Origanum majorana Essential Oil Inhalation during Neurofeedback Training Reduces Saliva Myeloperoxidase Activity at Session-1 in Bruxistic Patients
Abstract
1. Introduction
Aim
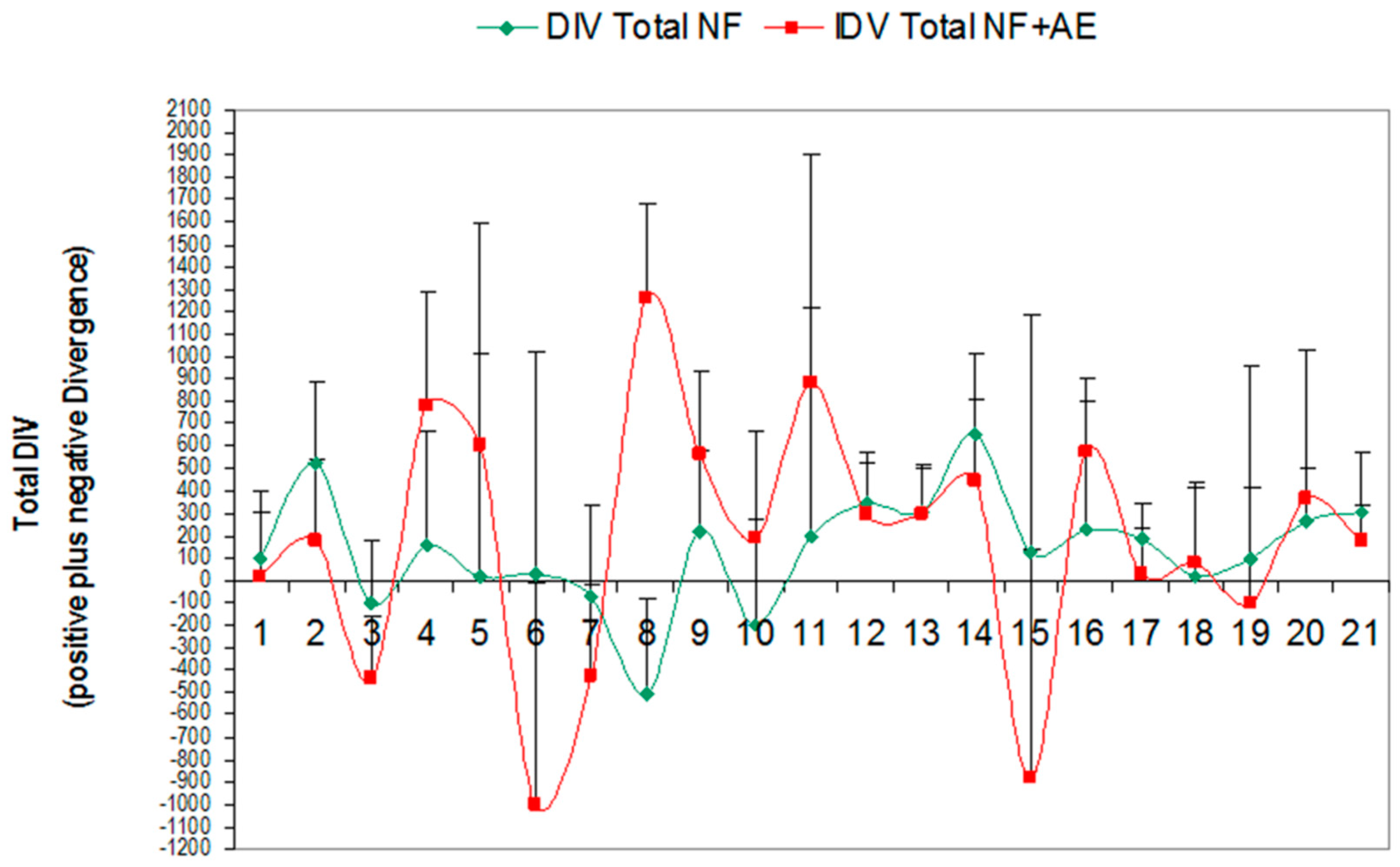
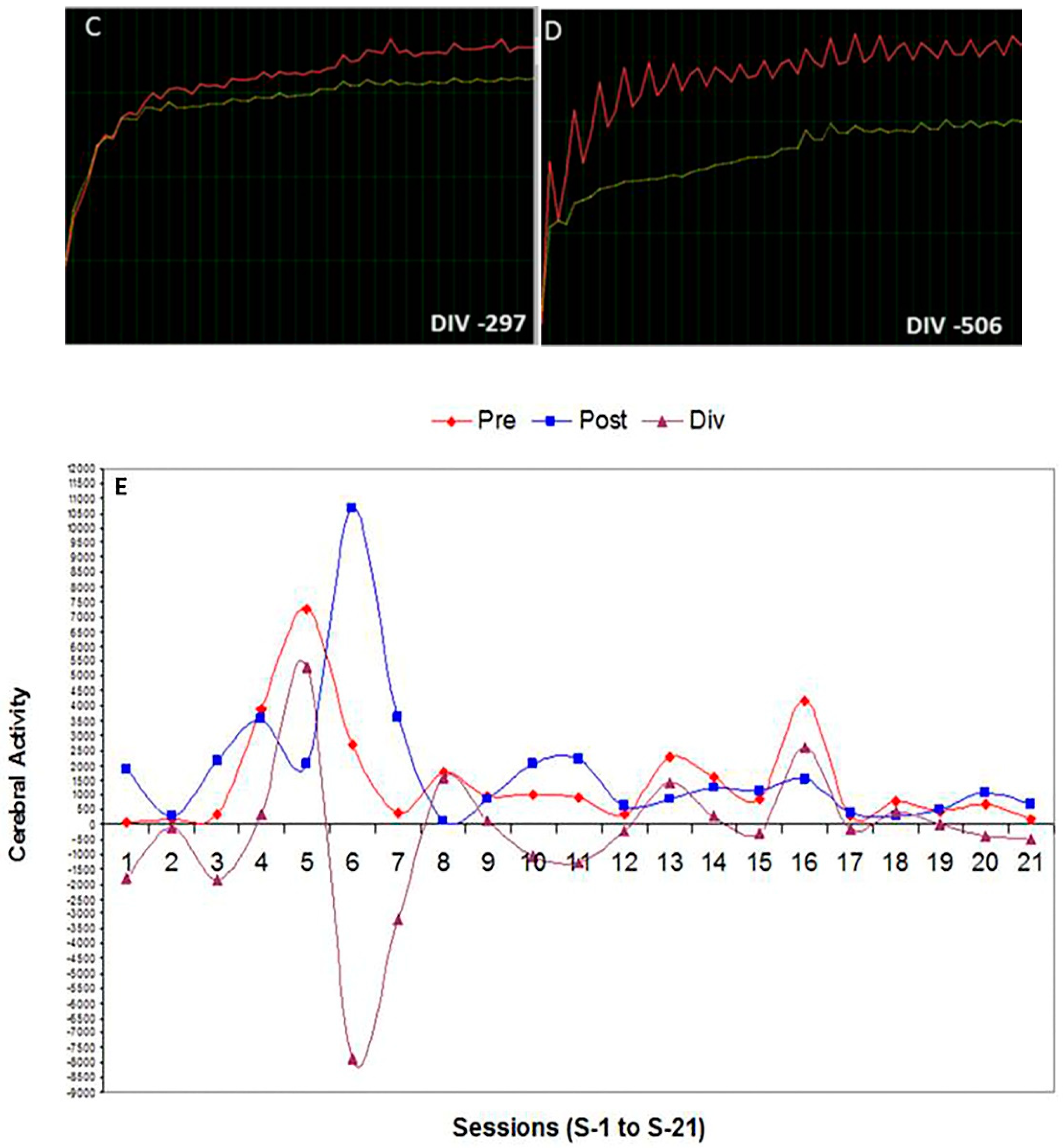
- We evaluate whether NO training in 21 consecutive training sessions could decrease cerebral activity (divergence) in bruxistic patients with high “intrinsic stress” and whether smelling Origanum majorana essential oil during NO training affect results.
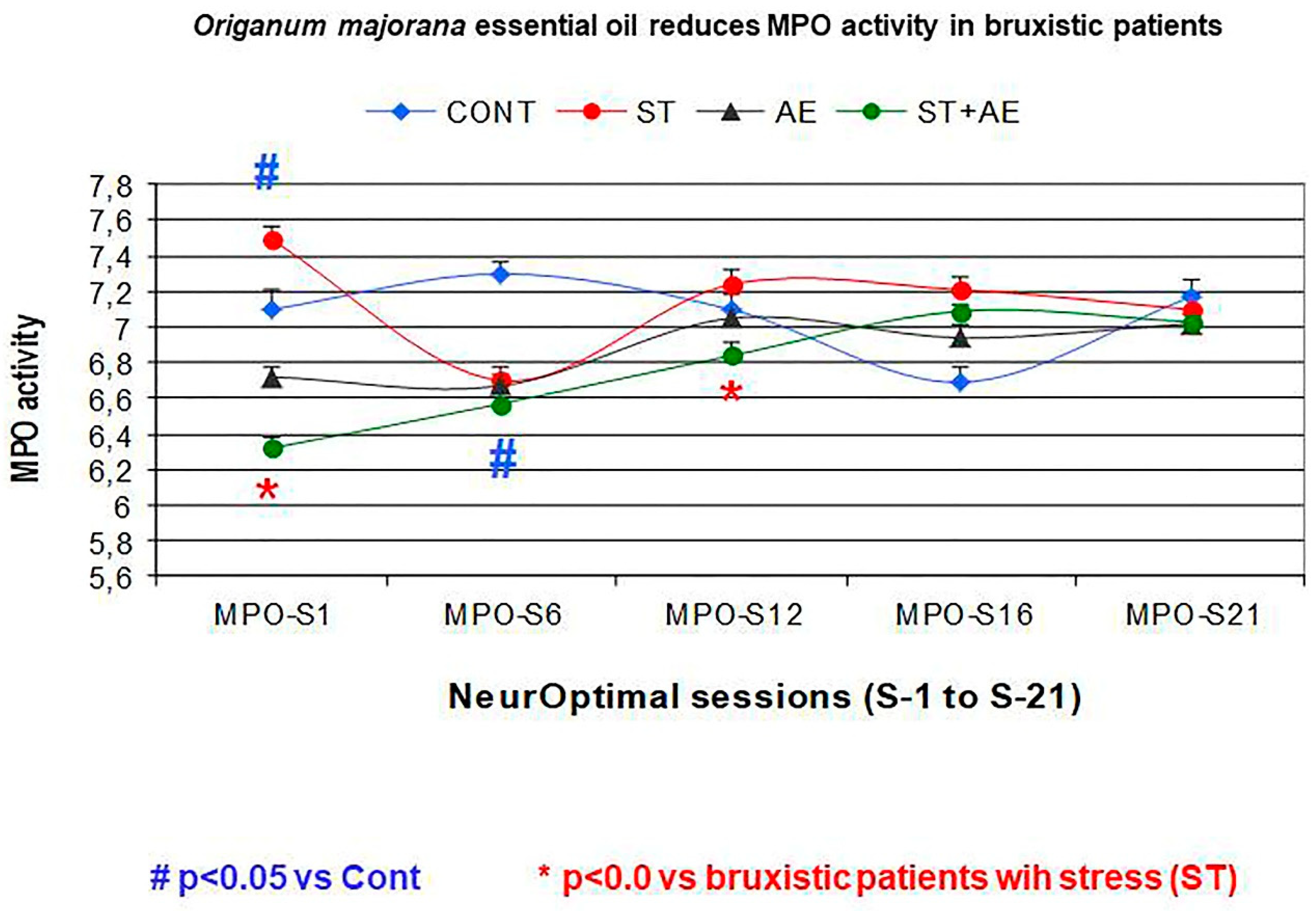
- We evaluated whether Origanum majorana essential oil (1% impregnated into nasal filter) could regulate their saliva MPO activity during 21 NO training sessions by measuring every six the NO sessions, including the first and last of 21 sessions (MPO: S1, 6, 12, 18, 21) in bruxistic patients exposed to this fragrance in 21 NO sessions and those NO trained bruxistic patients not exposed to this essential oil.
- We also evaluated whether Origanum majorana essential oil inhalation during 21 NO training sessions could reduce stress symptoms in bruxistic patients by decreasing their PSS (stress perceived scale) scores as compared to unexposed bruxistic patients to this fragance during 21 NO sessions.
2. Materials and Methods
2.1. Colorimetric Assay for Saliva Mieloperoxidase (MPO) Activity
2.2. Origanum Majorana Essential Oil
2.3. Sample Analysis
2.3.1. Study Groups: Design
2.3.2. Inclusion Criteria
- Q1
- In the last 3 months, has it been pointed out to you that you make a tooth grinding sound during sleep?
- Q2
- Do you experience orofacial jaw muscle fatigue or pain when you are awake?
- Q3
- When you are concentrating on something, or during work, do your upper and lower teeth
2.3.3. Exclusion Criteria
2.4. Depression, Anxiety and Stress Scale (DASS-42)
2.5. The Perceived Stress Scale (PSS)
2.6. NeurOptimal Technology: A Global Neurofeedback Technology that Measures Divergence (An Index of Cerebral Activity Capable of Predicting Brain Stability)
Neurofeedback (NO) Training Session
3. Results
3.1. Effect of Global Neurofeedback (NO) Overtraining during 21 Sessions on Total Divergence (Brain Activity) in Bruxistic Patients that Smell Origanum majorana Essential Oil
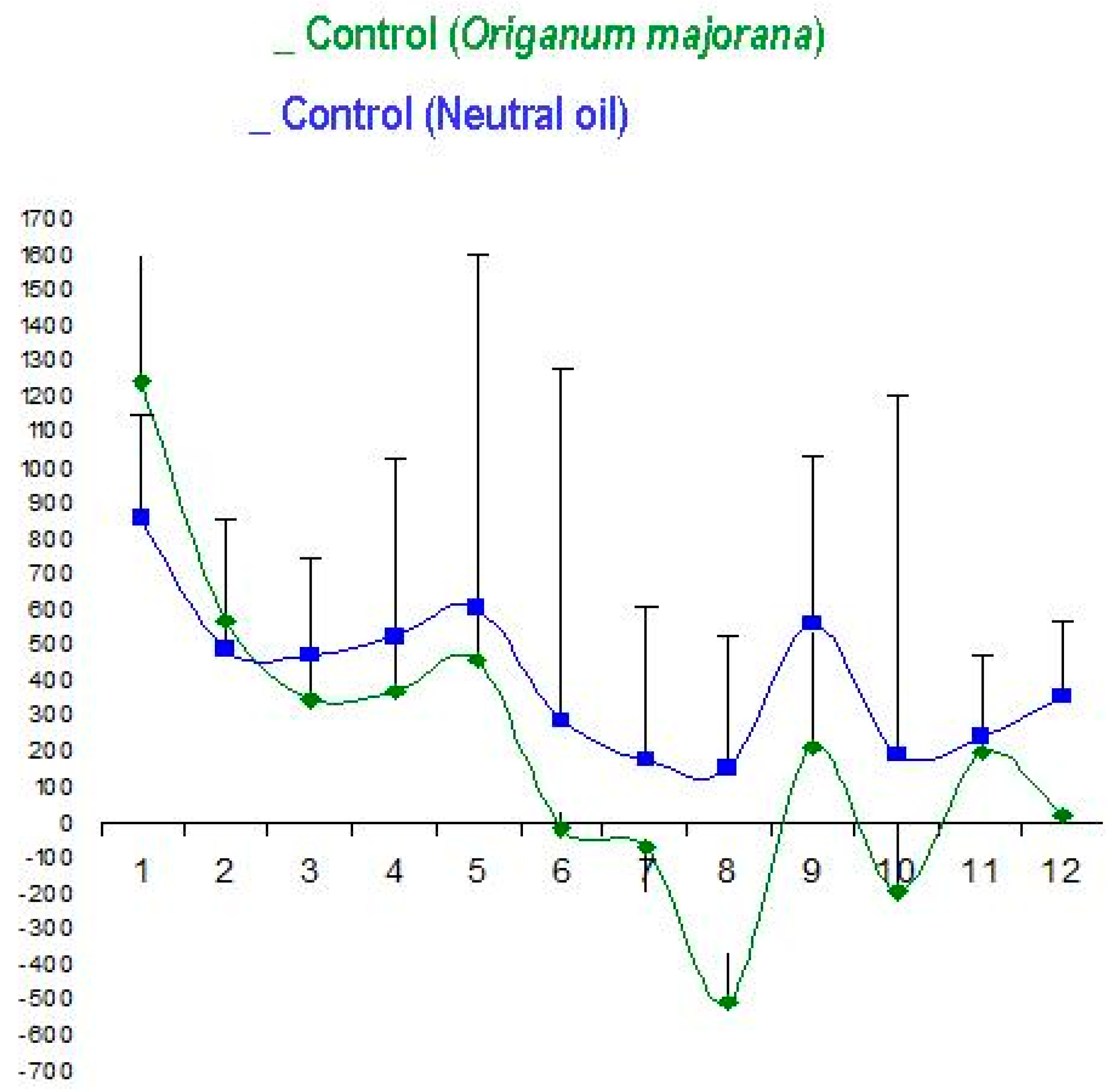
3.2. Origanum majorana Essential Oil Has not Effect on NO Training Sessions in controls (without Stress) as compared to Placebo-Treated Patients with a Neutral Oil in Controls (without Stress)
3.3. Effect of Neurofeedback Training (NO) and/or Origanum Majorana Exposure during NO Sessions on Myeloperoxidase (MPO) Activity
3.4. Effects of Neurofeedback Training (NO) and/or Origanum majorana Exposure on Stress Levels in the Stress Perceived Scale (PSS)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lobbezoo, F.; Ahlberg, K.J.; Raphael, A.G.; Wetselaar, A.G.; Glaros, T.; Kato, V.; Santiago, E.; Vinocur, A.; De Laat, R.; De Leeuw, K.; et al. International consensus on the assessment of bruxism: Report of a work in progress. J. Oral Rehabil. 2018, 45, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Lobbezoo, F.; Naeije, M. Bruxism is mainly regulated centrally, not peripherally. J. Oral Rehabil. 2001, 28, 1085–1091. [Google Scholar] [CrossRef]
