The Modified A-DIVA Scale as a Predictive Tool for Prospective Identification of Adult Patients at Risk of a Difficult Intravenous Access: A Multicenter Validation Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Setting
2.2. Participants
2.3. Sample Size Calculation
2.4. Procedure
2.5. Primary Outcome
2.6. Predictors
2.7. Statistical Analysis
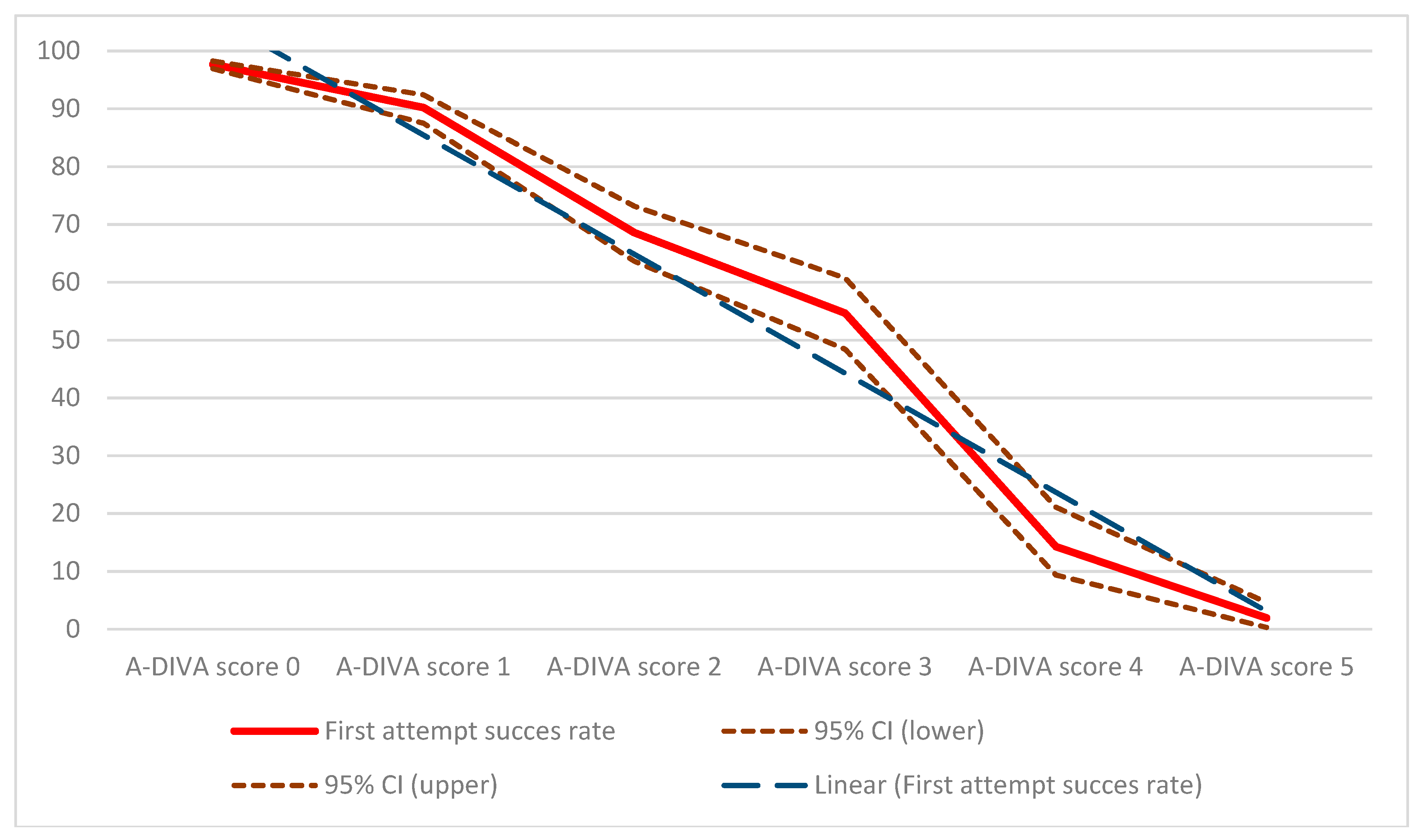
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dychter, S.S.; Gold, D.A.; Carson, D.; Haller, M. Intravenous therapy: A review of complications and economic considerations of peripheral access. J. Infus. Nurs. 2012, 35, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Vlaar, A.P.J.; Hunt, B.J. Improving peripheral intravenous catheter failure rates. Lancet (Lond. Engl.) 2018, 392, 366–367. [Google Scholar] [CrossRef]
- Ruegg, L.; Faucett, M.; Choong, K. Emergency inserted peripheral intravenous catheters: A quality improvement project. Br. J. Nurs. 2018, 27, S28–S30. [Google Scholar] [CrossRef] [PubMed]
- Loudermilk, R.A.; Steffen, L.E.; McGarvey, J.S. Strategically Applying New Criteria for Use Improves Management of Peripheral Intravenous Catheters. J. Healthc. Qual. 2018, 40, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Helm, R.E.; Klausner, J.D.; Klemperer, J.D.; Flint, L.M.; Huang, E. Accepted but unacceptable: Peripheral IV catheter failure. J. Infus. Nurs. 2015, 38, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, F.H.J.; Buise, M.P.; Claassen, J.J.F.; Dierick-van Daele, A.T.M.; Bouwman, A.R.A. Comparison of ultrasound guidance with palpation and direct visualization for peripheral vein cannulation in adult patients: A systematic review and meta-analysis. Br. J. Anaesth. 2018, 121, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, F.H.; Puijn, L.A.; van Aarle, W.H.; Dierick-van Daele, A.T.; Bouwman, A.R. Pain upon inserting a peripheral intravenous catheter: Size does not matter. J. Vasc Access 2018. [Google Scholar] [CrossRef] [PubMed]
- Bodenham Chair, A.; Babu, S.; Bennett, J.; Binks, R.; Fee, P.; Fox, B.; Johnston, A.J.; Klein, A.A.; Langton, J.A.; Mclure, H.; et al. Association of Anaesthetists of Great Britain and Ireland: Safe vascular access 2016. Anaesthesia 2016, 71, 573–585. [Google Scholar] [CrossRef]
- Au, A.K.; Rotte, M.J.; Grzybowski, R.J.; Ku, B.S.; Fields, J.M. Decrease in central venous catheter placement due to use of ultrasound guidance for peripheral intravenous catheters. Am. J. Emerg. Med. 2012, 30, 1950–1954. [Google Scholar] [CrossRef]
- Aponte, H.; Acosta, S.; Rigamonti, D.; Sylvia, B.; Austin, P.; Samolitis, T. The use of ultrasound for placement of intravenous catheters. AANA J. 2007, 75, 212–216. [Google Scholar]
- Loon FHJ van Puijn, L.A.P.M.; Houterman, S.; Bouwman, A.R.A. Development of the A-DIVA Scale: A Clinical Predictive Scale to Identify Difficult Intravenous Access in Adult Patients Based on Clinical Observations. Medicine 2016, 95, e3428. [Google Scholar] [CrossRef] [PubMed]
