; Garone, D.; Wellington, M.; Prozesky, H.; Zwahlen, M.; Schomaker, M.; Wandeler, G.; Kancheya, N. Tuberculosis and the risk of opportunistic infections and cancers in HIV-infected patients starting ART in Southern Africa. Trop. Med. Int. Health 2013, 18, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.-D.; Park, S.W.; Kim, H.B.; Kim, U.S.; Kim, N.J.; Choi, H.J.; Shin, D.H.; Lee, J.S.; Choe, K. Spectrum of opportunistic infections and malignancies in patients with human immunodeficiency virus infection in South Korea. Clin. Infect. Dis. 1999, 29, 1524–1528. [Google Scholar] [CrossRef] [PubMed]
- Raviglione, M.C.; Snider, D.E., Jr.; Kochi, A. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA 1995, 273, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Pathmanathan, I.; Dokubo, E.K.; Shiraishi, R.W.; Agolory, S.G.; Auld, A.F.; Onotu, D.; Odafe, S.; Dalhatu, I.; Abiri, O.; Debem, H.C.; et al. Incidence and predictors of tuberculosis among HIV-infected adults after initiation of antiretroviral therapy in Nigeria, 2004–2012. PLoS ONE 2017, 12, e0173309. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.Y.; Chen, M.Y.; Hsiao, C.F.; Hsieh, S.M.; Hung, C.C.; Chang, S.C. Endemic fungal infections caused by Cryptococcus neoformans and Penicillium marneffei in patients infected with human immunodeficiency virus and treated with highly active anti-retroviral therapy. Clin. Microbiol. Infect. 2006, 12, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Luft, B.J.; Remington, J.S. Toxoplasmic encephalitis in AIDS. Clin. Infect. Dis. 1992, 15, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Vanhove, M.; Beale, M.A.; Rhodes, J.; Chanda, D.; Lakhi, S.; Kwenda, G.; Molloy, S.; Karunaharan, N.; Stone, N.; Harrison, T.S.; et al. Genomic epidemiology of Cryptococcus yeasts identifies adaptation to environmental niches underpinning infection across an African HIV/AIDS cohort. Mol. Ecol. 2017, 26, 1991–2005. [Google Scholar] [CrossRef]
- Park, B.J.; Wannemuehler, K.A.; Marston, B.J.; Govender, N.; Pappas, P.G.; Chiller, T.M. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. Aids 2009, 23, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Firdaus, R.; Santra, P.; Pal, J.; Roy, A.; Bhattacharya, M.K.; Chakrabarti, S.; Sadhukhan, P.C. Recent pattern of Co-infection amongst HIV seropositive individuals in tertiary care hospital, Kolkata. Virol. J. 2011, 8, 116. [Google Scholar] [CrossRef]
- Puthanakit, T.; Aurpibul, L.; Oberdorfer, P.; Akarathum, N.; Kanjananit, S.; Wannarit, P.; Sirisanthana, T.; Sirisanthana, V. Hospitalization and mortality among HIV-infected children after receiving highly active antiretroviral therapy. Clin. Infect. Dis. 2007, 44, 599–604. [Google Scholar] [CrossRef]
- Katano, H.; Hishima, T.; Mochizuki, M.; Kodama, Y.; Oyaizu, N.; Ota, Y.; Mine, S.; Igari, T.; Ajisawa, A.; Teruya, K. The prevalence of opportunistic infections and malignancies in autopsied patients with human immunodeficiency virus infection in Japan. BMC Infect. Dis. 2014, 14, 229. [Google Scholar] [CrossRef]
- Schwarcz, L.; Chen, M.-J.; Vittinghoff, E.; Hsu, L.; Schwarcz, S. Declining incidence of AIDS-defining opportunistic illnesses: Results from 16 years of population-based AIDS surveillance. Aids 2013, 27, 597–605. [Google Scholar] [CrossRef]
- Ramaswami, R.; Chia, G.; Dalla Pria, A.; Pinato, D.J.; Parker, K.; Nelson, M.; Bower, M. Evolution of HIV-Associated Lymphoma Over 3 Decades. J. Acquir. Immune Defic. Syndr. 2016, 72, 177–183. [Google Scholar] [CrossRef]
- Organization, W.H. Global Tuberculosis Report 2017. Available online: http://apps.who.int/iris/bitstream/handle/10665/259366/9789241565516-eng.pdf;jsessionid=59F6D752DAF549242F08D3F5D6A3571A?sequence=1 (accessed on 10 December 2018).