- Bayar, G.R.; Tutuncu, R.; Acikel, C. Psychopathological profile of patients with different forms of bruxism. Clin. Oral Investig. 2012, 16, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Castroflorio, T.; Bargellini, A.; Rossini, G.; Cugliari, G.; Deregibus, A.; Manfredini, D. Agreement between clinical and portable EMG/ECG diagnosis of sleep bruxism. J. Oral Rehabil. 2015, 42, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Monaco, A.; Ciammella, N.M.; Marci, M.C.; Pirro, R.; Giannoni, M. The anxiety in bruxer child. A case-control study. Minerva Stomatol. 2002, 51, 247–250. [Google Scholar] [PubMed]
- Saulue, P.; Carra, M.C.; Laluque, J.F.; D’incau, E. Understanding bruxism in children and adolescents. Int. Orthod. 2015, 13, 489–506. [Google Scholar] [CrossRef] [PubMed]
- Vanderas, A.P.; Manetas, K.J. Relationship between malocclusion and bruxism in children and adolescents: A review. Pediatr. Dent. 1995, 17, 7–12. [Google Scholar] [PubMed]
- Katayoun, E.; Sima, F.; Naser, V.; Anahita, D. Study of the relationship of psychosocial disorders to bruxism in adolescents. J. Indian Soc. Pedod. Prev. Dent. 2008, 26 (Suppl. 3), S91–S97. [Google Scholar]
- Abekura, H.; Tsuboi, M.; Okura, T.; Kagawa, S.; Sadamori, S.; Akagawa, Y. Association between sleep bruxism and stress sensitivity in an experimental psychological stress task. Biomed. Res. 2011, 6, 395–399. [Google Scholar] [CrossRef]
- Weingerg, M.A.; Eskow, R. Periodontal terminology revised. J. Periodontol. 2003, 74, 563–565. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, D.; Landi, N.; Romagnoli, M.; Bosco, M. Psychic and occlusal factors in bruxers. Aust. Dent. J. 2004, 49, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Ortu, E.; Pietropaoli, D.; Marchetti, E.; Marchili, N.; Marzo, G.; Monaco, A. Bruxism in children: Use of the Functional Plane of Monaco (FPM). Eur. J. Paediatr. Dent. 2018, 19, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Khoury, S.; Carra, M.C.; Huynh, N.; Montplaisir, J.; Lavigne, G.J. Sleep Bruxism-Tooth Grinding Prevalence, Characteristics and Familial Aggregation: A Large Cross-Sectional Survey and Polysomnographic Validation. Sleep 2016, 39, 2049–2056. [Google Scholar] [CrossRef] [PubMed]
- Dawson, A.; Stensson, N.; Ghafouri, B.; Gerdle, B.; List, T.; Svensson, P.; Ernberg, M. Dopamine in plasma -a biomarker for myofascial TMD pain? J. Headache Pain 2016, 17, 65. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Kato, T.; Yoshizawa, S.; Suganuma, T.; Takaba, M.; Ono, Y.; Yoshizaw, A.; Yoshida, Y.; Kurihara, T.; Ishii, M.; Kawana, F.; et al. Effect of clonazepam and clonidine on primary sleep bruxism: A double-blind, crossover, placebocontrolled. J. Sleep Res. 2017, 26, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, C.C.; Alvarez, E.; Jaramillo, C.; Vélez, C.; Valencia, I. Effects of psychological techniques on bruxism in children with primary teeth. J. Oral Rehabil. 2001, 28, 354–360. [Google Scholar] [CrossRef]
