Efficacy Study of Anti-Endomysium Antibodies for Celiac Disease Diagnosis: A Retrospective Study in a Spanish Pediatric Population
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design and Participants
2.2. Methodology
2.2.1. Serology
2.2.2. Histopathological Assessment of Intestinal Mucosa
2.2.3. HLA
2.2.4. Statistical Analysis
3. Results
3.1. Sensitivity and Specificity
3.2. IDI and NRI
3.3. EMA Efficacy for CD Diagnosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Anti-DGP | Deamidated gliadin peptide antibodies |
| AGA | Anti-gliadin antibodies |
| Anti-TG2 | Anti-tissue transglutaminase antibodies |
| CD | Celiac Disease |
| CI | Confidence interval |
| DM | Diabetes mellitus |
| DOR | Diagnosis odds ratio |
| EMA | Anti-endomysium antibodies |
| ESPGHAN | European Society for Paediatric Gastroenterology, Hepatology and Nutrition |
| FITC | Fluorescein isothiocyanate |
| FN | False negative |
| FP | False positive |
| GFD | Gluten free diet |
| HLA | Human leukocyte antigen |
| IDI | Integrated Discrimination Improvement |
| IFI | Indirect immunofluorescence |
| IgA | immunoglobulin A |
| LR- | Negative Likelihood ratio |
| LR+ | Positive Likelihood ratio |
| ND | No data |
| NPV | Negative predictive value |
| NRI | Net Reclassification Improvement |
| PCR | Polymerase chain reaction |
| PPV | Positive predictive value |
| SIB | Small intestinal biopsy |
| SD | Standard deviation |
| TG2 | Tissue transglutaminase type 2 |
| TN | True negative |
| TP | True positive |
| ULN | Upper limit of normal |
References
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.R.; Mearin, M.L.; Phillips, A.; Shamir, R.; Troncone, R.; Giersiepen, K.; Branski, D.; Catassi, C.; et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 136–160. [Google Scholar] [CrossRef] [PubMed]