- Yang, C.; Gao, Q. Recent transmission of Mycobacterium tuberculosis in China: The implication of molecular epidemiology for tuberculosis control. Front. Med. 2018, 12, 76–83. [Google Scholar] [CrossRef]
- Hsueh, P.R.; Liu, Y.C.; So, J.; Liu, C.Y.; Yang, P.C.; Luh, K.T. Mycobacterium tuberculosis in Taiwan. J. Infect. 2006, 52, 77–85. [Google Scholar] [CrossRef]
- Lo, H.-Y.; Chou, P.; Yang, S.-L.; Lee, C.-Y.; Kuo, H.-S. Trends in tuberculosis in Taiwan, 2002–2008. J. Formos. Med. Assoc. 2011, 110, 501–510. [Google Scholar] [CrossRef]
- Department of Health, E.Y., R.O.C. (Taiwan). Mobilization Plan to Reduce Tuberculosis by Half in Ten Years. Available online: http://www.cdc.gov.tw/downloadfile.aspx?fid=ED4A28551F0CA7F5 (accessed on 10 December 2018).
- Department of Health, E.Y., R.O.C. (Taiwan). National Mobilization Plan to Halve TB in 10 Years—Phase 2. Available online: https://www.cdc.gov.tw/downloadfile_url.aspx?url=/Archives/c8966747-cf45-453d-a5ae-2c4b69f5ee09.pdf&filename=National+Mobilization+Plan+to+Halve+TB+in+10+Years+-+Phase+2 (accessed on 10 December 2018).
- Centers for Disease Control D.o.H., R.O.C.T. Statistics of Communicable Diseases and Surveillance Report Republic of China, 2005. Available online: http://61.57.41.133/uploads/files/212ed682-6f9b-465c-81fe-ba094a1f9ba8.pdf (accessed on 10 December 2018).
- Centers for Disease Control, D.o.H. (Taiwan), R.O.C. Taiwan Tuberculosis Control Report 2015. Available online: http://61.57.41.133/uploads/files/201702/785d5fb9-d98d-47d7-860f-9d5178668530.pdf (accessed on 10 December 2018).
- Sun, H.; Chen, M.; Hsieh, S.; Sheng, W.; Chang, S.; Hsiao, C.; Hung, C.; Chang, S. Changes in the clinical spectrum of opportunistic illnesses in persons with HIV infection in Taiwan in the era of highly active antiretroviral therapy. Jpn. J. Infect. Dis. 2006, 59, 311. [Google Scholar]
- Hung, C.-C.; Chen, M.-Y.; Hsieh, S.-M.; Sheng, W.-H.; Chang, S.-C. Clinical spectrum, morbidity, and mortality of acquired immunodeficiency syndrome in Taiwan: A 5-year prospective study. JAIDS J. Acquir. Immune Defic. Syndr. 2000, 24, 378–385. [Google Scholar] [CrossRef]
- Hsieh, S.M.; Hung, C.C.; Chen, M.Y.; Chang, S.C.; Hsueh, P.R.; Luh, K.T.; Chuang, C.Y. Clinical features of tuberculosis associated with HIV infection in Taiwan. J. Formos. Med. Assoc. 1996, 95, 923–928. [Google Scholar]
- Chiu, C.P.; Wong, W.W.; Kuo, B.; Tiao, T.M.; Fung, C.P.; Liu, C.Y. Clinical analysis of Mycobacterium tuberculosis infection in patients with acquired immunodeficiency syndrome. J. Microbiol. Immunol. Infect. 1999, 32, 250–256. [Google Scholar]
