Abstract
The proliferative capacity of residual breast cancer (BC) disease indicates the existence of partial treatment resistance and higher probability of tumor recurrence. We explored the therapeutic potential of adding neoadjuvant metformin as an innovative strategy to decrease the proliferative potential of residual BC cells in patients failing to achieve pathological complete response (pCR) after pre-operative therapy. We performed a prospective analysis involving the intention-to-treat population of the (Metformin and Trastuzumab in Neoadjuvancy) METTEN study, a randomized multicenter phase II trial of women with primary, non-metastatic (human epidermal growth factor receptor 2) HER2-positive BC evaluating the efficacy, tolerability, and safety of oral metformin (850 mg twice-daily) for 24 weeks combined with anthracycline/taxane-based chemotherapy and trastuzumab (arm A) or equivalent regimen without metformin (arm B), before surgery. We centrally evaluated the proliferation marker Ki67 on sequential core biopsies using visual assessment (VA) and an (Food and Drug Administration) FDA-cleared automated digital image analysis (ADIA) algorithm. ADIA-based pre-operative values of high Ki67 (≥20%), but not those from VA, significantly predicted the occurrence of pCR in both arms irrespective of the hormone receptor status (p = 0.024 and 0.120, respectively). Changes in Ki67 in residual tumors of non-pCR patients were significantly higher in the metformin-containing arm (p = 0.025), with half of all patients exhibiting high Ki67 at baseline moving into the low-Ki67 (<20%) category after neoadjuvant treatment. By contrast, no statistically significant changes in Ki67 occurred in residual tumors of the control treatment arm (p = 0.293). There is an urgent need for innovative therapeutic strategies aiming to provide the protective effects of decreasing Ki67 after neoadjuvant treatment even if pCR is not achieved. Metformin would be evaluated as a safe candidate to decrease the aggressiveness of residual disease after neoadjuvant (pre-operative) systemic therapy of BC patients.
1. Introduction
Pathological complete response (pCR), the primary endpoint for a majority of neoadjuvant trials in breast cancer (BC), has been commonly adopted as a surrogate marker of long-term treatment benefit [1,2,3]. However, in patients with BC who fail to achieve pCR and have a worse prognosis, other biological markers are urgently needed to identify those at high-risk who could benefit from additional, customized therapeutic strategies. Central to these issues is Ki67, a nuclear protein associated with cellular proliferation, which is a well-established prognostic and predictive biomarker in patients with BC treated with neoadjuvant therapies.
Pre-therapeutic Ki67 positivity is a predictive marker for pCR. Indeed, a majority of studies have identified a significant association between high Ki67-associated proliferation at baseline and higher rates of pCR after neoadjuvant chemotherapy, especially in the triple-negative and (human epidermal growth factor receptor 2) HER2-positive BC subtypes [4,5,6,7]. Although the potential prognostic value of Ki67 after neoadjuvant therapy has been less characterized, there is strong evidence to suggest that changes in Ki67 index between pre- and post-neoadjuvant hormonal and chemotherapy might be a strong predictor of outcome for patients who do not achieve a pCR. Accordingly, the evaluation not only of absolute Ki67 values, but also of any differences in specific Ki67 levels between pre- and post-neoadjuvant therapy, might predict early recurrence in BC [8,9,10,11]. Patients showing a decrease in Ki67 index from biopsy to surgery have a better prognosis than patients with high levels of Ki67 expression at surgery [8]. A difference in Ki67 expression after versus before neoadjuvant therapy might be an important predictor of early metastasis and worse outcome [10,11]. Therefore, the finding that patients with BC whose tumors have low Ki67 expression after neoadjuvant therapy show better overall and disease-free survival compared with those whose tumors maintain high Ki67 expression [8,9,11] supports the notion that patients without pCR after neoadjuvant therapy are clinically heterogeneous and could be classified according to changes in Ki67 into good and poor prognostic groups [12,13]. High post-neoadjuvant treatment-associated Ki67 index therefore indicates the need for innovative therapeutic strategies aiming to provide the protective effects of decreasing Ki67 index even if pCR is not achieved.
Here, we sought to clarify whether the anti-diabetic biguanide metformin might be employed as a safe candidate to diminish the proliferative potential of residual BC disease after neoadjuvant therapy even if pCR is not achieved. We undertook a prospective analysis involving a well-characterized intention-to-treat (ITT) cohort of the (Metformin and Trastuzumab in Neoadjuvancy) METTEN study, a randomized multicenter phase II clinical trial for patients with HER2-positive BC receiving either metformin combined with anthracycline/taxane-based chemotherapy and trastuzumab (arm A), or an equivalent regimen without metformin (arm B), before surgery [14,15,16]. We first evaluated the function of a pre-therapeutic Ki67 labeling index as a predictive marker for pCR after adding neoadjuvant metformin. We then assessed the effect of adding metformin on the change of Ki67 on sequential core biopsies in patients with residual disease after neoadjuvant treatment. Considering the open debate regarding the reproducibility of Ki67 scoring by visual analysis (VA) in multicenter settings [17,18,19,20,21], we centrally re-evaluated Ki67 using VA and an (Food and Drug Administration) FDA-cleared Ki67 automated digital image analysis (ADIA) algorithm simultaneously. Here we present preliminary evidence highlighting the clinical potential of metformin as a safe candidate to prevent and/or treat the proliferative potential of residual BC disease after neoadjuvant therapy.
2. Experimental Section
2.1. Subjects
The METTEN study was registered with the EU Clinical Trials Register and is available online (EudraCT number 2011-000490-30). The study assessed the efficacy, tolerability, and safety of adding metformin to neoadjuvant chemotherapy plus trastuzumab in early HER2-positive BC [14]. Briefly, patients were randomly assigned to receive daily oral metformin (850 mg twice-daily) for 24 weeks concurrently with 12 cycles of weekly paclitaxel (80 mg/m2) plus trastuzumab (4 mg/kg loading dose followed by 2 mg/kg) followed by four cycles of 3-weekly fluorouracil (600 mg/m2), epirubicin (75 mg/m2), cyclophosphamide (600 mg/m2) with concomitant trastuzumab (6 mg/kg) (arm A), or equivalent sequential chemotherapy plus trastuzumab without metformin (arm B), followed by surgery. Patients had surgery within 4–5 weeks of the last cycle of neoadjuvant treatment. Post-surgery, patients received thrice-weekly trastuzumab to complete 1 year of neoadjuvant-adjuvant treatment according to institutional practice.
The primary endpoint was the pCR rate in the per-protocol efficacy population. pCR was defined as absence of invasive tumor cells on hematoxylin and eosin evaluation of the complete resected breast specimen (and all sample regional lymph nodes if a lymphadenectomy or sentinel lymph node biopsy was performed) following the completion of neoadjuvant systemic therapy. Residual ductal carcinoma in situ only was included in the definition of pCR (ypT0/is, ypN0) [14]. Ki67 scoring was carried out in the ITT population (n = 79), which included all randomly assigned patients who received at least one dose of study medication.