- Raigrodski, A.J.; Mohamed, S.E.; Gardiner, D.M. The effect of amitriptyline on pain intensity and perception of stress in bruxers. J. Prosthodont. 2001, 10, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Da Rosa, C.; Câmara, S.G.; Béria, J.U. Representations and use intention of phytotherapy in primary health care. Cienc. Saude Colet. 2011, 16, 311–318. [Google Scholar]
- Carvalho, B.; Cordeiro da Silva, F.; Consolação, S.; Motta, L.J.; Curiki, L.M.; Agnelli, R.; Mesquita-Ferarri, K.; Santos Fernandes, P.; Bussadori, S.K. Evaluation of electromyographic signals in children with bruxism before and after therapy with Melissa Officinalis L—A randomized controlled clinical trial. J. Phys. Ther. Sci. 2016, 28, 738–742. [Google Scholar]
- Palombo, E.A. Traditional medicinal plant extracts and natural products with activity against oral bacteria: Potential application in the prevention and treatment of oral diseases. Evid. Based Complement. Altern. Med. 2011, 2011, 680354. [Google Scholar] [CrossRef]
- Groppo, F.C.; Bergamaschi, C.C.; Cogo, K.; Franz-Montan, M.; Motta, R.H.; de Andrade, E.E. Use of phytotherapy in dentistry. Phytother. Res. 2008, 22, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Babpour, E.; Angaji, S.A.; Angaji, S.M. Antimicrobial effects of four medicinal plants on dental plaque. J. Med. Plants Res. 2009, 3, 132–137. [Google Scholar]
- Shaelb, F.; Khan, S.N.; Thakur, M.; Kohan-Ghard, H.R.; Drewlo, S.; Saed, G.M.; Pennathur, S.; Abu Soud, H.M. The impact of Myeloperoxidase and Activated Macrophages on Metaphase II mouse oocyte quality. PLoS ONE 2016, 11, e0151160. [Google Scholar]
- Cabassi, A.; Binno, S.M.; Tedeschi, S.; Graiani, G.; Galizia, C.; Bianconcini, M.; Coghi, P.; Fellini, F.; Ruffini, L.; Govoni, P.; Piepoli, M.; Perlini, S.; et al. Myeloperoxidase-related chlorination activity is positively associated with circulating ceruloplasmin in chronic heart failure patients: Relationship with neurohormonal, inflammatory, and nutritional parameters. BioMed Res. Int. 2015, 2015, 691693. [Google Scholar]
- Marcaccini, A.M.; Amato, P.A.; Leão, F.V.; Gerlach, R.F.; Ferreira, J.T. Myeloperoxidase activity is increased in gingival crevicular fluid and whole saliva after fixed orthodontic appliance activation. Am. J. Orthod. Dentofac. Orthop. 2010, 138, 613–616. [Google Scholar] [CrossRef]
- Moss, M.; Cook, J.; Wesnes, K.; Duckett, P. Aromas of rosemary and lavender essential oils differentially affect cognition and mood in healthy adults. Int. J. Neurosci. 2003, 113, 15–38. [Google Scholar] [CrossRef]