- Rice, B.; Elford, J.; Yin, Z.; Kruijshaar, M.; Abubakar, I.; Lipman, M.; Pozniak, A.; Kall, M.; Delpech, V. Decreasing incidence of tuberculosis among heterosexuals living with diagnosed HIV in England and Wales. Aids 2013, 27, 1151–1157. [Google Scholar] [CrossRef]
- Grant, A.D.; Bansi, L.; Ainsworth, J.; Anderson, J.; Delpech, V.; Easterbrook, P.; Fisher, M.; Gazzard, B.; Gilson, R.; Gompels, M.; et al. Tuberculosis among people with HIV infection in the United Kingdom: Opportunities for prevention? Aids 2009, 23, 2507–2515. [Google Scholar] [CrossRef]
- Winter, J.R.; Stagg, H.R.; Smith, C.J.; Brown, A.E.; Lalor, M.K.; Lipman, M.; Pozniak, A.; Skingsley, A.; Kirwan, P.; Yin, Z.; et al. Injecting drug use predicts active tuberculosis in a national cohort of people living with HIV. Aids 2017, 31, 2403–2413. [Google Scholar] [CrossRef]
- Lesko, C.R.; Cole, S.R.; Zinski, A.; Poole, C.; Mugavero, M.J. A systematic review and meta-regression of temporal trends in adult CD4+ cell count at presentation to HIV care, 1992–2011. Clin. Infect. Dis. 2013, 57, 1027–1037. [Google Scholar] [CrossRef]
- Barry, S.M.; Lloyd-Owen, S.J.; Madge, S.J.; Cozzi-Lepri, A.; Evans, A.J.; Phillips, A.N.; Johnson, M.A. The changing demographics of new HIV diagnoses at a London centre from 1994 to 2000. HIV Med. 2002, 3, 129–134. [Google Scholar] [CrossRef]
- Selik, R.M.; Mokotoff, E.D.; Branson, B.; Owen, S.M.; Whitmore, S.; Hall, H.I. Revised Surveillance Case Definition for HIV Infection—United States, 2014. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6303a1.htm (accessed on 10 December 2018).
- Jeong, S.J.; Italiano, C.; Chaiwarith, R.; Ng, O.T.; Vanar, S.; Jiamsakul, A.; Saphonn, V.; Nguyen, K.V.; Kiertiburanakul, S.; Lee, M.P. Late presentation into care of HIV disease and its associated factors in Asia: Results of TAHOD. AIDS Res. Hum. Retrovir. 2016, 32, 255–261. [Google Scholar] [CrossRef]
- Iroezindu, M.O. Disparities in the Magnitude of Human Immunodeficiency Virus-related Opportunistic Infections Between High and Low/Middle-income Countries: Is Highly Active Antiretroviral Therapy Changing the Trend? Ann. Med. Health Sci. Res. 2016, 6, 4–18. [Google Scholar] [CrossRef]
- Yazdanpanah, Y.; Chene, G.; Losina, E.; Goldie, S.J.; Merchadou, L.D.; Alfandari, S.; Seage, G.R., 3rd; Sullivan, L.; Marimoutou, C.; Paltiel, A.D.; et al. Incidence of primary opportunistic infections in two human immunodeficiency virus-infected French clinical cohorts. Int. J. Epidemiol. 2001, 30, 864–871. [Google Scholar] [CrossRef]
- Organization, W.H. WHO Policy on Collaborative TB/HIV Activities: Guidelines for National Programmes and Other Stakeholders. Available online: https://www.ncbi.nlm.nih.gov/books/NBK131887/ (accessed on 10 December 2018).