- Werkstetter, K.J.; Korponay-Szabó, I.R.; Popp, A.; Villanacci, V.; Salemme, M.; Heilig, G.; Lillevang, S.T.; Mearin, M.L.; Ribes-Koninckx, C.; Thomas, A.; et al. Accuracy of tests for antibodies against tissue-transglutaminase in diagnosis of celiac disease, without biopsy. Gastroenterology 2017, 153, 924–935. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Petroff, D.; Richter, T.; Auth, M.K.H.; Uhlig, H.H.; Laass, M.W.; Lauenstein, P.; Krahl, A.; Händel, N.; de Laffolie, J.; et al. Validation of Antibody-Based Strategies for Diagnosis of Pediatric Celiac Disease without Biopsy. Gastroenterology 2017, 153, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.; Kurppa, K.; Mearin, M.L.; Ribes-Koninckx, C.; Shamir, R.; Troncone, R.; Auricchio, R.; Castillejo, G.; et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Coeliac Disease 2020. J. Pediatr. Gastroenterol. Nutr. 2019. [Google Scholar] [CrossRef] [PubMed]
- Giersiepen, K.; Lelgemann, M.; Stuhldreher, N.; Ronfani, L.; Husby, S.; Koletzko, S.; Korponay-Szabó, I.R.; ESPGHAN Working Group on Coeliac Disease Diagnosis. Accuracy of Diagnostic Antibody test for Coeliac Disease in Children: Summary of an Evidence Report. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Revised Criteria for Diagnosis of Coeliac Disease. Report of Working Group of European Society of Paediatric Gastroenterology and Nutrition. Arch. Dis. Child. 1990, 65, 909–911. [Google Scholar] [CrossRef] [PubMed]
- Oberhuber, G.; Granditsch, G.; Vogelsang, H. The histopathology of coeliac disease: Time for a standardized report scheme for pathologists. Eur. J. Gastroenterol. Hepatol. 1999, 11, 1185–1194. [Google Scholar] [CrossRef]
- Olerup, O.; Zetterquist, H. HLA DR typing by PCR amplification with sequence-specific primers (PCR-SSP) in 2 hours: An alternative to serological DR typing in clinical practice including donor-recipient matching in cadaveric transplantation. Tissue Antigens 1992, 39, 225–235. [Google Scholar] [CrossRef]
- Olerup, O.; Aldener, A.; Fogdell, A. HLA-DQB1 and -DQA1 typing by PCR amplification with sequence-specific primers (PCR-SSP) in 2 hours. Tissue Antigens 1993, 41, 119–134. [Google Scholar] [CrossRef]
- Kawasaki, E.S. Sample Preparation from Blood, Cells, and other Fluids. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; p. 146. [Google Scholar]