- Mitropoulou, G.; Fitsiou, E.; Stavropoulou, E.; Papavassilopoulou, E.; Vamvakias, M.; Pappa, A.; Oreopoulou, A.; Kourkoutas, Y. Composition, antimicrobial, antioxidant, and antiproliferative activity of Origanum dictamnus (dittany) essential oil. Microb. Ecol. Health Dis. 2015, 6, 26543. [Google Scholar]
- Bina, F.; Rahimi, R. Sweet Marjoram: A Review of Ethnopharmacology, Phytochemistry, and Biological Activities. J. Evid. Based Complement. Altern. Med. 2016, 22, 175–185. [Google Scholar] [CrossRef]
- Tepe, B.; Cakir, A.; Sihoglu, A. Medicinal Uses, Phytochemistry, and Pharmacology of Origanum onites (L.): A Review. Chem. Biodivers. 2016, 13, 504–520. [Google Scholar] [CrossRef]
- Assaf, M.H.; Ali, A.A.; Makboul, M.A. Preliminary study of phenolic glycosides from Origanum majorana; quantitative estimation of arbutin; cytotoxic activity of hydroquinone. Planta Med. 1987, 53, 343–345. [Google Scholar] [CrossRef]
- Koush, Y.; Meskaldji, D.E.; Pichon, S.; Rey, G.; Rieger, S.W.; Linden, D.E.; Van De Ville, D.; Vuilleumier, P.; Scharnowski, F. Learning Control Over Emotion Networks Through Connectivity-Based Neurofeedback. Cereb. Cortex 2015, 27, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Kamarch, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Lundqvist, L.O.; Carlsson, F.; Hilmersson, P.; Juslin, P.N. Emotional responses to music: Experience, expression, and physiology. Psychol. Music 2009, 37, 61–90. [Google Scholar] [CrossRef]
- Auer, T.; Schweizer, R.; Frahm, J. Training Efficiency and Transfer Success in an Extended Real-Time Functional MRI Neurofeedback Training of the Somatomotor Cortex of Healthy Subjects. Front. Hum. Neurosci. 2015, 9, 547. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sokhadze, E.M.; El-Baz, A.S.; Li, X.; Sears, L.; Casanova, M.F.; Tasman, A. Relative Power of Specific EEG Bands and Their Ratios during Neurofeedback Training in Children with Autism Spectrum Disorder. Front. Hum. Neurosci. 2016, 14, 723. [Google Scholar] [CrossRef] [PubMed]
- Gruber, T.; Keil, A.; Müller, M.M. Modulation of induced gamma band responses and phase synchrony in a paired associate learning task in the human EEG. Neurosci. Lett. 2001, 316, 29–32. [Google Scholar] [CrossRef]
- Gruzelier, J.; Egner, T. Critical validation studies of neurofeedback. Child Adolesc. Psychiatr. Clin. N. Am. 2005, 14, 83–104. [Google Scholar] [CrossRef] [PubMed]
- Linden, M.; Habib, T.; Radojevic, V. A controlled study of the effects of EEG biofeedback on cognition and behavior of children with attention deficit disorder and learning disabilities. Biofeedback Self Regul. 1996, 21, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Zotev, V.; Yuan, H.; Misaki, M.; Phillips, R.; Young, K.D.; Feldner, M.T.; Bodurka, J. Correlation between amygdala BOLD activity and frontal EEG asymmetry during real-time fMRI neurofeedback training in patients with depression. Neuroimage Clin. 2016, 12, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Azarpaikan, A.; Taheri Torabi, H. Effect of somatosensory and neurofeedback training on balance in older healthy adults: A preliminary investigation. Aging Clin. Exp. Res. 2017, 30, 745–753. [Google Scholar] [CrossRef]