2.2. Ki67 Immunohistochemistry
Ki67 was evaluated by immunohistochemistry (IHC) on three-micron-thick sections of formalin-fixed paraffin-embedded (FFPE) tissue sections from diagnostic core and approximately one week before surgery sequential biopsies were obtained from all participating institutions of the METTEN study (Figure 1), and were subjected to Ki67 staining using the CONFIRM anti-Ki67 (30-9) rabbit monoclonal primary antibody on a Benchmark XT platform (Ventana Medical Systems Inc., Tucson, AZ, USA).
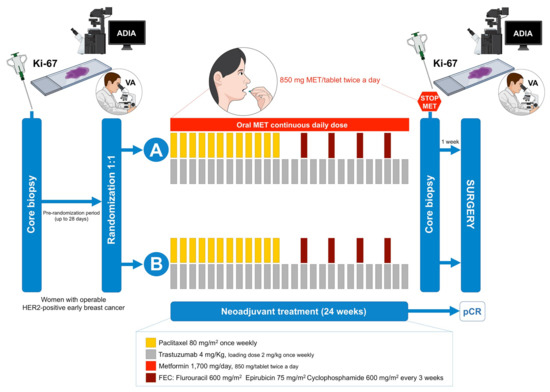
Figure 1.
METTEN study design. Figure shows the treatment and sequential biopsies schedules of the METTEN study (MET: metformin; pCR: pathological complete response; ADIA: automated digital image analysis; VA: visual analysis).
2.2.1. Visual Assessment
Ki67 was centrally evaluated by an experienced breast pathologist (ELB, Department of Anatomical Pathology, Dr. Josep Trueta Hospital of Girona, Girona, Spain), blinded to both the original pathology reports and the METTEN clinical trial database throughout the entire procedure. Manual counting of negative and positive cells was carried out by “eyeballed” estimates involving a more global average and not only hot spots.
2.2.2. Automated Digital Image Analysis
An FDA-cleared Ki67 image analysis algorithm was employed for determining Ki67. First, all of the stained slides were scanned into digital images using the VENTANA iScan System Version 1.0 (Ventana Medical Systems, Inc., Sunnyvale, CA, USA). Then, the VENTANA Companion Algorithm Ki67 (30-9) image analysis application was employed with the VENTANA iScan Coreo Au scanner running the Virtuoso Digital Pathology Image Analysis software. The same breast pathologist who evaluated Ki67 scores by VA selected the regions of interest for ADIA-based Ki67 scoring, taking into consideration confounding factors of digital scoring such as intratumor heterogeneity, presence of intermixed inflammatory cells, carcinoma in situ, or the occurrence of other non-cancer proliferating cells.
2.3. Statistical Analysis
Descriptive data were summarized using percentages, medians or means with their respective 25th and 75th percentiles, or standard deviations, as appropriate. Clinical baseline characteristics between treatment arms were assessed using the Chi-square or Fisher’s exact test for categorical variables, Student’s t-test for continuous variables with normal distribution, or the Mann–Whitney U test for non-normal distributions. The assumption of normality was evaluated with the Shapiro–Wilk test in parametric testing.
Agreement between VA and ADIA of Ki67 scoring was assessed using the Bland–Altman plot with 95% limits of agreement (LOA). Bland–Altman plots were constructed to enable visual observation for agreement between the two methods and to determine the 95% LOA. A non-parametric Passing–Bablok regression analysis, in which the slope of the regression line is calculated as a shifted median of all possible slopes between pairs of points that is resistant to outliers thereby giving an unbiased slope estimate solely if both methods have constant coefficients of variation, was applied to determine the interchangeability between the VA- and the ADIA-based Ki67 scoring by assessing systematic error: (a) constant bias was confirmed when the 95% confidence interval (CI) for intercept included value “0” and (b) proportional bias when 95% CI for slope included value “1.” Intra-class correlation coefficient (ICC) with a 95% CI was estimated using two-way mixed effect models to assess the consistency between VA and ADIA assessment of Ki67 scoring according to the two score methods. A higher ICC indicates better consistency. Although there is no universally accepted, standardized criteria for the ICC, we used the following kappa coefficient-like criteria to aid interpretation (20): 0.00–0.20 was interpreted as slight correlation; 0.21–0.40 as fair correlation; 0.41–0.60 as moderate correlation; 0.61–0.80 as substantial correlation; and >0.80 as excellent (almost perfect) correlation.
Binary logistic regression was used to assess the prognostic effect of Ki67 scoring on pCR. Unadjusted and adjusted odds ratios (ORs) with their relative 95% CIs were reported as a measure of association. All tests were two-sided and a p-value < 0.05 was set as statistically significant. Statistical analyses were carried out using SPSS (IBM Corp. released 2017. IBM SPSS Statistics for Windows, Version 25.0; Armonk, NY, USA) and STATA (StataCorp. 2013. Stata Statistical Software: Release 13; StataCorp LP, College Station, TX, USA).
2.4. Ethics Statement
The hospital (Dr. Josep Trueta Hospital, Girona, Spain) ethics committee (Clinical Investigation Ethic Committee, CIEC) and independent institutional review boards at each site participating in the METTEN study approved the protocol and any amendments. All procedures were in accordance with the ethical standards of the institutional research committees and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
3. Results
3.1. Study Participants
The present study was designed to evaluate the therapeutic activity of neoadjuvant metformin with respect to Ki67 on sequential core biopsies obtained from patients belonging to the ITT population of the METTEN trial (Figure 1), which included all randomly assigned HER2-positive BC patients who received at least one dose of study medication (n = 79; Table 1). The baseline characteristics and clinical-pathological variables at diagnosis of those ITT patients who achieved pCR after neoadjuvant therapy and those who did not have been reported previously [15,16].

Table 1.
Characteristics of patients at baseline according to treatment arm.
3.2. Correlation between Visual Assessment and Automated Digital Image Analysis for Ki67 Scoring
Of the 84 tissue samples included in the VA-ADIA correlation study (n = 69 from available diagnostic core biopsies and n = 15 from available sequential biopsies obtained following preoperative therapy and approximately one week before surgery), the mean VA Ki67 score from an experienced breast pathologist was 40.36% (95% CI: 35.76%–44.95%), whereas the mean Ki67 score by ADIA was 39.44% (95% CI: 34.39%–44.49%) (Figure 2A). VA and ADIA scoring of Ki67 was highly correlated (Spearman’s correlation coefficient 0.839 (p < 0.001)). Passing–Bablok regression analysis showed a strong linear relationship between VA and ADIA methods (Pearson’s correlation coefficient 0.852 (p < 0.001)), with a constant (intercept −3.35; 95% CI −10.0–0.26) and non-proportional bias (slope 1.08; 95% CI 0.97–1.22) (Figure 2B). The Bland–Altman plot (difference plot) with 95% LOA showed a small and balanced spread of the relative difference between VA and ADIA, with an estimated bias (mean difference) of −0.92 (95% LOA −24.95 to −23.11). Only 3 out of 84 values (3.6%) felt outside the LOA (Figure 2C).