- Pencina, M.J.; D’Agostino, R.B.; D’Agostino, R.B.; Vasan, R.S. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat. Med. 2008, 27, 157–172. [Google Scholar] [CrossRef]
- Collin, P.; Kaukinen, K.; Vogelsang, H.; Korponay-Szabó, I.; Sommer, R.; Schreier, E.; Volta, U.; Granito, A.; Veronesi, L.; Mascart, F.; et al. Antiendomisyal and antihuman recombinant tissue transglutaminase antibodies in the diagnosis of celiac disease: A biopsy-proven European multicentre study. Eur. J. Gastroenterol. Hepatol. 2005, 17, 85–91. [Google Scholar] [CrossRef]
- Lagerqvist, C.; Dahlbom, I.; Hansson, T.; Jidell, E.; Juto, P.; Olcén, P.; Stenlund, H.; Hernell, O.; Ivarsson, A. Antigliadin immunoglobulin a best in finding celiac disease in children younger than 18 months of age. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 428–435. [Google Scholar] [CrossRef]
- Al-Hussaini, A.; Troncone, R.; Khormi, M.; AlTuraiki, M.; Alkhamis, W.; Alrajhi, M.; Halal, T.; Fagih, M.; Alharbi, S.; Bashir, M.S.; et al. Mass Screening for Celiac Disease Among School-aged Children: Toward Exploring Celiac Iceberg in Saudi Arabia. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 646–651. [Google Scholar] [CrossRef]
- Hogen Esch, C.E.; Csizmadia, G.D.; van Hoogstraten, I.M.; Schreurs, M.W.; Mearin, M.L.; von Blomberg, B.M. Childhood coeliac disease: Towards an improved serological mass screening strategy. Aliment. Pharmacol. Ther. 2010, 31, 760–766. [Google Scholar] [CrossRef]
- Lionetti, E.; Castellaneta, S.; Francavilla, R.; Pulvirenti, A.; Naspi Catassi, G.; Catassi, C.; SIGENP Working Group of Weaning and CD Risk. Long-Term Outcome of Potential Celiac Disease in Genetically at-Risk Children: The Prospective CELIPREV Cohort Study. J. Clin. Med. 2019, 8, 186. [Google Scholar] [CrossRef]
- Simell, S.; Hoppu, S.; Hekkala, A.; Simell, T.; Stahlberg, M.R.; Viander, M.; Yrjänäinen, H.; Grönlund, J.; Markula, P.; Simell, V.; et al. Fate of five celiac disease-associated antibodies during normal diet in genetically at-risk children observed from birth in a natural history study. Am. J. Gastroenterol. 2007, 102, 2026–2035. [Google Scholar] [CrossRef]