- Marakay, M.M.; Seulgi, E.; Kyungmo, P. Self-regulation of primary motor cortex activity with motor imagery induces functional connectivity modulation: A real-time fMRI neurofeedback study. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2017, 2017, 4147–4150. [Google Scholar]
- Pulli, B.; Ali, M.; Forghani, R.; Schob, S.; Hsieh, K.L.; Wojtkiewicz, G.; Linnoila, J.J.; Chen, J.W. Measuring myeloperoxidase activity in biological samples. PLoS ONE 2013, 5, e67976. [Google Scholar] [CrossRef] [PubMed]
- Cabaña-Muñoz, M.E.; Pérez Laso, C.; Parmigiani-Izquierdo, J.M.; Merino, J.J. Origanum majorana Essential Oil Reduces VAS score and Modulates Cerebral Activity during 10 NeurOptimal® Sessions in Patients. Int. J. Sci. Res. (IJSR) 2016, 2, 2319–7064. [Google Scholar]
- Nakayama, R.; Nishiyama, A.; Shimada, M. Bruxism-Related Signs and Periodontal Disease: A Preliminary Study. Open Dent. J. 2018, 12, 400–405. [Google Scholar] [CrossRef]
- Crawford, J.R.; Henry, J.D. The Depression Anxiety Stress scales (DASS): Normative data and latent structure in a large non-clinical sample-British. J. Clin. Psychol. 2003, 42, 11–131. [Google Scholar] [CrossRef] [PubMed]
- Joss, A.; Adler, R.; Lang, N.P. Bleeding on probing. A parameter for monitoring periodontal conditions in clinical practice. J. Clin. Periodontal 1994, 21, 402–408. [Google Scholar] [CrossRef]
- Manfredini, D.; Ahlberg, J.; Mura, R.; Lobbezoo, F. Bruxism is unlikely to cause damage to the periodontium: Findings from a systematic literature assessment. J. Periodontol. 2015, 86, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Botero, J.E.; Bedoya, E. Determinantes del diagnóstico periodontal. Rev. Clin. Periodoncia Implantol. Rehabil. Oral 2010, 3, 94–99. [Google Scholar] [CrossRef]
- Bragger, U.; Nyman, S.; Lang, N.P.; von Wyttenbach, T.; Salvi, G.; Schurch, E., Jr. The significance of alveolar bone in periodontal disease. A long-term observation in patients with cleft lip, alveolus and palate. J. Clin. Periodontal 1990, 17, 379–384. [Google Scholar] [CrossRef]
- Ainamo, J.; Bay, Y. Problems and proposals for recording gingivitis and plaque. Int. Dent. 1975, 25, 229–233. [Google Scholar]
- Lang, N.P.; Joss, A.; Tonetti, M.S. Monitoring disease during supportive periodontal treatment by bleeding on probing. Periodontal 1996, 12, 44–48. [Google Scholar] [CrossRef]
- Salvi, G.E.; Lindhe, J.; Lang, N.P. Examination of patients with periodontal disease. In Clinical Periodontology and Implants Dentistry, 5th ed.; Lindh, J., Lan, N.P., Karring, T., Eds.; Wiley: Oxford, UK, 2008; pp. 573–586. [Google Scholar]