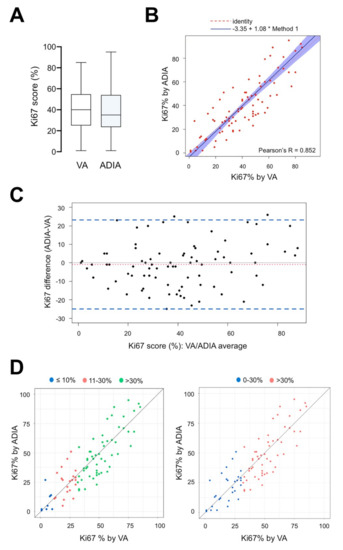
Figure 2.
Performance fit comparison between manual visual assessment and automated digital image analysis of Ki67 score on diagnostic and pre-operative (approx. 1-week before surgery) core biopsies in the METTEN study (n = 84). (A) Box plots of Ki67 scores (%) according to visual assessment (VA) and automated digital image analysis (ADIA) methods. Horizontal lines inside the boxes represent the median value; box limits indicate the 25th and 75th percentiles; whiskers extend 1.5 times the interquartile range from the 25th and 75th percentiles. (B) Scatter plot of the Passing–Bablok regression analysis of paired Ki67 scores obtained by VA and ADIA. Blue-shaded areas represent 95% confidence intervals. R2 value and regression equation are indicated in the figure. (C) Limits of agreement (LOA). The difference in Ki67 measurements made in paired analysis was plotted against the average of two methods compared. Figure depicts the Bland–Altman scatter plot of agreement between Ki67 scores obtained by VA and ADIA. General agreement between methods is evident with minimal variance (LOA, area between dashed lines). (D) Correlation between VA and ADIA of Ki67 scores stratified by VA values.
To evaluate whether there was non-uniformity within specific data ranges, the consistency between VA and ADIA was analyzed using the ICC. We selected the two-way mixed-effects model to compute both the absolute agreement (AA, when different raters assign the same score to the same subject) and the consistency (C, when raters’ scores to the same group of subjects are correlated in an additive manner) definitions of ICC. By ICC analysis, a good agreement was demonstrated between VA and ADIA Ki67 scoring in the whole cohort of our study (ICC = 0.849 (AA)/0.848 (C), 95% CI: 0.777–0.899, p < 0.001). Because Ki67 cut-offs ranging from 10% to 30% have been widely employed when classifying patients into Ki67 high- or low-risk groups for clinical decision making, we re-classified Ki67 scoring into three groups (≤10%, 11%–30% and >30% Ki67), stratified by VA values, according to the two score methods (Figure 2D). The 11%–30% and ≤10% (ICC = 0.281 (AA)/277 (C) and 0.117/0.114, p = 0.100 and 0.386, respectively; Table 2) groups showed notably poorer consistency than the >30% group, which reached a substantial correlation (ICC = 0.735 (AA)/0.739 (C), p < 0.001; Table 2). When Ki67 scoring was stratified into two groups (low 0%–30%; high >30%), we concluded the level of reliability to be moderate for low Ki67 values (ICI = 0.563 (AA)/0.565 (C), p < 0.001) in comparison with the substantial level of correlation observed for high Ki67 values.

Table 2.
Intra-class correlation coefficient between visual assessment and automated digital image analysis of Ki67 scoring.
3.3. Association between VA- and ADIA-Based Ki67 Scoring and Pathological Response
Because the clinically relevant Ki67 cut-off is 20% as defined by the St. Gallen criteria [22], we applied this cut-off to investigate the association between Ki67 expression, treatment arm, and pCR (Supplementary Table S1). In bivariate analysis, we observed the predictive capacity of baseline ADIA-based Ki67 ≥20% to significantly associate with the probability of achieving pCR (OR = 4.76, 95% CI = 1.13–20.09, p = 0.034; Figure 3). The direction and/or intensity of the predictive relationship between baseline ADIA-based Ki67 ≥20% and pCR occurred independently of the treatment arm (Supplementary Tables S1–S3). After additional adjustment for potential confounding characteristics such as the hormone receptor status, the relationship between baseline ADIA-based Ki67 ≥20% and the ability of neoadjuvant trastuzumab-based chemotherapy to achieve a pCR in patients remained significant (OR = 5.57, 95% CI = 1.26–24.74, p = 0.024; Figure 3; Supplementary Tables S4–S6). We failed to observe such predictive capacities of Ki67 ≥20% when Ki67 scoring was stratified using the VA values (Figure 3; Supplementary Tables S1 and S4).
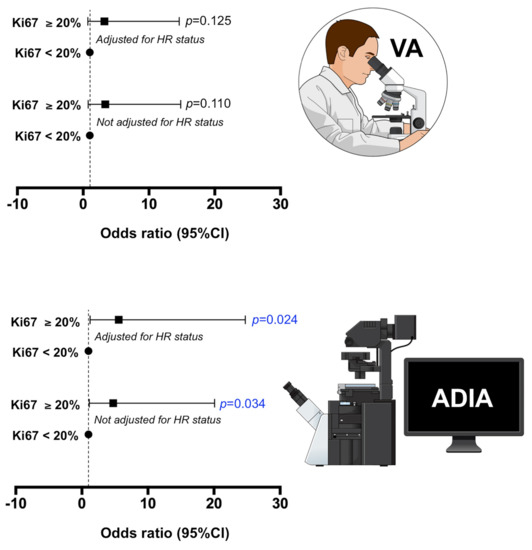
Figure 3.
Association of baseline Ki67 scores obtained by visual assessment and automated digital image analysis and the ability of neoadjuvant treatment to achieve pathological complete response (pCR) at surgery in the METTEN study. We applied the clinically relevant Ki67 cut-off of 20% (St. Gallen criteria) to investigate the association between low (<20%) and high (≥20%) Ki67 expression at baseline and the ability of neoadjuvant treatment to achieve pCR at surgery. The association was further adjusted by a well-known predictive factor of pCR in the neoadjuvant treatment of breast cancer, such as the hormonal receptor status.