- Day, C.L.; Abrahams, D.A.; Harris, L.D.; van Rooyen, M.; Stone, L.; de Kock, M.; Hanekom, W.A. HIV-1 Infection Is Associated with Depletion and Functional Impairment of Mycobacterium tuberculosis-Specific CD4 T Cells in Individuals with Latent Tuberculosis Infection. J. Immunol. 2017, 199, 2069–2080. [Google Scholar] [CrossRef]
- Armenta, R.F.; Collins, K.M.; Strathdee, S.A.; Bulterys, M.A.; Munoz, F.; Cuevas-Mota, J.; Chiles, P.; Garfein, R.S. Mycobacterium tuberculosis infection among persons who inject drugs in San Diego, California. Int. J. Tuberc. Lung Dis. 2017, 21, 425–431. [Google Scholar] [CrossRef]
- Valenca, M.S.; Scaini, J.L.; Abileira, F.S.; Goncalves, C.V.; von Groll, A.; Silva, P.E. Prevalence of tuberculosis in prisons: Risk factors and molecular epidemiology. Int. J. Tuberc. Lung Dis. 2015, 19, 1182–1187. [Google Scholar] [CrossRef]
- Biadglegne, F.; Rodloff, A.C.; Sack, U. Review of the prevalence and drug resistance of tuberculosis in prisons: A hidden epidemic. Epidemiol. Infect. 2015, 143, 887–900. [Google Scholar] [CrossRef]
- Akolo, C.; Adetifa, I.; Shepperd, S.; Volmink, J. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst. Rev. 2010. [Google Scholar] [CrossRef]
- Naing, C.; Mak, J.W.; Maung, M.; Wong, S.F.; Kassim, A.I. Meta-analysis: The association between HIV infection and extrapulmonary tuberculosis. Lung 2013, 191, 27–34. [Google Scholar] [CrossRef]
- Jones, B.E.; Young, S.M.; Antoniskis, D.; Davidson, P.T.; Kramer, F.; Barnes, P.F. Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection. Am. Rev. Respir. Dis. 1993, 148, 1292–1297. [Google Scholar] [CrossRef]
- Du Bruyn, E.; Wilkinson, R.J. The Immune Interaction between HIV-1 Infection and Mycobacterium tuberculosis. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Melsew, Y.A.; Doan, T.N.; Gambhir, M.; Cheng, A.C.; McBryde, E.; Trauer, J.M. Risk factors for infectiousness of patients with tuberculosis: A systematic review and meta-analysis. Epidemiol. Infect. 2018, 146, 345–353. [Google Scholar] [CrossRef]
- Centers for Disease Control. Taiwan National Infectious Disease Statistics System. Available online: https://nidss.cdc.gov.tw/en/Default.aspx (accessed on 10 December 2018).

| Total (2001–2015) n = 1757 | Period 1 (2001–2005) n = 194 | Period 2 (2006–2010) n = 492 | Period 3 (2011–2015) n = 1071 | p-Value | |
|---|---|---|---|---|---|
| Follow-up period, median (IQR; days) | 741 (961) | 724 (880) | 551 (893) | 829 (981) | <0.001 |
| Male, n (%) | 1709 (97.3) | 186 (95.9) | 468 (95.1) | 1055 (98.5) | <0.001 |
| Mean age at presentation of HIV, years (SD) | 30.21 (9.75) | 31.54 (10.09) | 31.43 (10.32) | 29.41 (9.33) | <0.001 |
| HIV transmission route, n (%) | |||||
| Homosexual | 1367 (77.8) | 130 (67.0) | 358 (72.8) | 879 (82.1) | <0.001 |
| Heterosexual | 228 (13.0) | 38 (19.6) | 80 (16.3) | 110 (10.3) | <0.001 |
| Bisexual | 88 (5.0) | 0 (0.0) | 24 (4.9) | 64 (6.0) | 0.002 |
| IVDU | 65 (3.7) | 26 (13.4) | 26 (5.3) | 13 (1.2) | <0.001 |