- Bracha, H.S.; Ralston, T.C.; Williams, A.E.; Yamashita, J.M.; Bracha, A.S. The clenching-grinding spectrum and fear circuitry disorders: Clinical insights from the neuroscience/paleoanthropology interface. CNS Spectr. 2005, 10, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Gungormus, Z.; Erciyas, K. Evaluation of the relationship between anxiety and depression and bruxism. J. Int. Med. Res. 2009, 37, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Takemura, T.; Takahashi, M.; Fukuda, M.; Ohnuki, T. A psychological study on patients with masticatory muscle disorder and sleep bruxism. J. Cranio Mandibular Sleep Pract. 2006, 24, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Lis-Balchin, M.; Hart, S. Studies on the mode of action of the essential oil of Lavender (LaParetvandula angustifolia P. Miller). Phytother. Res. 1999, 13, 540–542. [Google Scholar] [CrossRef]
- Moskowitz, H.R.; Gerbers, C.L. Functional properties of the olfactory system: Psychophysics. Dimensional salience of odors. Ann. N. Y. Acad. Sci. 1974, 237, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zholobenko, A.; Mouithys-Mickalad, A.; Modriansky, M.; Serteyn, D.; Franck, T. Polyphenols from Silybum marianum inhibit in vitro the oxidant response of equine neutrophils and myeloperoxidase activity. J. Vet. Pharmacol. Ther. 2016, 39, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, T.; Tonosaki, K. Smelling lavender and rosemary increases free radical scavenging activity and decreases cortisol level in saliva. Psychiatry Res. 2007, 150, 89–96. [Google Scholar] [CrossRef]
- Lehrner, J.; Marwinski, G.; Lehr, S.; Johren, P.; Deecke, L. Ambient odors of orange and Lavender reduce anxiety and improve mood in a dental office. Physiol. Behav. 2005, 86, 92–95. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Graham, J.E.; Malarkey, W.B.; Porter, K.; Lemeshow, S.; Glaser, R. Olfactory influences on mood and autonomic, endocrine, and immune function. Psychoneuroendocrinology 2008, 33, 328–339. [Google Scholar] [CrossRef]
- Armfield, J.M.; Heaton, L.J. Management of fear and anxiety in the dental clinic: A review. Aust. Dent. J. 2013, 58, 390–407. [Google Scholar] [CrossRef] [PubMed]
- Zabirunnisa, M.; Gadagi, J.S.; Gadde, P.; Myla, N.; Koneru, J.; Thatimatla, C. Dental patient anxiety: Possible deal with Lavender fragrance. J. Res. Pharm. Pract. 2014, 3, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Manley, C.H. Psychophysiological effect of odor. Crit. Rev. Food Sci. Nutr. 1993, 33, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Palacios, A.; García-López, E.; Meza-Rios, A.; Armendariz-Borunda, J.; Sandoval-Rodríguez, A. Myeloperoxidase enzymatic activity is increased in patients with different levels of dental crowding after initial orthodontic activation. Am. J. Orthod. Dentofac. Orthop. 2014, 146, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Vukelić, M.; Gharabaghi, A. Self-regulation of circumscribed brain activity modulates spatially selective and frequency specific connectivity of distributed resting state networks. Front. Behav. Neurosci. 2015, 14, 181. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.M.; van Deusen, A. A new neurofeedback protocol for depression. Span. J. Psychol. 2011, 14, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.; Birbaumer, N.; Lutzenberger, W.; Gruzelier, J.H.; Kaiser, J. Neurofeedback treatment for attention-deficit/hyperactivity disorder in children: A comparison with methylphenidate. Appl. Psychophysiol. Biofeedback 2003, 28, 1–12. [Google Scholar] [CrossRef]