3.4. Impact of Neoadjuvant Metformin on Ki67 Expression in Non-pCR Patients
We analyzed the decrease (or lack of decrease) in the percentage of Ki67-positive cancer cells between paired samples of core biopsies samples at baseline and approximately one week before surgery obtained from non-pCR patients in whom both VA and ADIA-based Ki67 scoring was available. The median baseline Ki67 level in this population (n = 14) was 42% (VA)/34.5% (ADIA) before neoadjuvant trastuzumab-based chemotherapy, which significantly decreased to a median of 19.5% (VA; p = 0.014)/17.0% (ADIA; p = 0.009) after treatment (Figure 4). To determine whether the addition of metformin significantly impacted Ki67 expression during neoadjuvant trastuzumab-based chemotherapy, we re-evaluated the relative change in Ki67 scoring from baseline to end-point treatment in non-pCR patients (Figure 4). The Ki67 decreases were largest in the metformin-containing arm A (n = 8), from 42.5% (VA)/34.5% (ADIA) at baseline to 11% (VA; p = 0.025)/11% (ADIA; p = 0.035) at surgery (Figure 4). Conversely, no significant differences were observed in the control treatment arm B (n = 6), in which Ki67 decreased from 36.0% (VA)/38.5% (ADIA) at baseline to 28.5% (VA; p = 0.293)/25.0% (ADIA; p = 0.080).
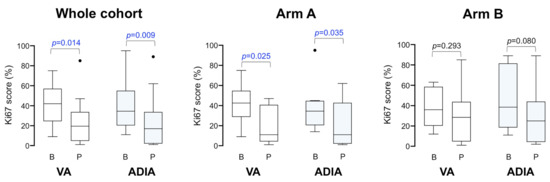
Figure 4.
Global changes in Ki67 scores among METTEN study patients with a residual tumor after neoadjuvant treatment. Box plots showing the distribution of Ki67 values in baseline (B) and pre-operative (P) core biopsies in the whole population and stratified by treatment arms. The figure shows the median values (horizontal bars within boxes) and 25th and 75th percentile (lower and upper horizontal lines of the boxes); whiskers extend 1.5 times the interquartile range from the 25th and 75th percentiles. Circles: outliers.
Finally, we evaluated the change of Ki67 category (high ≥20% versus low <20%) by treatment arm, finding that 57% (VA)/50% (ADIA) of high-Ki67, non-pCR patients moved into the low-Ki67 category following neoadjuvant treatment in the metformin-containing arm A (Figure 5). Conversely, 67% (VA)/80% (ADIA) of high-ki67, non-pCR patients remained unchanged following neoadjuvant treatment in the control arm B (Figure 5). This numerically higher pre- and post-treatment change of Ki67 scoring categories in the metformin-containing arm A was not statistically significant compared with that observed in the standard treatment arm B (p = 0.5210 VA; p = 0.3582 ADIA).
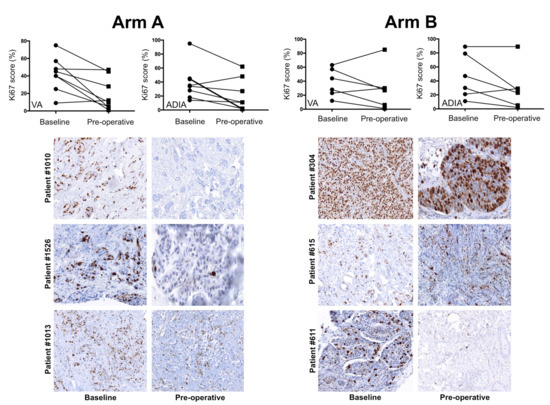
Figure 5.
Individual changes in Ki67 scores among METTEN study patients with a residual tumor after neoadjuvant treatment. Top: changes in Ki67 score for individual patients at baseline and post-treatment according to treatment arm and scoring method. Bottom: representative images of Ki67 expression staining are shown for each pair of biopsies at baseline and approximately one week before surgery (pre-operative) in individual patients.
4. Discussion
The prognosis of patients with BC is better for those who obtain pCR with neoadjuvant therapy than for those who do not [23,24]. For non-pCR patients—a clinically heterogenous population in terms of prognosis—there are few available therapeutic strategies (i.e., capecitabine in HER2-negative BC [25] and trastuzumab emtansine in HER2-positive BC [26]) that can be added to neoadjuvant therapy regimens to prevent recurrence. Ki67 is a validated biomarker of recurrence-free survival in BC residual disease after neoadjuvant therapy [27]. To the best of our knowledge, this is the first study reporting that the addition of neoadjuvant metformin to a well-established pre-operative treatment involving chemotherapy and targeted therapy significantly impacts the short-term changes in Ki67 post-neoadjuvant therapy.
Metformin, a biguanide derivative that has long been a cornerstone in the treatment of type 2 diabetes, has recently become incorporated into the armamentarium against cancer. Epidemiological and preclinical evidence is beginning to suggest that metformin may reduce overall cancer risk and mortality, with particularly significant effects in BC [28,29,30,31,32,33,34,35,36,37]. Accordingly, many clinical studies, including proof-of-principle studies in the prevention setting and phase 2 trials in the adjuvant and metastatic settings, have been planned and/or are currently under way to test the causal nature of the suggested correlation between metformin and clinical benefit in cancer. Among them, several pre-operative window-of-opportunity trials have consistently demonstrated the ability of metformin, at conventional anti-diabetic doses, to reduce Ki67 expression in non-diabetic cancer patients, which might vary with host and tumor characteristics [31,38,39,40,41]. Meta-analyses of randomized clinical trials investigating the effect of metformin on biomarkers associated with BC outcomes have suggested a potential benefit from metformin treatment in reducing Ki67 expression in these patients [42,43]. However, no study to date had explored whether the addition of metformin to neoadjuvant regimens in BC might impact the Ki67-measured proliferative activity of residual tumors when pCR is not achieved. Because the quality of evidence regarding the potential benefit of metformin treatment in reducing Ki67 proliferation rates has recently been questioned due to inadequate methodology [44], and considering the open debate regarding the reproducibility of Ki67 technical assessment, interpretation, and scoring in multicenter settings [17,18,19,20,21], we decided to centrally re-evaluate Ki67 using both VA and ADIA approaches simultaneously. Because the consistency over time of paired, sequential biomarker measurements (e.g., Ki67) can be affected not only by intra-tumoral heterogeneity but also by the biospecimen type [45], we employed sequential core biopsies (but not core versus resection), which have been shown to be more consistent and appropriate to assess the effects of drug therapy in vivo on Ki67 using immunohistochemistry [46]. Such an augmentation of the level of evidence suggests that metformin’s ability to reduce the post-neoadjuvant treatment Ki67 index is a real biological phenomenon rather than an analytical artifact or a tissue sampling bias. Although the mean Ki67 suppression was similar between the two treatment arms, a statistically larger decrease in the proliferative capacity of residual tumor cells from baseline values was observed only in the non-pCR patients belonging to the metformin-containing arm, thereby suggesting that metformin might be considered as a safe candidate to prevent and/or treat the proliferative potential of residual BC disease after neoadjuvant therapy.