- Gorski, L.A. The 2016 Infusion Therapy Standards of Practice. Home Healthc. Now 2017, 35, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.S.; de Groot, J.A.; Dutton, S.; Omar, O.; Shanyinde, M.; Tajar, A.; Voysey, M.; Wharton, R.; Yu, L.; Moons, K.G.; et al. External validation of multivariable prediction models: A systematic review of methodological conduct and reporting. BMC Med. Res. Methodol. 2014, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- Moons, K.G.M.; Kengne, A.P.; Grobbee, D.E.; Royston, P.; Vergouwe, Y.; Altman, D.G.; Woodward, M. Risk prediction models: II. External validation, model updating, and impact assessment. Heart 2012, 98, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Moons, K.G.M.; Altman, D.G.; Vergouwe, Y.; Royston, P. Prognosis and prognostic research: Application and impact of prognostic models in clinical practice. BMJ 2009, 338, b606. [Google Scholar] [CrossRef] [PubMed]
- Debray, T.P.A.; Moons, K.G.M.; Ahmed, I.; Koffijberg, H.; Riley, R.D. A framework for developing, implementing, and evaluating clinical prediction models in an individual participant data meta-analysis. Stat. Med. 2013, 32, 3158–3180. [Google Scholar] [CrossRef] [PubMed]
- Bleeker, S.E.; Moll, H.A.; Steyerberg, E.W.; Donders, A.R.T.; Derksen-Lubsen, G.; Grobbee, D.E.; Moons, K.G.M. External validation is necessary in prediction research: A clinical example. J. Clin. Epidemiol. 2003, 56, 826–832. [Google Scholar] [CrossRef]
- Moons, K.G.M.; Altman, D.G.; Reitsma, J.B.; Collins, G.S. New Guideline for the Reporting of Studies Developing, Validating, or Updating a Multivariable Clinical Prediction Model: The TRIPOD Statement. Adv. Anat. Pathol. 2015, 22, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Ogundimu, E.O.; Altman, D.G.; Collins, G.S. Adequate sample size for developing prediction models is not simply related to events per variable. J. Clin. Epidemiol. 2016, 76, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, W.; Zuithoff, N.P.A.; Mallett, S.; Geerlings, M.I.; Vergouwe, Y.; Steyerberg, E.W.; Altman, D.G.; Moons, K.G. Reporting and methods in clinical prediction research: A systematic review. PLoS Med. 2012, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Tian, L. Joint confidence region estimation for area under ROC curve and Youden index. Stat. Med. 2014, 33, 985–1000. [Google Scholar] [CrossRef] [PubMed]
- Paul, P.; Pennell, M.L.; Lemeshow, S. Standardizing the power of the Hosmer-Lemeshow goodness of fit test in large data sets. Stat. Med. 2013, 32, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, G.; D’Amico, R.; Nardi, D.; Tinazzi, A.; Apolone, G. One model, several results: The paradox of the Hosmer-Lemeshow goodness-of-fit test for the logistic regression model. J. Epidemiol. Biostat. 2000, 5, 251–253. [Google Scholar] [PubMed]
- Lemeshow, S.; Hosmer, D.W.J. A review of goodness of fit statistics for use in the development of logistic regression models. Am. J. Epidemiol. 1982, 115, 92–106. [Google Scholar] [CrossRef]