| Unknown | 9 (0.5) | 0 (0.0) | 4 (0.8) | 5 (0.5) | 0.384 |
| Comorbidities, n (%) | |||||
| Chronic kidney disease | 7 (0.4) | 0 (0.0) | 2 (0.4) | 5 (0.5) | 0.637 |
| Diabetes mellitus | 41 (2.3) | 7 (3.6) | 16 (3.3) | 18 (1.7) | 0.074 |
| Congestive heart failure | 3 (0.2) | 0 (0.0) | 0 (0.0) | 3 (0.3) | 0.382 |
| Solid tumour | 13 (0.7) | 1 (0.5) | 4 (0.8) | 8 (0.7) | 0.919 |
| Haematological cancer | 5 (0.3) | 2 (1.0) | 2 (0.4) | 1 (0.1) | 0.066 |
| Autoimmune disease | 12 (0.7) | 3 (1.5) | 3 (0.6) | 6 (0.6) | 0.300 |
| Organ transplantation | 2 (0.1) | 2 (1.0) | 0 (0.0) | 0 (0.0) | <0.001 |
| Mean CD4+ count at presentation, cells/L (SD) | 283 (214) | 319 (229) | 284 (227) | 275 (204) | 0.031 |
| Subgroup of CD4+ count, cells/L (%) a | |||||
| ≤200 | 685 (39.0) | 70 (36.1) | 199 (40.4) | 416 (38.8) | 0.566 |
| 201–350 | 492 (28.0) | 52 (26.8) | 122 (24.8) | 318 (29.7) | 0.125 |
| 351–500 | 320 (18.2) | 25 (12.9) | 99 (20.1) | 196 (18.3) | 0.086 |
| ≥501 | 260 (14.8) | 47 (24.2) | 72 (14.6) | 141 (13.2) | <0.001 |
| HIV VL≥100,000 copies/mL, n (%) a | 698 (39.7) | 86 (44.3) | 205 (41.7) | 407 (38.0) | 0.148 |
| HIV stage at presentation by 2014 CDC definition [48], n (%) | |||||
| Stage 0 (Acute HIV) | 124 (7.1) | 7 (3.6) | 36 (7.3) | 81 (7.6) | 0.136 |
| Stage 1 (CD4+ count ≥500 cells/L) | 231 (13.1) | 41 (21.1) | 66 (13.4) | 124 (11.6) | 0.001 |
| Stage 2 (CD4+ count 200–499 cells/L) | 729 (41.5) | 69 (35.6) | 195 (39.6) | 465 (43.7) | 0.077 |
| Stage 3 (AIDS) | 673 (38.3) | 77 (39.7) | 195 (39.6) | 401 (37.4) | 0.649 |
| AOIs during observation period | |||||
| Patients with AOI(s) at HIV presentation, n (%) | 337 (19.2) | 42 (21.6) | 100 (20.3) | 195 (18,2) | 0.4 |
| Patients who developed AOI(s) within the study period, n (%) | 414 (23.6) | 50 (25.8) | 129 (26.2) | 235 (21.9) | 0.134 |
| Total | 2001–2005 | 2006–2010 | 2011–2015 | p | ||
|---|---|---|---|---|---|---|
| No. of Episodes, n = 635 | CD4+ T cell, cell/mm3 (Median, IQR) | No. of Episodes, n = 85 | No. of Episodes, n = 207 | No. of Episodes, n = 343 | ||
| Opportunistic infection, n (%) | ||||||
| Pneumocystis jirovecii pneumonia | 266 (57.8) | 27 (51) | 33 (58.9) | 84 (57.1) | 149 (58.0) | 0.971 |
| Cytomegalovirus disease (other than liver, spleen, or nodes) | 55 (12.0) | 25 (75) | 3 (5.4) | 14 (9.5) | 38 (14.8) | 0.078 |
| Mycobacterium tuberculosis infection | 78 (17.0) | 87 (167) | 22 (39.3) | 32 (21.8) | 24 (9.3) | <0.001 |
| Wasting syndrome | 47 (10.2) | 50.5 (95) | 6 (10.7) | 12 (8.2) | 29 (11.3) | 0.604 |
| Candidiasis (oesophagus, bronchi, trachea, lung) | 54 (11.7) | 22 (56) | 13 (23.2) | 17 (11.6) | 24 (9.3) | 0.014 |
| Cryptococcosis, extrapulmonary | 37 (8.0) | 31 (45) | 4 (7.1) | 15 (10.2) | 18 (7.0) | 0.505 |
| Disseminated Mycobacterium avium complex infection or M. kansasii | 30 (6.5) | 35 (56) | 2 (3.6) | 11 (7.5) | 17 (6.6) | 0.599 |
| Cryptosporidiosis, chronic intestinal | 6 (1.3) | 28 (149) | 0 (0.0) | 1 (0.7) | 5 (1.9) | 0.367 |