Neoadjuvant therapy is known to affect the Ki67 index based on the ability of therapeutic agents to act either on cycling cells (e.g., cytotoxic chemotherapeutics) or on major oncogene-driven proliferative pathways (e.g., HER2-targeted drugs). The numerically higher pCR rate observed in the METTEN patients receiving neoadjuvant metformin compared with the reference arm did not reach statistical significance [14]. However, adding neodjuvant metformin appears to provide the protective effects of decreasing Ki67 in the residual tissue that remains after treatment with conventional anthracycline/taxane cytotoxic chemotherapy plus anti-HER2 trastuzumab neoadjuvant therapy. These findings, overall, might suggest that the ability of metformin to target the relationship between lowered Ki67 and inhibition of tumor growth could occur in the absence of objective tumor regression and likely involves pathobiological features of post-therapy residual BC disease that are different to treatment-naïve BC. Beyond the intrinsic (genomic) and/or acquired (nongenomic) mechanisms driving the survival of residual tumors after neoadjuvant therapy, we are accumulating evidence that the ability of cancer cells to flexibly rewire their mitochondrial metabolism (e.g., enhanced oxidative phosphorylation, lipid metabolism, and serine/glycine one-carbon metabolism) critically contribute to therapy resistance in residual disease [47,48,49]. Because all these mitochondria-centric traits can be targeted by metformin [50,51,52,53], it is tempting to speculate that adding metformin to complex neoadjuvant regimens involving chemotherapy and targeted therapy might impede the activation of compensatory metabolic programs linked to the maintenance of proliferative capacity in residual disease [47,48,49]. Nonetheless, because the inhibition of Ki67 after neoadjuvant therapy has a prognostic role [27], it would be of interest to test whether this greater proliferation inhibition that resulted in half of all patients exhibiting high-Ki67 at baseline moving into the low-Ki67 (<20%) category at surgery will be influential in recurrence-free survival among patients not achieving pCR. If future studies will conclude that the neoadjuvant addition of metformin is effective in prolonging disease-free survival among the patients who had residual invasive disease on pathological testing, metformin should become a safe candidate to decrease the aggressiveness of residual disease in the preoperative systemic treatment of breast cancer patients. Evaluation of safety and tolerability of the six-month intervention with metformin as part of a complex neoadjuvant combination including chemotherapy and trastuzumab showed no difference with that of the equivalent regimen without metformin [14]. The most common adverse effects (AEs) of grade ≥3 were neutropenia in both arms and diarrhea in the metformin-containing arm, while none of the serious AEs was deemed to be metformin-related [14]. Although a major concern regarding the clinical usage of metformin is its known ability to induce gastrointestinal upset and diarrhea, which might limit patient compliance when combined with cytotoxic chemotherapy [54], it should be noted that the dropout rate in the metformin arm was much lower than the expected (25%)—solely 13% of patients withdrew because of metformin-related upset and diarrhea, whereas 75% of patients completed the triple regimen of metformin, chemotherapy, and trastuzumab [14].
5. Limitations of the Study
Our current findings must be interpreted with caution and at least two major limitations should be borne in mind. First, we acknowledge that our conclusions would be at best hypothesis generating given both the post-hoc nature of the study for which the original design was not powered and the small sample size evaluated. Second, both the variation in the Ki67 scoring system between VA and ADIA and the lack of Ki67 scores of <14% on diagnostic core biopsies precluded further analyses using recommendations from the International Ki67 in Breast Cancer working group [55,56].
6. Conclusions
Classic histopathological parameters such as Ki67 status provide valuable prognostic and predictive information when assessed in residual BC tissue after neoadjuvant therapy. The proliferative capacity of residual BC disease indicates the existence of partial treatment resistance and higher probability of tumor recurrence, which is supported by the negative prognostic value associated with the persistence of tumor cells following neoadjuvant therapies [12,27,57,58,59]. Because the decision-making process for the choice of adjuvant treatment in BC is rapidly transitioning from the use of therapeutic approaches based on the assessment of pre-neoadjuvant therapy features to being guided by the parameters of the residual disease after neoadjuvant therapy, further research is warranted on the potential of metformin as a preemptive treatment of residual BC proliferation.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/8/12/2180/s1, Table S1: Association of the interaction between baseline Ki67 score and pCR by treatment arm, Table S2: Association of the interaction between baseline Ki67 score and pCR in the metformin-containing arm A, Table S3: Association of the interaction between baseline Ki67 score and pCR in the control arm B, Table S4: Association of the interaction between baseline Ki67 score and pCR by treatment arm adjusted by HR status, Table S5: Association of the interaction between baseline Ki67 score and pCR in the metformin-containing arm A adjusted by HR status, Table S6: Association of the interaction between baseline Ki67 score and pCR in the control arm B adjusted by HR status.
Author Contributions
Conception and design: B.M.-C., J.A.M.; study coordination: B.M.-C.; acquisition of data (e.g., acquired and managed patients, performed immunohistochemical determinations): E.L.-B., S.P., J.D., I.Á., S.M., J.M.P.-G., N.B.-L., C.A.R.-S., K.A., S.D., M.L., I.M., A.S., G.V., J.C., G.O., C.M., L.C., J.B., J.J.; analysis and data interpretation (e.g., development of methodology, statistical analysis, biostatistics): M.B., E.C., S.V., J.A.M.; administrative and technical support (e.g., reporting or organizing data, constructing databases): S.S., M.G., M.B. writing, review, and/or revision of the manuscript: J.A.M., M.B., E.C., E.L.-B., B.M.-C. All authors read and approved the final manuscript for submission.