- Keszei, A.P.; Novak, M.; Streiner, D.L. Introduction to health measurement scales. J. Psychosom. Res. 2010, 68, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet (Lond. Engl.) 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Parker, S.I.A.; Benzies, K.M.; Hayden, K.A.; Lang, E.S. Effectiveness of interventions for adult peripheral intravenous catheterization: A systematic review and meta-analysis of randomized controlled trials. Int. Emerg. Nurs. 2017, 31, 15–21. [Google Scholar] [CrossRef]
- Bensghir, M.; Chkoura, K.; Mounir, K.; Drissi, M.; Elwali, A.; Ahtil, R.; Méziane, M.; Alaoui, H.; Elmoqadem, A.; Lahlou, J.; et al. Peripheral intravenous access in the operating room: Characteristics and predictors of difficulty. Ann. Fr. Anesth. Reanim. 2012, 31, 600–604. [Google Scholar] [CrossRef]
- Egan, G.; Healy, D.; O’Neill, H.; Clarke-Moloney, M.; Grace, P.A.; Walsh, S.R. Ultrasound guidance for difficult peripheral venous access: Systematic review and meta-analysis. Emerg. Med. J. 2013, 30, 521–526. [Google Scholar] [CrossRef]
- Carr, P.J.; Rippey, J.C.R.; Budgeon, C.A.; Cooke, M.L.; Higgins, N.; Rickard, C.M. Insertion of peripheral intravenous cannulae in the Emergency Department: Factors associated with first-time insertion success. J. Vasc. Access 2016, 17, 182–190. [Google Scholar] [CrossRef]
- Wallis, M.C.; McGrail, M.; Webster, J.; Marsh, N.; Gowardman, J.; Playford, E.G.; Rickard, C.M. Risk factors for peripheral intravenous catheter failure: A multivariate analysis of data from a randomized controlled trial. Infect. Control Hosp. Epidemiol. 2014, 35, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Rippey, J.C.; Carr, P.J.; Cooke, M.; Higgins, N.; Rickard, C.M. Predicting and preventing peripheral intravenous cannula insertion failure in the emergency department: Clinician “gestalt” wins again. Emerg. Med. Australas. 2016, 28, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Carr, P.J.; Rippey, J.C.R.; Cooke, M.L.; Bharat, C.; Murray, K.; Higgins, N.S.; Foale, A.; Rickard, C.M. Development of a clinical prediction rule to improve peripheral intravenous cannulae first attempt success in the emergency department and reduce post insertion failure rates: The Vascular Access Decisions in the Emergency Room (VADER) study protocol. BMJ Open 2016, 6, e009196. [Google Scholar] [CrossRef] [PubMed]
- Armenteros-Yeguas, V.; Gárate-Echenique, L.; Tomás-López, M.A.; Cristóbal-Domínguez, E.; Moreno-de Gusmão, B.; Miranda-Serrano, E.; Moraza-Dulanto, M.I. Prevalence of difficult venous access and associated risk factors in highly complex hospitalised patients. J. Clin. Nurs. 2017, 26, 4267–4275. [Google Scholar] [CrossRef] [PubMed]