- Vriezinga, S.L.; Auricchio, R.; Bravi, E.; Castillejo, G.; Chmielewska, A.; Crespo Escobar, P.; Kolaček, S.; Koletzko, S.; Korponay-Szabo, I.R.; Mummert, E.; et al. Randomized feeding Intervention in infants at high risk for celiac disease. N. Engl. J. Med. 2014, 371, 1304–1315. [Google Scholar] [CrossRef]
- Stene, L.C.; Honeyman, M.C.; Hoffenberg, E.J.; Haas, J.E.; Sokol, R.J.; Emery, L.; Taki, I.; Norris, J.M.; Erlich, H.A.; Eisenbarth, G.S.; et al. Rotavirus infection frequency and risk of celiac disease autoimmunity in early childhood: A longitudinal study. Am. J. Gastroenterol. 2006, 101, 2333–2340. [Google Scholar] [CrossRef]
- Ferrara, F.; Quaglia, S.; Caputo, I.; Esposito, C.; Lepretti, M.; Pastore, S.; Giorgi, R.; Martelossi, S.; Dal Molin, G.; Di Toro, N.; et al. Anti-transglutaminase antibodies in non-coeliac children suffering from infectious diseases. Clin. Exp. Immunol. 2010, 159, 217–223. [Google Scholar] [CrossRef]
- Farrace, M.G.; Picarelli, A.; Di Tola, M.; Sabbatella, L.; Marchione, O.P.; Ippolito, G.; Piacentini, M. Presence of anti-“tissue” transglutaminase antibodies in inflammatory intestinal diseases: An apoptosis-associated event? Cell Death. Diff. 2001, 8, 767–770. [Google Scholar] [CrossRef][Green Version]
- Drastich, P.; Honsová, E.; Lodererová, A.; Jarešová, M.; Pekáriková, A.; Hoffmanová, I.; Tučková, L.; Tlaskalová-Hogenová, H.; Spičák, J.; Sánchez, D. Celiac disease markers in patients with liver diseases: A single center large scale screening study. World J. Gastroenterol. 2012, 18, 6255–6262. [Google Scholar] [CrossRef] [PubMed]
- Maglio, M.; Florian, F.; Vecchiet, M.; Auricchio, R.; Paparo, F.; Spadaro, R.; Zanzi, D.; Rapacciuolo, L.; Franzese, A.; Sblattero, D.; et al. Majority of Children with Type 1 Diabetes Produce and Deposit Anti-Tissue Transglutaminase Antibodies in the Small Intestine. Diabetes 2009, 58, 1578–1584. [Google Scholar] [CrossRef] [PubMed]
- Lerma, J.C.; Escobar, P.C.; Simo, E.M.; Aliaga, E.D.; Miguel, B.P.; Ribes-Koninckx, C. Low gluten consumption by young children from families with a history of coeliac disease. J. Pediatr. Gastroenterol. Nutr. 2014, 58, e50. [Google Scholar] [CrossRef] [PubMed]