Funding
This work was supported by Grants from the Ministerio de Sanidad, Servicios Sociales e Igualdad (EC10-125, Ayudas para el Fomento de la Investigación Clínica Independiente to Begoña Martin-Castillo). Work in the Menendez laboratory is supported by the Spanish Ministry of Science and Innovation (Grant SAF2016-80639-P, Plan Nacional de l+D+I, founded by the European Regional Development Fund, Spain) and by an unrestricted research grant from the Fundació Oncolliga Girona (Lliga catalana d’ajuda al malalt de càncer, Girona). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Acknowledgments
The METTEN study was conceived and designed by Begoña Martin-Castillo and Javier A. Menendez, and was sponsored by the Consortium for the Support of Biomedical Research Network (CAIBER) and the Catalan Institute of Oncology (ICO). The Unit of Clinical Research at the ICO Girona and the Unit for Statistical and Methodological Assessment at the Girona Biomedical Research Institute (IDIBGI) were responsible for central data gathering and analysis. All authors had responsibility for the decision to submit for publication. The authors would like to thank Dr. Kenneth McCreath for editorial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Esserman, L.J.; Berry, D.A.; DeMichele, A.; Carey, L.; Davis, S.E.; Buxton, M.; Hudis, C.; Gray, J.W.; Perou, C.; Yau, C.; et al. Pathological complete response predicts recurrence-free survival more effectively by cancer subset: Results from the I-SPY 1 TRIAL—CALGB 150007/150012, ACRIN 6657. J. Clin. Oncol. 2012, 30, 3242–3249. [Google Scholar] [CrossRef]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBCpooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- DeMichele, A.; Yee, D.; Berry, D.A.; Albain, K.S.; Benz, C.C.; Boughey, J.; Buxton, M.; Chia, S.K.; Chien, A.J.; Chui, S.Y.; et al. The Neoadjuvant Model Is Still the Future for Drug Development in Breast Cancer. Clin. Cancer Res. 2015, 21, 2911–2915. [Google Scholar] [CrossRef]
- Fernández-Sánchez, M.; Gamboa-Dominguez, A.; Uribe, N.; García-Ulloa, A.C.; Flores-Estrada, D.; Candelaria, M.; Arrieta, O. Clinical and pathological predictors of the response to neoadjuvant anthracycline chemotherapy in locally advanced breast cancer. Med. Oncol. 2006, 23, 171–183. [Google Scholar] [CrossRef]
- Colleoni, M.; Bagnardi, V.; Rotmensz, N.; Viale, G.; Mastropasqua, M.; Veronesi, P.; Cardillo, A.; Torrisi, R.; Luini, A.; Goldhirsch, A. A nomogram based on the expression of Ki-67, steroid hormone receptors status and number of chemotherapy courses to predict pathological complete remission after preoperative chemotherapy for breast cancer. Eur. J. Cancer 2010, 46, 2216–2224. [Google Scholar] [CrossRef]
- Fasching, P.A.; Heusinger, K.; Haeberle, L.; Niklos, M.; Hein, A.; Bayer, C.M.; Rauh, C.; Schulz-Wendtland, R.; Bani, M.R.; Schrauder, M.; et al. Ki67, chemotherapy response, and prognosis in breast cancer patients receiving neoadjuvant treatment. BMC Cancer 2011, 11, 486. [Google Scholar] [CrossRef]
- Horimoto, Y.; Arakawa, A.; Tanabe, M.; Sonoue, H.; Igari, F.; Senuma, K.; Tokuda, E.; Shimizu, H.; Kosaka, T.; Saito, M. Ki67 expression and the effect of neo-adjuvant chemotherapy on luminal HER2-negative breast cancer. BMC Cancer 2014, 14, 550. [Google Scholar] [CrossRef]
- Montagna, E.; Bagnardi, V.; Viale, G.; Rotmensz, N.; Sporchia, A.; Cancello, G.; Balduzzi, A.; Galimberti, V.; Veronesi, P.; Luini, A.; et al. Changes in PgR and Ki-67 in residual tumour and outcome of breast cancer patients treated with neoadjuvant chemotherapy. Ann. Oncol. 2015, 26, 307–313. [Google Scholar] [CrossRef]
- Diaz-Botero, S.; Espinosa-Bravo, M.; Gonçalves, V.R.; Esgueva-Colmenarejo, A.; Peg, V.; Perez, J.; Cortes, J.; Rubio, I.-T. Different Prognostic Implications of Residual Disease after Neoadjuvant Treatment: Impact of Ki 67 and Site of Response. Ann. Surg. Oncol. 2016, 23, 3831–3837. [Google Scholar] [CrossRef]
- Tokuda, E.; Horimoto, Y.; Arakawa, A.; Himuro, T.; Senuma, K.; Nakai, K.; Saito, M. Differences in Ki67 expressions between pre- and post-neoadjuvant chemotherapy specimens might predict early recurrence of breast cancer. Hum. Pathol. 2017, 63, 40–45. [Google Scholar] [CrossRef]
- Cabrera-Galeana, P.; Muñoz-Montaño, W.; Lara-Medina, F.; Alvarado-Miranda, A.; Pérez-Sánchez, V.; Villarreal-Garza, C.; Quintero, R.M.; Porras-Reyes, F.; Bargallo-Rocha, E.; Del Carmen, I.; et al. Ki67 Changes Identify Worse Outcomes in Residual Breast Cancer Tumors after Neoadjuvant Chemotherapy. Oncologist 2018, 23, 670–678. [Google Scholar] [CrossRef]
- Jones, R.L.; Salter, J.; A’Hern, R.; Nerurkar, A.; Parton, M.; Reis-Filho, J.S.; Smith, I.E.; Dowsett, M. The prognostic significance of Ki67 before and after neoadjuvant chemotherapy in breast cancer. Breast Cancer Res. Treat. 2009, 116, 53–68. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Schmitt, W.D.; Loibl, S.; Müller, B.M.; Blohmer, J.U.; Sinn, B.V.; Eidtmann, H.; Eiermann, W.; Gerber, B.; Tesch, H.; et al. Ki67 measured after neoadjuvant chemotherapy for primary breast cancer. Clin. Cancer Res. 2013, 19, 4521–4531. [Google Scholar] [CrossRef]
- Martin-Castillo, B.; Pernas, S.; Dorca, J.; Álvarez, I.; Martínez, S.; Pérez-Garcia, J.M.; Batista-López, N.; Rodríguez-Sánchez, C.A.; Amillano, K.; Domínguez, S.; et al. A phase 2 trial of neoadjuvant metformin in combination with trastuzumab and chemotherapy in women with early HER2-positive breast cancer: The METTEN study. Oncotarget 2018, 9, 35687–35704. [Google Scholar] [CrossRef]
- Cuyàs, E.; Buxó, M.; Ferri Iglesias, M.J.; Verdura, S.; Pernas, S.; Dorca, J.; Álvarez, I.; Martínez, S.; Pérez-Garcia, J.M.; Batista-López, N.; et al. The C Allele of ATM rs11212617 Associates with Higher Pathological Complete Remission Rate in Breast Cancer Patients Treated with Neoadjuvant Metformin. Front. Oncol. 2019, 9, 193. [Google Scholar] [CrossRef]