- Piredda, M.; Biagioli, V.; Barrella, B.; Carpisassi, I.; Ghinelli, R.; Giannarelli, D.; de Marinis, M.G. Factors affecting difficult peripheral intravenous cannulation in adults: A prospective observational study. J. Clin. Nurs. 2017, 26, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Prottengeier, J.; Maier, J.-N.; Gall, C.; Heinrich, S.; Schmidt, J.; Birkholz, T. Does it matter who places the intravenous? An inter-professional comparison of prehospital intravenous access difficulties between physicians and paramedics. Eur. J. Emerg. Med. 2017, 24, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Calero, M.A.; Fernandez-Fernandez, I.; Molero-Ballester, L.J.; Matamalas-Massanet, C.; Moreno-Mejias, L.; de Pedro-Gomez, J.E.; Blanco-Mavillard, I.; Morales-Asencio, J.M. Risk factors for difficult peripheral venous cannulation in hospitalised patients. Protocol for a multicentre case-control study in 48 units of eight public hospitals in Spain. BMJ Open 2018, 8, e020420. [Google Scholar] [CrossRef]
- Fields, J.M.; Piela, N.E.; Au, A.K.; Ku, B.S. Risk factors associated with difficult venous access in adult ED patients. Am. J. Emerg. Med. 2014, 32, 1179–1182. [Google Scholar] [CrossRef]
- Sabri, A.; Szalas, J.; Holmes, K.S.; Labib, L.; Mussivand, T. Failed attempts and improvement strategies in peripheral intravenous catheterization. Biomed. Mater. Eng. 2013, 23, 93–108. [Google Scholar]
- Stolper, E.; van de Wiel, M.; van Royen, P.; van Bokhoven, M.; van der Weijden, T.; Dinant, G.J. Gut feelings as a third track in general practitioners’ diagnostic reasoning. J. Gen. Intern. Med. 2011, 26, 197–203. [Google Scholar] [CrossRef]
- Stolper, E.; van Bokhoven, M.; Houben, P.; van Royen, P.; van de Wiel, M.; van der Weijden, T.; Jan Dinant, G. The diagnostic role of gut feelings in general practice. A focus group study of the concept and its determinants. BMC Fam. Pract. 2009, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Stolper, E.; van Royen, P.; van de Wiel, M.; van Bokhoven, M.; Houben, P.; van der Weijden, T.; Jan Dinant, G. Consensus on gut feelings in general practice. BMC Fam. Pract. 2009, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Civetta, G.; Cortesi, S.; Mancardi, M.; de Pirro, A.; Vischio, M.; Mazzocchi, M.; Scudeller, L.; Bottazzi, A.; Iotti, G.A.; Palo, A. EA-DIVA score (Enhanced Adult DIVA score): A new scale to predict difficult preoperative venous cannulation in adult surgical patients. J. Vasc. Access. 2018. [Google Scholar] [CrossRef] [PubMed]
- Yen, K.; Riegert, A.; Gorelick, M.H. Derivation of the DIVA score: A clinical prediction rule for the identification of children with difficult intravenous access. Pediatr. Emerg. Care 2008, 24, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Riker, M.W.; Kennedy, C.; Winfrey, B.S.; Yen, K.; Dowd, M.D. Validation and refinement of the difficult intravenous access score: A clinical prediction rule for identifying children with difficult intravenous access. Acad. Emerg. Med. 2011, 18, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Lian, A.; Rippey, J.C.R.; Carr, P.J. Teaching medical students ultrasound-guided vascular access—Which learning method is best? J. Vasc. Access. 2017, 18, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.; Lawrence, M. Ultrasound-Guided Peripheral Intravenous Access Program for Emergency Physicians, Nurses, and Corpsmen (Technicians) at a Military Hospital. Mil. Med. 2016, 181, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Duran-Gehring, P.; Bryant, L.; Reynolds, J.A.; Aldridge, P.; Kalynych, C.J.; Guirgis, F.W. Ultrasound-guided peripheral intravenous catheter training results in physician-level success for emergency department technicians. J. Ultrasound Med. 2016, 35, 2343–2352. [Google Scholar] [CrossRef] [PubMed]