- Poddar, U.; Ram Thapa, B.; Kanwal Nain, C.; Singh, K. Is tissue transglutaminase autoantibody the best for diagnosing celiac disease in children of developing countries? J. Clin. Gastroenterol. 2008, 42, 147–151. [Google Scholar] [CrossRef]
- Llorente, M.J.; Sebastián, M.; Fernández-Aceñero, M.J.; Prieto, G.; Villanueva, S. IgA antibodies against tissue transglutaminase in the diagnosis of celiac disease: Concordance with intestinal biopsy in children and adults. Clin. Chem. 2004, 50, 451–453. [Google Scholar] [CrossRef][Green Version]
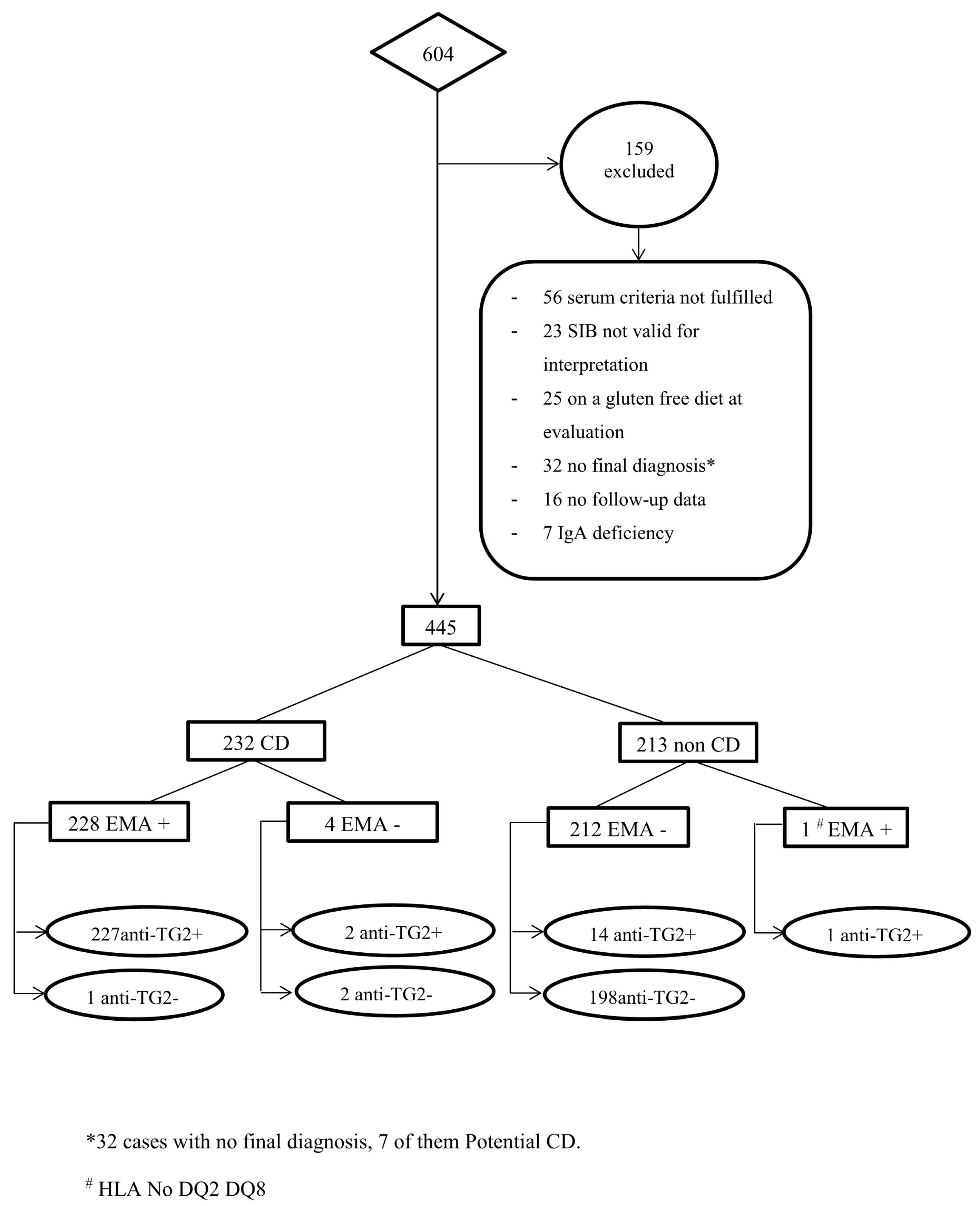
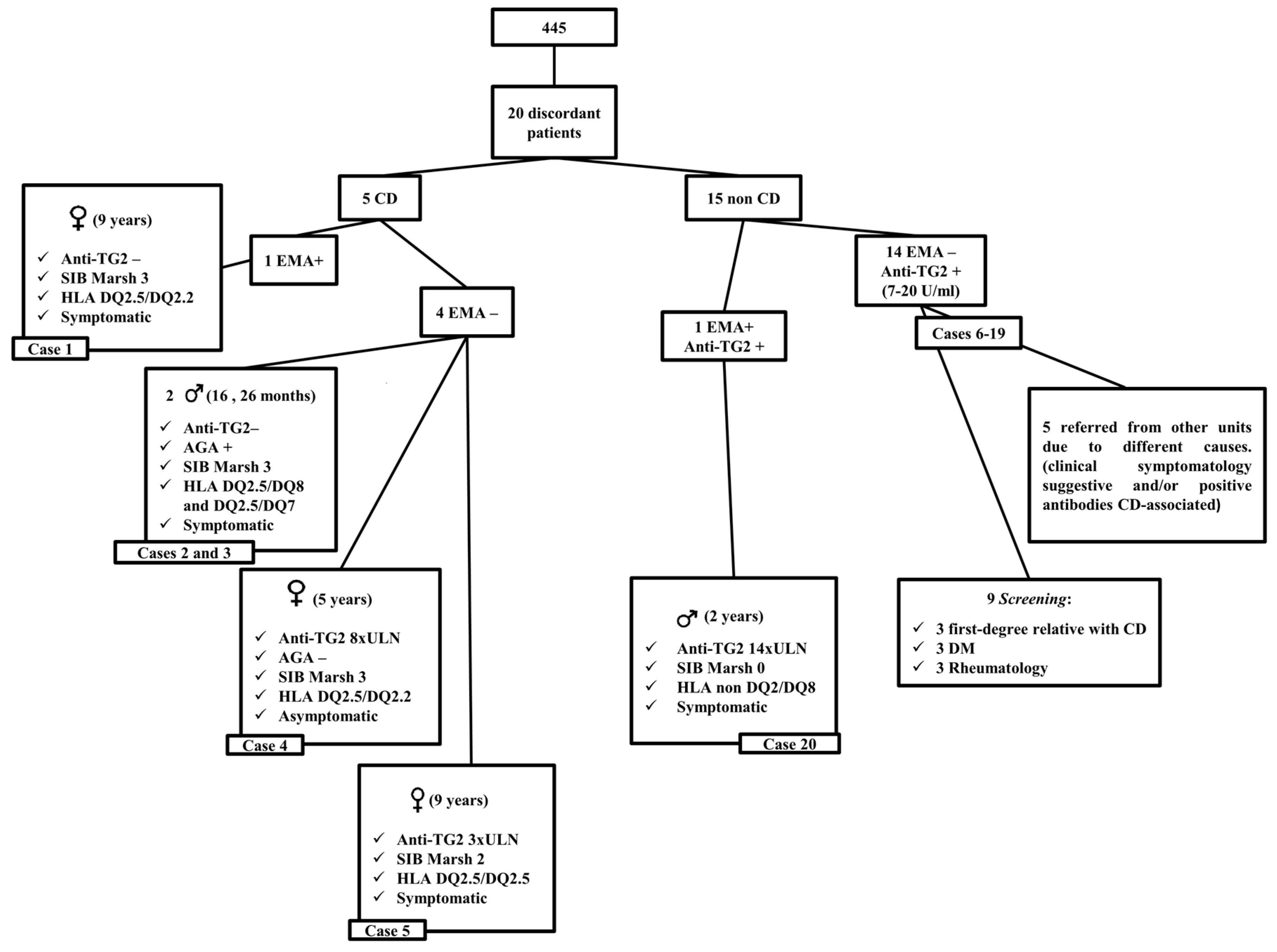
| EMA+ | EMA− | Anti-TG2+ | Anti-TG2− | |
|---|---|---|---|---|
| CD patients (n = 232) | 228 | 4 | 229 | 3 |
| Non-CD patients (n = 213) | 1 | 212 | 15 | 198 |
| Sensitivity a (%) | 98.3 (95.6–99.5) | 98.7 (96.3–99.7) | ||
| Specificity a (%) | 99.5 (97.4–100) | 93.0 (88.7–96) | ||
| PPV a (%) | 99.6 (97.6–100) | 93.9 (90.1–96.5) | ||
| NPV a (%) | 98.1 (95.3–99.4) | 98.5 (95.7–99.7) | ||
| LR+ a | 209.3 (29.6–1479.3) | 14.0 (8.6–22.8) | ||
| LR− a | 0.02 (0.01–0.05) | 0.01 (0.00–0.04) | ||
| DOR a | 12,084 (1339.9–10,8978.8) | 1007.6 (287.5–3531.3) | ||
| Conditions | N | TP | FP | FN | TN | Prevalence [95%CI] | Sensitivity [95%CI] | Specificity [95%CI] | PPV [95%CI] | LR+[95%CI] |
|---|---|---|---|---|---|---|---|---|---|---|
| Anti-TG2 ≥10 × ULN EMA+ Symptoms HLA compatible | 284 | 132 | 0 | 71 | 81 | 71.4 (65.8, 76.7) | 65.0 (58.0, 71.6) | 100 (95.5, 100) | 100 (97.2, 100) | ∞ |
| Anti-TG2 ≥10 × ULN EMA+ Symptoms | 445 | 154 | 1 | 78 | 212 | 52.1 (47.4, 56.9) | 66.4 (59.9, 72.4) | 99.5 (97.4, 100) | 99.5 (97.1, 100) | 141.4 (20.0, 1001.3) |
| Anti-TG2 ≥10 × ULN EMA+ | 445 | 191 | 1 | 41 | 212 | 52.1 (47.4, 56.9) | 82.3 (76.8, 87.0) | 99.5 (97.4, 100) | 99.5 (97.1, 100) | 175.4 (24.8, 1240.3) |
| EMA+ Symptoms | 445 | 181 | 1 | 51 | 212 | 52.1 (47.4, 56.9) | 78.0 (72.1, 83.2) | 99.5 (97.4, 100) | 99.5 (97.0, 100) | 166.2 (23.5, 1175.7) |
| Anti-TG2 ≥10 × ULN Symptoms | 445 | 154 | 1 | 78 | 212 | 52.1 (47.4, 56.9) | 66.4 (59.9, 72.4) | 99.5 (97.4, 100) | 99.4 (96.5, 100) | 141.4 (20.0, 1001.3) |