- Cuyàs, E.; Fernández-Arroyo, S.; Buxó, M.; Pernas, S.; Dorca, J.; Álvarez, I.; Martínez, S.; Pérez-Garcia, J.M.; Batista-López, N.; Rodríguez-Sánchez, C.A.; et al. Metformin induces a fasting- and antifolate-mimicking modification of systemic host metabolism in breast cancer patients. Aging (Albany NY) 2019, 11, 2874–2888. [Google Scholar] [CrossRef]
- Guarneri, V.; Dieci, M.V.; Bisagni, G.; Frassoldati, A.; Bianchi, G.V.; De Salvo, G.L.; Orvieto, E.; Urso, L.; Pascual, T.; Paré, L.; et al. De-escalated therapy for HR+/HER2+ breast cancer patients with Ki67 response after 2 weeks letrozole: Results of the PerELISA neoadjuvant study. Ann. Oncol. 2019, 30, 921–926. [Google Scholar] [CrossRef]
- Kwon, A.Y.; Park, H.Y.; Hyeon, J.; Nam, S.J.; Kim, S.W.; Lee, J.E.; Yu, J.H.; Lee, S.K.; Cho, S.Y.; Cho, E.Y. Practical approaches to automated digital image analysis of Ki-67 labeling index in 997 breast carcinomas and causes of discordance with visual assessment. PLoS ONE 2019, 14, e0212309. [Google Scholar] [CrossRef]
- Laurinavicius, A.; Plancoulaine, B.; Laurinaviciene, A.; Herlin, P.; Meskauskas, R.; Baltrusaityte, I.; Besusparis, J.; Dasevicius, D.; Elie, N.; Iqbal, Y.; et al. A methodology to ensure and improve accuracy of Ki67 labelling index estimation by automated digital image analysis in breast cancer tissue. Breast Cancer Res. 2014, 16, R35. [Google Scholar] [CrossRef]
- Zhong, F.; Bi, R.; Yu, B.; Yang, F.; Yang, W.; Shui, R. A Comparison of Visual Assessment and Automated Digital Image Analysis of Ki67 Labeling Index in Breast Cancer. PLoS ONE 2016, 11, e0150505. [Google Scholar] [CrossRef]
- Koopman, T.; Buikema, H.J.; Hollema, H.; de Bock, G.H.; van der Vegt, B. Digital image analysis of Ki67 proliferation index in breast cancer using virtual dual staining on whole tissue sections: Clinical validation and inter-platform agreement. Breast Cancer Res. Treat. 2018, 169, 33–42. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.J.; Panel Members. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef]
- Kuerer, H.M.; Newman, L.A.; Smith, T.L.; Ames, F.C.; Hunt, K.K.; Dhingra, K.; Theriault, R.L.; Singh, G.; Binkley, S.M.; Sneige, N.; et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J. Clin. Oncol. 1999, 17, 460–469. [Google Scholar] [CrossRef]
- Hennessy, B.T.; Hortobagyi, G.N.; Rouzier, R.; Kuerer, H.; Sneige, N.; Buzdar, A.U.; Kau, S.W.; Fornage, B.; Sahin, A.; Broglio, K.; et al. Outcome after pathologic complete eradication of cytologically proven breast cancer axillary node metastases following primary chemotherapy. J. Clin. Oncol. 2005, 23, 9304–9311. [Google Scholar] [CrossRef]
- Masuda, N.; Lee, S.J.; Ohtani, S.; Im, Y.H.; Lee, E.S.; Yokota, I.; Kuroi, K.; Im, S.A.; Park, B.W.; Kim, S.B.; et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Huang, C.S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- Penault-Llorca, F.; Radosevic-Robin, N. Biomarkers of residual disease after neoadjuvant therapy for breast cancer. Nat. Rev. Clin. Oncol. 2016, 13, 487–503. [Google Scholar] [CrossRef]
- Goodwin, P.J.; Stambolic, V.; Lemieux, J.; Chen, B.E.; Parulekar, W.R.; Gelmon, K.A.; Hershman, D.L.; Hobday, T.J.; Ligibel, J.A.; Mayer, I.A.; et al. Evaluation of metformin in early breast cancer: A modification of the traditional paradigm for clinical testing of anti-cancer agents. Breast Cancer Res. Treat. 2011, 126, 215–220. [Google Scholar] [CrossRef]
- Goodwin, P.J.; Parulekar, W.R.; Gelmon, K.A.; Shepherd, L.E.; Ligibel, J.A.; Hershman, D.L.; Rastogi, P.; Mayer, I.A.; Hobday, T.J.; Lemieux, J.; et al. Effect of metformin vs placebo on and metabolic factors in NCIC CTG MA.32. J. Natl. Cancer Inst. 2015, 107, djv006. [Google Scholar] [CrossRef]
- Decensi, A.; Puntoni, M.; Goodwin, P.; Cazzaniga, M.; Gennari, A.; Bonanni, B.; Gandini, S. Metformin and cancer risk in diabetic patients: A systematic review and meta-analysis. Cancer Prev. Res. (Phila) 2010, 3, 1451–1461. [Google Scholar] [CrossRef]
- DeCensi, A.; Puntoni, M.; Gandini, S.; Guerrieri-Gonzaga, A.; Johansson, H.A.; Cazzaniga, M.; Pruneri, G.; Serrano, D.; Schwab, M.; Hofmann, U.; et al. Differential effects of metformin on breast cancer proliferation according to markers of insulin resistance and tumor subtype in a randomized presurgical trial. Breast Cancer Res. Treat. 2014, 148, 81–90. [Google Scholar] [CrossRef]
- Del Barco, S.; Vazquez-Martin, A.; Cufí, S.; Oliveras-Ferraros, C.; Bosch-Barrera, J.; Joven, J.; Martin-Castillo, B.; Menendez, J.A. Metformin: Multi-faceted protection against cancer. Oncotarget 2011, 2, 896–917. [Google Scholar] [CrossRef]
- Col, N.F.; Ochs, L.; Springmann, V.; Aragaki, A.K.; Chlebowski, R.T. Metformin and breast cancer risk: A meta-analysis and critical literature review. Breast Cancer Res. Treat. 2012, 135, 639–646. [Google Scholar] [CrossRef]
- Pollak, M.N. Investigating metformin for cancer prevention and treatment: The end of the beginning. Cancer Discov. 2012, 2, 778–790. [Google Scholar] [CrossRef]
- Pollak, M. Potential applications for biguanides in oncology. J. Clin. Investig. 2013, 123, 3693–3700. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From mechanisms of Action to therapies. Cell Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef]
- Gandini, S.; Puntoni, M.; Heckman-Stoddard, B.M.; Dunn, B.K.; Ford, L.; DeCensi, A.; Szabo, E. Metformin and cancer risk and mortality: A systematic review and meta-analysis taking into account biases and confounders. Cancer Prev. Res. (Phila) 2014, 7, 867–885. [Google Scholar] [CrossRef]
- Niraula, S.; Dowling, R.J.; Ennis, M.; Chang, M.C.; Done, S.J.; Hood, N.; Escallon, J.; Leong, W.L.; McCready, D.R.; Reedijk, M.; et al. Metformin in early breast cancer: A prospective window of opportunity neoadjuvant study. Breast Cancer Res. Treat. 2012, 135, 821–830. [Google Scholar] [CrossRef]