- Song, J.W.; Chung, K.C. Observational studies: Cohort and case-control studies. Plast. Reconstr. Surg. 2010, 126, 2234–2242. [Google Scholar] [CrossRef]
- Boyko, E.J. Observational research--opportunities and limitations. J. Diabetes Complicat. 2013, 27, 642–648. [Google Scholar] [CrossRef]
| Variable | Category | Development Cohort (N = 1255) | Evaluation Cohort (N = 2332) |
|---|---|---|---|
| Gender | Male Female | 598 (48%) 657 (52%) | 1052 (45%) 1280 (55%) |
| Age | Years | 55 (17) | 59 (18) |
| Weight | Kilograms | 83 (22) | 81 (20) |
| Length | Centimeters | 171 (10) | 171 (11) |
| ASA classification | ASA 1 ASA 2 ASA 3 ASA 4 | 312 (25%) 581 (46%) 355 (28%) 7 (1%) | 549 (24%) 1356 (58%) 362 (16%) 65 (2%) |
| Race | Asian Caucasian Mediterranean and Arabic Afro-European | 0 (0%) 1119 (89%) 111 (9%) 25 (2%) | 215 (9%) 1866 (80%) 168 (7%) 83 (4%) |
| Dominant side | Left Right | 120 (10%) 1135 (90%) | 167 (7%) 2165 (93%) |
| Variable | Category | Successful First Attempt (N = 2923) | Unsuccessful First Attempt (N = 664) |
|---|---|---|---|
| Number of attempts | 1 (0) | 2 (1) | |
| Side of cannulation | Left Right | 1549 (53%) 1374 (47%) | 412 (62%) 252 (38%) |
| Place of cannulation | Dorsum of the hand Lower arm Elbow crease Upper arm | 1900 (65%) 702 (24%) 292 (10%) 29 (1%) | 378 (57%) 179 (27%) 100 (15%) 7 (1%) |
| Size of the catheter | 22 gauge 20 gauge 18 gauge 16 gauge 14 gauge | 117 (4%) 1491 (51%) 1140 (39%) 146 (5%) 29 (1%) | 73 (11%) 391 (59%) 153 (23%) 27 (4%) 20 (3%) |
| History of difficult intravenous cannulation | Yes No | 409 (14%) 2514 (86%) | 412 (62%) 252 (38%) |
| Practitioners expectation of difficult intravenous access | Yes No | 234 (8%) 2689 (92%) | 266 (40%) 398 (60%) |
| Palpable vein after tourniquet placement | Yes No | 2748 (94%) 175 (6%) | 219 (33%) 445 (67%) |
| Visible vein after tourniquet placement | Yes No | 2718 (93%) 205 (7%) | 139 (21%) 525 (79%) |
| Both palpable and visible vein after tourniquet placement | Yes No | 2835 (97%) 88 (3%) | 286 (43%) 378 (57%) |
| Diameter of the vein after tourniquet placement | Millimeters | 3 (1) | 2 (1) |
| Practitioner | Physician Nurse | 234 (8%) 2689 (92%) | 73 (11%) 591 (89%) |
| Pain score on a verbal numeric rating scale | 0–10 | 2 (2) | 5 (3) |
| Factor | ß | SE | p Value | Odds Ratio | 95% CI |
|---|---|---|---|---|---|
| History of a difficult intravenous cannulation | 0.976 | 0.180 | <0.001 | 2.7 | 1.6 to 4.4 |
| Practitioner’s expectation of a difficult intravenous access | 0.936 | 0.191 | <0.001 | 2.6 | 1.6 to 4.0 |
| No palpable vein after tourniquet placement | 1.670 | 0.187 | <0.001 | 4.8 | 2.5 to 8.1 |