| HIV encephalopathy | 7 (1.5) | 121 (207) | 0 (0.0) | 3 (2.0) | 4 (1.6) | 0.568 |
| HSV, chronic ulcer greater >1 month; or bronchitis, pneumonitis, or oesophagitis | 5 (1.1) | 66.5 (137) | 0 (0.0) | 0 (0.0) | 5 (1.9) | 0.136 |
| Salmonellosis septicaemia, recurrent | 5 (1.1) | 32 (31) | 0 (0.0) | 1 (0.7) | 4 (1.6) | 0.505 |
| Recurrent pneumonia | 3 (0.7) | 25 | 0 (0.0) | 1 (0.7) | 2 (0.8) | 0.806 |
| Progressive multifocal leukoencephalopathy | 3 (0.7) | 120 | 1 (1.8) | 0 (0.0) | 2 (0.8) | 0.343 |
| Toxoplasma encephalitis | 2 (0.4) | 45 | 1 (1.8) | 0 (0.0) | 1 (0.4) | 0.221 |
| Talaromyces marneffei, disseminated or extrapulmonary | 2 (0.4) | 18 | 0 (0.0) | 1 (0.7) | 1 (0.4) | 0.794 |
| Histoplasmosis, disseminated or extrapulmonary | 0 (0.0) | N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) | N/A |
| Coccidioidomycosis, disseminated or extrapulmonary | 0 (0.0) | N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) | N/A |
| Isosporiasis, chronic intestinal (>1 month) | 0 (0.0) | N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) | N/A |
| Opportunistic malignancy | ||||||
| Kaposi’s sarcoma | 16 (3.5) | 24 (207) | 0 (0.0) | 9 (6.1) | 7 (2.7) | 0.063 |
| Lymphoma | 18 (3.9) | 109 (153) | 0 (0.0) | 5 (3.4) | 13 (5.1) | 0.194 |
| Invasive cervical cancer | 1 (0.2) | N/A | 0 (0.0) | 1 (0.7) | 0 (0.0) | 0.344 |
| Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| Crude OR (95% CI) | p | Adjusted OR (95% CI) | p | |
| Age, per 10-year increase | 1.68 (1.38–2.04) | <0.001 | 1.40 (1.08–1.79) | 0.009 |
| Male | 0.4 (0.14–1.15) | 0.089 | 0.90 (0.27–3.01) | 0.86 |
| HIV transmission route | ||||
| Homosexual | Reference | Reference | ||
| Heterosexual | 2.50 (1.38–4.55) | 0.003 | 1.01 (0.49–2.12) | 0.97 |
| Bisexual | 0.77 (0.18–3.25) | 0.72 | 0.62 (0.14–2.73) | 0.53 |
| IVDU | 3.37 (1.38–8.27) | 0.008 | 2.56 (0.92–7.12) | 0.072 |
| Unknown | 0.00 (N/A) | 0.99 | 0.00 (N/A) | 0.99 |
| Period | ||||
| Period 1 (2001–2005) | Reference | Reference | ||
| Period 2 (2006–2010) | 0.47 (0.25–0.86) | 0.015 | 0.43 (0.22–0.83) | 0.012 |
| Period 3 (2011–2015) | 0.16 (0.08–0.30) | <0.001 | 0.16 (0.08–0.32) | <0.001 |
| Subgroup of CD4 cell count at presentation | ||||
| CD4 count ≥ 501 cells/µL | Reference | Reference | ||
| CD4 count 351–500 cells/µL | 0.54 (0.09–3.25) | 0.50 | 0.78 (0.13–4.80) | 0.79 |
| CD4 count 201–350 cells/µL | 1.06 (0.26–4.26) | 0.94 | 1.43 (0.34–5.96) | 0.62 |
| CD4 count < 200 cells/µL | 7.18 (2.22–23.20) | 0.001 | 7.71 (2.20–27.05) | 0.001 |
| VL≥100,000 copies/mL | 3.50 (2.04–5.98) | <0.001 | 1.60 (0.88–2.90) | 0.12 |
| Diabetes mellitus | 0.66 (0.09–4.85) | 0.68 | 0.23 (0.03–1.87) | 0.17 |
| Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| Crude HR (95% CI) | p | Adjusted HR (95% CI) | p | |
| Age, per 10-year increase | 1.12 (0.69–1.81) | 0.66 | 0.96 (0.50–1.86) | 0.91 |
| Male | 21.07 (0.00–9,912,364.81) | 0.647 | 371,294.35 (0.00–∞) | 0.99 |