- Hadad, S.M.; Coates, P.; Jordan, L.B.; Dowling, R.J.; Chang, M.C.; Done, S.J.; Purdie, C.A.; Goodwin, P.J.; Stambolic, V.; Moulder-Thompson, S.; et al. Evidence for biological effects of metformin in operable breast cancer: Biomarker analysis in a pre-operative window of opportunity randomized trial. Breast Cancer Res. Treat. 2015, 150, 149–155. [Google Scholar] [CrossRef]
- Sivalingam, V.; McVey, R.; Gilmour, K.; Ali, S.; Roberts, C.; Renehan, A.; Kitchener, H.; Crosbie, E. A presurgical window-of-opportunity study of metformin in obesity-driven endometrial cancer. Lancet 2015, 385 (Suppl. 1), S90. [Google Scholar] [CrossRef]
- Sivalingam, V.N.; Kitson, S.; McVey, R.; Roberts, C.; Pemberton, P.; Gilmour, K.; Ali, S.; Renehan, A.G.; Kitchener, H.C.; Crosbie, E.J. Measuring the biological effect of presurgical metformin treatment in endometrial cancer. Br. J. Cancer 2016, 114, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, J.; Manzari, N.; Thompson, J.; Gudi, S.K.; Chhabra, M.; Naik, G.; Mousavi, S.M.; Varkaneh, H.K.; Clark, C.; Zhang, Y. The effect of metformin on biomarkers associated with breast cancer outcomes: A systematic review, meta-analysis, and dose-response of randomized clinical trials. Clin. Transl. Oncol. 2019, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Yuan, J.; Bi, Y.; Wang, C.; Liu, Y. The effect of metformin on biomarkers and survivals for breast cancer- a systematic review and meta-analysis of randomized clinical trials. Pharmacol. Res. 2019, 141, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Kitson, S.J.; Maskell, Z.; Sivalingam, V.N.; Allen, J.L.; Ali, S.; Burns, S.; Gilmour, K.; Latheef, R.; Slade, R.J.; Pemberton, P.W.; et al. PRE-surgical Metformin In Uterine Malignancy (PREMIUM): A Multi-Center, Randomized Double-Blind, Placebo-Controlled Phase III Trial. Clin. Cancer Res. 2019, 25, 2424–2432. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Akcakanat, A.; Chen, H.; Sahin, A.; Tarco, E.; Carkaci, S.; Adrada, B.E.; Singh, G.; Do, K.A.; Garces, Z.M.; et al. Influence of biospecimen variables on proteomic biomarkers in breast cancer. Clin. Cancer Res. 2014, 20, 3870–3883. [Google Scholar] [CrossRef]
- Hadad, S.M.; Jordan, L.B.; Roy, P.G.; Purdie, C.A.; Iwamoto, T.; Pusztai, L.; Moulder-Thompson, S.L.; Thompson, A.M. A prospective comparison of ER, PR, Ki67 and gene expression in paired sequential core biopsies of primary, untreated breast cancer. BMC Cancer 2016, 16, 745. [Google Scholar] [CrossRef]
- Havas, K.M.; Milchevskaya, V.; Radic, K.; Alladin, A.; Kafkia, E.; Garcia, M.; Stolte, J.; Klaus, B.; Rotmensz, N.; Gibson, T.J.; et al. Metabolic shifts in residual breast cancer drive tumor recurrence. J. Clin. Investig. 2017, 127, 2091–2105. [Google Scholar] [CrossRef]
- Porporato, P.E.; Filigheddu, N.; Pedro, J.M.B.; Kroemer, G.; Galluzzi, L. Mitochondrial metabolism and cancer. Cell Res. 2018, 28, 265–280. [Google Scholar] [CrossRef]
- Echeverria, G.V.; Ge, Z.; Seth, S.; Zhang, X.; Jeter-Jones, S.; Zhou, X.; Cai, S.; Tu, Y.; McCoy, A.; Peoples, M.; et al. Resistance to neoadjuvant chemotherapy in triple-negative breast cancer mediated by a reversible drug-tolerant state. Sci. Transl. Med. 2019, 11, eaav0936. [Google Scholar] [CrossRef]
- Sancho, P.; Burgos-Ramos, E.; Tavera, A.; Bou Kheir, T.; Jagust, P.; Schoenhals, M.; Barneda, D.; Sellers, K.; Campos-Olivas, R.; Graña, O.; et al. MYC/PGC-1α Balance Determines the Metabolic Phenotype and Plasticity of Pancreatic Cancer Stem Cells. Cell Metab. 2015, 22, 590–605. [Google Scholar] [CrossRef]
- Sancho, P.; Barneda, D.; Heeschen, C. Hallmarks of cancer stem cell metabolism. Br. J. Cancer 2016, 114, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Cuyàs, E.; Fernández-Arroyo, S.; Verdura, S.; García, R.Á.; Stursa, J.; Werner, L.; Blanco-González, E.; Montes-Bayón, M.; Joven, J.; Viollet, B.; et al. Metformin regulates global DNA methylation via mitochondrial one-carbon metabolism. Oncogene 2018, 37, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Jagust, P.; de Luxán-Delgado, B.; Parejo-Alonso, B.; Sancho, P. Metabolism-Based Therapeutic Strategies Targeting Cancer Stem Cells. Front. Pharmacol. 2019, 10, 203. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.K.; Arya, A.; Malecek, M.K.; Shin, D.S.; Carneiro, B.; Chandra, S.; Kaplan, J.; Kalyan, A.; Altman, J.K.; Platanias, L.; et al. Repurposing metformin for cancer treatment: Current clinical studies. Oncotarget 2016, 7, 40767–40780. [Google Scholar] [CrossRef]
- Cheang, M.C.; Chia, S.K.; Voduc, D.; Gao, D.; Leung, S.; Snider, J.; Watson, M.; Davies, S.; Bernard, P.S.; Parker, J.S.; et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J. Natl. Cancer Inst. 2009, 101, 736–750. [Google Scholar] [CrossRef]
- Dowsett, M.; Nielsen, T.O.; A’Hern, R.; Bartlett, J.; Coombes, R.C.; Cuzick, J.; Ellis, M.; Henry, N.L.; Hugh, J.C.; Lively, T.; et al. International Ki-67 in Breast Cancer Working Group. Assessment of Ki67 in breast cancer: Recommendations from the International Ki67 in Breast Cancer working group. J. Natl. Cancer Inst. 2011, 103, 1656–1664. [Google Scholar] [CrossRef]
- Penault-Llorca, F.; Abrial, C.; Raoelfils, I.; Chollet, P.; Cayre, A.; Mouret-Reynier, M.A.; Thivat, E.; Mishellany, F.; Gimbergues, P.; Durando, X. Changes and predictive and prognostic value of the mitotic index, Ki-67, cyclin D1, and cyclo-oxygenase-2 in 710 operable breast cancer patients treated with neoadjuvant chemotherapy. Oncologist 2008, 13, 1235–1245. [Google Scholar] [CrossRef]
- Diaz, J.; Stead, L.; Shapiro, N.; Newell, R.; Loudig, O.; Lo, Y.; Sparano, J.; Fineberg, S. Mitotic counts in breast cancer after neoadjuvant systemic chemotherapy and development of metastatic disease. Breast Cancer Res. Treat. 2013, 138, 91–97. [Google Scholar] [CrossRef]
- Yoshioka, T.; Hosoda, M.; Yamamoto, M.; Taguchi, K.; Hatanaka, K.C.; Takakuwa, E.; Hatanaka, Y.; Matsuno, Y.; Yamashita, H. Prognostic significance of pathologic complete response and Ki67 expression after neoadjuvant chemotherapy in breast cancer. Breast Cancer 2015, 22, 185–191. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).