| No visible vein after tourniquet placement | 1.879 | 0.192 | <0.001 | 5.9 | 2.5 to 10.1 |
| Diameter of the vein less than 3 millimeters after tourniquet placement | 1.247 | 0.094 | <0.001 | 3.5 | 2.7 to 4.4 |
| Factor | Score |
|---|---|
| Is there a known history of a difficult intravenous access? | 1 |
| Do you expect a failed first attempt or a difficult intravenous access? | 1 |
| Is there an inability to identify a dilated vein by palpating the upper extremity? | 1 |
| Is there an inability to identify a dilated vein by visualizing the upper extremity? | 1 |
| Has the largest dilated vein a diameter less than 3 millimeters? | 1 |
| Unit (Hospital and Department) | Success Rate on the First Attempt | AUC of the ROC Curve | SE of the AUC | Hosmer-Lemeshow χ2 | Hosmer-Lemeshow p Value |
|---|---|---|---|---|---|
| Overall cohort (N = 3587) | 83% | 97% | 0.003 | 15.58 | 0.086 |
| Unit 1 a (N = 1212) | 85% | 96% | 0.002 | 10.04 | 0.190 |
| Unit 2 a (N = 848) | 80% | 98% | 0.003 | 2.94 | 0.201 |
| Unit 3 a (N = 598) | 86% | 98% | 0.007 | 3.00 | 0.223 |
| Unit 4 a (N = 433) | 81% | 97% | 0.009 | 4.49 | 0.344 |
| Unit 5 a (N = 230) | 83% | 96% | 0.012 | 11.34 | 0.096 |
| Unit 6 b (N = 162) | 84% | 93% | 0.011 | 9.82 | 0.076 |
| Unit 7 c (N = 104) | 71% | 77% | 0.078 | 21.86 | 0.054 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Loon, F.H.J.; van Hooff, L.W.E.; de Boer, H.D.; Koopman, S.S.H.A.; Buise, M.P.; Korsten, H.H.M.; Dierick-van Daele, A.T.M.; Bouwman, A.R.A. The Modified A-DIVA Scale as a Predictive Tool for Prospective Identification of Adult Patients at Risk of a Difficult Intravenous Access: A Multicenter Validation Study. J. Clin. Med. 2019, 8, 144. https://doi.org/10.3390/jcm8020144
van Loon FHJ, van Hooff LWE, de Boer HD, Koopman SSHA, Buise MP, Korsten HHM, Dierick-van Daele ATM, Bouwman ARA. The Modified A-DIVA Scale as a Predictive Tool for Prospective Identification of Adult Patients at Risk of a Difficult Intravenous Access: A Multicenter Validation Study. Journal of Clinical Medicine. 2019; 8(2):144. https://doi.org/10.3390/jcm8020144
Chicago/Turabian Stylevan Loon, Fredericus H. J., Loes W. E. van Hooff, Hans D. de Boer, Seppe S. H. A. Koopman, Marc P. Buise, Hendrikus H. M. Korsten, Angelique T. M. Dierick-van Daele, and Arthur R. A. Bouwman. 2019. "The Modified A-DIVA Scale as a Predictive Tool for Prospective Identification of Adult Patients at Risk of a Difficult Intravenous Access: A Multicenter Validation Study" Journal of Clinical Medicine 8, no. 2: 144. https://doi.org/10.3390/jcm8020144
APA Stylevan Loon, F. H. J., van Hooff, L. W. E., de Boer, H. D., Koopman, S. S. H. A., Buise, M. P., Korsten, H. H. M., Dierick-van Daele, A. T. M., & Bouwman, A. R. A. (2019). The Modified A-DIVA Scale as a Predictive Tool for Prospective Identification of Adult Patients at Risk of a Difficult Intravenous Access: A Multicenter Validation Study. Journal of Clinical Medicine, 8(2), 144. https://doi.org/10.3390/jcm8020144