| EMA and anti-TG2 in symptomatic versus asymptomatic patients | ||||||||||
| EMA in Asymptomatics | 109 | 47 | 0 | 1 | 61 | 44.0 (34.5, 53.9) | 97.9 (88.9, 100) | 100 (94.1, 100) | 100 (92.5, 100) | ∞ |
| EMA in Symptomatics | 336 | 181 | 1 | 3 | 151 | 54.8 (49.3, 60.2) | 98.4 (95.3, 99.7) | 99.3 (96.4, 100) | 99.5 (97.0, 100) | 149.5 (21.2, 1054.7) |
| Anti-TG2 ≥10 × ULN in Asymptomatics | 109 | 37 | 0 | 11 | 61 | 44.0 (34.5, 53.9) | 77.1 (62.7, 88.0) | 100 (94.1, 100) | 100 (90.5, 100) | ∞ |
| Anti-TG2 ≥10 × ULN in Symptomatics | 336 | 154 | 1 | 30 | 151 | 54.8 (49.3, 60.2) | 83.7 (77.5, 88.7) | 99.3 (96.4, 100) | 99.4 (96.5, 100) | 127.2 (18.0, 898.2) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roca, M.; Donat, E.; Marco-Maestud, N.; Masip, E.; Hervás-Marín, D.; Ramos, D.; Polo, B.; Ribes-Koninckx, C. Efficacy Study of Anti-Endomysium Antibodies for Celiac Disease Diagnosis: A Retrospective Study in a Spanish Pediatric Population. J. Clin. Med. 2019, 8, 2179. https://doi.org/10.3390/jcm8122179
Roca M, Donat E, Marco-Maestud N, Masip E, Hervás-Marín D, Ramos D, Polo B, Ribes-Koninckx C. Efficacy Study of Anti-Endomysium Antibodies for Celiac Disease Diagnosis: A Retrospective Study in a Spanish Pediatric Population. Journal of Clinical Medicine. 2019; 8(12):2179. https://doi.org/10.3390/jcm8122179
Chicago/Turabian StyleRoca, María, Ester Donat, Natalia Marco-Maestud, Etna Masip, David Hervás-Marín, David Ramos, Begoña Polo, and Carmen Ribes-Koninckx. 2019. "Efficacy Study of Anti-Endomysium Antibodies for Celiac Disease Diagnosis: A Retrospective Study in a Spanish Pediatric Population" Journal of Clinical Medicine 8, no. 12: 2179. https://doi.org/10.3390/jcm8122179
APA StyleRoca, M., Donat, E., Marco-Maestud, N., Masip, E., Hervás-Marín, D., Ramos, D., Polo, B., & Ribes-Koninckx, C. (2019). Efficacy Study of Anti-Endomysium Antibodies for Celiac Disease Diagnosis: A Retrospective Study in a Spanish Pediatric Population. Journal of Clinical Medicine, 8(12), 2179. https://doi.org/10.3390/jcm8122179