- Diego, M.A.; Jones, N.A.; Field, T.; Hernandez-Reif, M.; Schanberg, S.; Kuhn, C.; McAdam, V.; Galamaga, R.; Galamaga, M. Aromatherapy positively affects mood, EEG patterns of alertness and math computations. Int. J. Neurosci. 1998, 96, 217–224. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Kim, S. Influence of Fragrances on Human Psychophysiological Activity: With Special Reference to Human Electroencephalographic Response. Sci. Pharm. 2016, 84, 724. [Google Scholar] [CrossRef]
- Micoulaud-Franchi, J.A.; McGonigal, A.; Lopez, R.; Daudet, C.; Kotwas, I.; Bartolomei, F. Electroencephalographic neurofeedback: Level of evidence in mental and brain disorders and suggestions for good clinical practice. Neurophysiol. Clin. 2015, 45, 423–433. [Google Scholar] [CrossRef]


| Study Groups Total. 104 Participants and 500 Saliva Samples for Mieloperoxidase (MPO) Determination | n | Saliva Samples |
|---|---|---|
| (a) Trained Bruxistic participants (with high “intrinsic stress”) who smell Origanum majorana (AE) essential oil during 21 NO sessions. | 12 | 120 |
| (b) Trained Bruxistic participants (with high “intrinsic stress”) who do not smell O. majorana essential oil during 21 NO sessions. | 12 | 120 |
| (c) Bruxistic participants (with high “intrinsic stress”) without neurofeedback training | 30 | 120 |
| (d) Unstreased and not bruxistic patients (without NO training) | 5 | 50 |
| (e) Controls (unstressed and non bruxistic patients) without NO training. | 30 | 60 |
| (f) Controls (without stress) exposed to O.majorana during 12 NO sessions | 5 | 15 |
| (g) Control (placebo) patients who smell a neutral oil during 12 NO sessions | 5 | 15 |
| NeurOptimal (Trained Bruxistic Patients) | NeurOptimal (Trained Controls) | ||
|---|---|---|---|
| + AE | + AE | ||
| Inclusion Criteria | Inclusion Criteria | Inclusion Criteria | Inclusion Criteria |
| Bruxistic patients (n = 12, 120 saliva) | Bruxistic patients (n = 12, 120 saliva) | Control Subjects (n = 5, 15 saliva) | Control Subjects (n = 5, 15 saliva) |
| With stress DASS-42 > 16–25 (Stress item) | With stress DASS-42 > 16–25 (Stress item) | Without stress DASS-42 (0–14) (for the Stress item) | Without stress DASS-42 (0–14) (for the Stress item) |
| Bruxism test and underwent oral examination (a good healthy state) | Bruxism test and underwent oral examination (a good healthy state) | Without Bruxism (oral examination and a good healthy state) | Without Bruxism |
| Trained in NeurOptimal (NO): 21 sessions | Trained in NO (21 sessions) | Trained in NO (12 sessions) | Trained in NO (12 sessions) |