| HIV transmission route | ||||
| Homosexual | Reference | Reference | ||
| Heterosexual | 0.60 (0.08–4.72) | 0.63 | 0.59 (7–4.91) | 0.63 |
| Bisexual | 0.00 (0.00–∞) | 0.98 | 0.00 (0.00–∞) | 0.99 |
| IVDU | 9.29 (2.91–29.65) | 5.63 (1.44–21.99) | 0.013 | |
| Unknown | 0.00 (N/A) | 0.99 | 0.00 (N/A) | 0.99 |
| Period | ||||
| Period 1 (2001–2005) | Reference | Reference | ||
| Period 2 (2006–2010) | 1.27 (0.35–4.71) | 0.72 | 1.08 (0.28–4.14) | 0.91 |
| Period 3 (2011–2015) | 0.16 (0.03–0.78) | 0.02 | 0.18 (0.04–0.91) | 0.038 |
| Subgroup of CD4 cell count at presentation | ||||
| CD4 count ≥ 501 cells/µL | Reference | Reference | ||
| CD4 count 351–500 cells/µL | 0.41 (0.07–2.22) | 0.30 | 0.68 (0.12–3.88) | 0.67 |
| CD4 count 201–350 cells/µL | 0.51 (0.13–2.03) | 0.34 | 0.80 (0.19–3.29) | 0.75 |
| CD4 count < 200 cells/µL | 0.52 (0.14–1.94) | 0.33 | 1.01 (0.23–4.45) | 0.99 |
| VL≥100,000 copies/mL | 1.09 (0.39–3.06) | 0.87 | 1.20 (0.38–3.74) | 0.76 |
| Diabetes mellitus | 0.05 (0.00–170,215.51) | 0.693 | 0.00 (0.00–∞) | 0.99 |
| Total (2001–2015) n = 78 | Period 1 (2001–2005) n = 22 | Period 2 (2006–2010) n = 32 | Period 3 (2011–2015) n = 24 | p-Value | |
|---|---|---|---|---|---|
| Age at event, n (%) | 36.1 (10.2) | 35.9 (10.7) | 36.8 (10.3) | 35.3 (10.2) | 0.863 |
| Male, n (%) | 73 (93.6) | 22 (100) | 28 (87.5) | 23 (95.8) | 0.158 |
| Event within 90 days of enrolment, n (%) | 66 (84.6) | 20 (90.9) | 27 (84.4) | 19 (79.2) | 0.544 |
| With extrapulmonary TB, n (%) | 31 (39.7) | 8 (36.4) | 11 (34.4) | 12 (50.0) | 0.462 |
| CD4+ counts <200 cells/L, n (%) | 61 (78.2) | 17 (77.3) | 25 (78.1) | 19 (79.2) | 0.988 |
| VL on event (>100,000 copies/mL), n (%) | 47 (60.3) | 13 (59.1) | 20 (62.5) | 14 (58.3) | 0.943 |
| Attributable mortality, n (%) | 8 (10.3) | 3 (13.6) | 3 (9.4) | 2 (8.3) | 0.820 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-Y.; Wu, P.-H.; Lu, P.-L.; Tsai, H.-C. Changing Spectrum of Opportunistic Illnesses among HIV-Infected Taiwanese Patients in Response to a 10-Year National Anti-TB Programme. J. Clin. Med. 2019, 8, 163. https://doi.org/10.3390/jcm8020163
Lee C-Y, Wu P-H, Lu P-L, Tsai H-C. Changing Spectrum of Opportunistic Illnesses among HIV-Infected Taiwanese Patients in Response to a 10-Year National Anti-TB Programme. Journal of Clinical Medicine. 2019; 8(2):163. https://doi.org/10.3390/jcm8020163
Chicago/Turabian StyleLee, Chun-Yuan, Pei-Hua Wu, Po-Liang Lu, and Hung-Chin Tsai. 2019. "Changing Spectrum of Opportunistic Illnesses among HIV-Infected Taiwanese Patients in Response to a 10-Year National Anti-TB Programme" Journal of Clinical Medicine 8, no. 2: 163. https://doi.org/10.3390/jcm8020163
APA StyleLee, C.-Y., Wu, P.-H., Lu, P.-L., & Tsai, H.-C. (2019). Changing Spectrum of Opportunistic Illnesses among HIV-Infected Taiwanese Patients in Response to a 10-Year National Anti-TB Programme. Journal of Clinical Medicine, 8(2), 163. https://doi.org/10.3390/jcm8020163