| Exposed to Origanum majorana essential oil during 21 sessions | They underwent 21 NO sessions without Origanum majorana essential oil | Exposed to Origanum majorana essential oil during 12 sessions | Exposed to placebo (neutral oil) in 12 NO |
| Inclusion Criteria | Inclusion Criteria | Exclusion Criteria (for all groups) | Inclusion Criteria |
| Bruxistic patients (n = 30, 120 salive) | Control Subjects (n = 5, 50 saliva) | Periodontal disease or gingivitis | Control Subjects (n = 30, 60 saliva) |
| With stress: DASS-42 > 16–25 (Stress item) | Without stress DASS-42 (0–14) (for the Stress item) | presence of bacterial plaque | Without stress: DASS-42 (0–14) (for the Stress item) |
| Bruxism test and underwent oral examination (a good healthy state) | Without Bruxism (oral examination and a good healthy state). | Orthodontic devices | Without Bruxism |
| Without NeurOptimal (NO) training | Without Origanum majorana essential oil exposure | the use of removable partial denture | Without NO training |
| Unexposed to Origanum majorana | |||
| Normal | Mild | Moderate | Severe | Very Severe | |
|---|---|---|---|---|---|
| Stress | 0–14 | 15–18 | 19–25 | 26–33 | >34 |
| Questionnaire (Bruxistic Patients) | 0 (Never) | 1 (Hardly Ever) | 2 Occasionally | 3 Fairly Often | 4 Very Often |
|---|---|---|---|---|---|
| Q1 | 75% | 20% | 5% | 0% | 0% |
| Q2 | 65% | 15% | 20% | 0% | 0% |
| Q3 | 28% | 12% | 22% | 28% | 10% |
| Item | Never Hardly Ever Occasionally (0 Score) | Fairly Often Very Often (1 Score) |
|---|---|---|
| Q1 | 100% | 0% |
| Q2 | 100% | 0% |
| Q1 = 1 or Q2 = 1 (high-SBRS) | 0% | 0% |
| Q3 (high-ABRS) | 62% | 38% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merino, J.J.; Parmigiani-Izquierdo, J.M.; López-Oliva, M.E.; Cabaña-Muñoz, M.E. Origanum majorana Essential Oil Inhalation during Neurofeedback Training Reduces Saliva Myeloperoxidase Activity at Session-1 in Bruxistic Patients. J. Clin. Med. 2019, 8, 158. https://doi.org/10.3390/jcm8020158
Merino JJ, Parmigiani-Izquierdo JM, López-Oliva ME, Cabaña-Muñoz ME. Origanum majorana Essential Oil Inhalation during Neurofeedback Training Reduces Saliva Myeloperoxidase Activity at Session-1 in Bruxistic Patients. Journal of Clinical Medicine. 2019; 8(2):158. https://doi.org/10.3390/jcm8020158
Chicago/Turabian StyleMerino, José Joaquín, José María Parmigiani-Izquierdo, María Elvira López-Oliva, and María Eugenia Cabaña-Muñoz. 2019. "Origanum majorana Essential Oil Inhalation during Neurofeedback Training Reduces Saliva Myeloperoxidase Activity at Session-1 in Bruxistic Patients" Journal of Clinical Medicine 8, no. 2: 158. https://doi.org/10.3390/jcm8020158
APA StyleMerino, J. J., Parmigiani-Izquierdo, J. M., López-Oliva, M. E., & Cabaña-Muñoz, M. E. (2019). Origanum majorana Essential Oil Inhalation during Neurofeedback Training Reduces Saliva Myeloperoxidase Activity at Session-1 in Bruxistic Patients. Journal of Clinical Medicine, 8(2), 158. https://doi.org/10.3390/jcm8020158




