Abstract
Rheumatoid arthritis is a chronic inflammatory disease characterized by the development of osseous and cartilaginous damage. The correct differentiation between a true erosion and other entities—then often called “pseudoerosions”—is essential to avoid misdiagnosing rheumatoid arthritis and to correctly interpret the progress of the disease. The aims of this systematic review were as follows: to create a definition and delineation of the term “pseudoerosion”, to point out morphological pitfalls in the interpretation of images, and to report on difficulties arising from choosing different imaging modalities. A systematic review on bone erosions in rheumatoid arthritis was performed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The following search terms were applied in PubMed and Scopus: “rheumatoid arthritis”, “bone erosion”, “ultrasonography”, “radiography”, “computed tomography” and “magnetic resonance imaging”. Appropriate exclusion criteria were defined. The systematic review registration number is 138826. The search resulted ultimately in a final number of 25 papers. All indications for morphological pitfalls and difficulties utilizing imaging modalities were recorded and summarized. A pseudoerosion is more than just a negative definition of an erosion; it can be anatomic (e.g., a normal osseous concavity) or artefact-related (i.e., an artificial interruption of the calcified zones). It can be classified according to their configuration, shape, content, and can be described specifically with an anatomical term. “Calcified zone” is a term to describe the deep components of the subchondral, subligamentous and subtendinous bone, and may be applied for all non-cancellous borders of a bone, thus representing a third type of the bone matrix beside the cortical and the trabecular bone.
1. Introduction
Rheumatoid arthritis (RA) manifests with three types of structural joint damage: joint space narrowing, erosions, and capsular abnormalities in the form of synovial proliferation and subluxations [1,2,3,4]. The diagnosis of erosions and their quantification as part of radiographic scoring systems is an accepted surrogate biomarker of structural progression of arthritis [4,5]. Erosions in RA have been defined in consensus statements and in studies with high-resolution peripheral quantitative computed tomography (HRpqCT) as cortical defects, breaks, or other discontinuities with underlying trabecular bone loss and characteristic locations that can be identified with imaging [6,7,8,9,10,11,12]. On radiographs, according to the 2010 ACR/EULAR (American College of Rheumatology/European League Against Rheumatism) rheumatoid arthritis classification criteria [13,14], erosions have to be seen at least at three separate joints at the interphalangeal (PIP), metacarpophalangeal (MCP), wrist (counted as one joint), or metatarsophalangeal (MTP) joints [15,16]. For ultrasound (US) and magnetic resonance imaging (MRI), the operational OMERACT (outcome measures in rheumatology) definition requests the abnormality being visible in two planes [17,18].
At the wrist, the most frequent locations are the capitate, ulna, lunate, triquetrum, and scaphoid [19,20,21,22,23,24,25,26], at the ankle the distal fibular notch, the navicular, cuneiform and cuboid bones are often involved, the talus and calcaneus less frequently [27,28]. Why erosions occur at these sites is commonly explained by immunological and anatomical models [29,30,31]. The latter mainly refer to the thinning of cartilage near capsular insertions at bones (bare areas) and to microdamage [32,33,34,35,36]. Following immunologically-based concepts, erosion formation is explained by increased bone resorption and decreased bone formation at certain locations in the subchondral bone [37]. Werner et al. [32] showed a correlation between cortical micro-channels and the occurrence of bone erosions in bare areas.
Especially in early, preclinical or undifferentiated arthritis with small or no erosions, it is necessary to differentiate a true rheumatic erosion from the various forms of normal erosion-simulating concavities of the bony surface and therefor avoid false-positive statements [38,39]. Such so-called pseudoerosions [40] have been described to be smooth and well demarcated on radiographs, ultrasound, computed tomography (CT) and MRI [41]. The effect of misinterpreting a normal anatomic concavity as an erosion or vice versa may be estimated from the intra- and inter-reader variations of scoring systems and has been directly mentioned for the RAMRIS (rheumatoid arthritis MRI score) [42,43]. The spectrum of MRI “erosion-like” lesions is broad: Ejbjerg et al. [44] observed them in 1.9% of healthy persons, whereas Olech et al. [45] saw them in 65%. Rothschild [46] questioned if such findings should be interpreted as true erosions, old erosions from earlier diseases without clinical significance, or other. For the US, a 30% false-positive rate of erosion detection has been reported [47]. The computer-assisted assessment of erosions was considered helpful, but difficulties in discriminating those from normal bony concavities were observed [48,49].
The aim of this systematic review was (1) to evaluate the frequency of specifically stated difficulties arising in the interpretation of imaging modalitis in search for bone erosions, (2) to define the characteristic anatomic appearances and patterns of pseudoerosions with respect to the potential pitfalls in the diagnosis of RA as reported in the literature and (3) to develop an anatomic concept for improving the accuracy and precision of imaging assessment.
2. Materials and Methods
A systematic review on bone erosions in RA was performed based on the guidelines of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement and was registered accordingly (No. 138826) [50].
2.1. Search Strategy
The search was performed in PubMed (Medline) and Scopus with the following search terms: “rheumatoid arthritis”, “bone erosion”, “ultrasonography”, “radiography”, “computed tomography” and “magnetic resonance imaging” (example for search in PubMed: “rheumatoid arthritis” AND bone AND erosion AND (ultrasonography OR radiography OR “computed tomography” OR “magnetic resonance imaging”). No specific date was defined as starting point, the end of search was 31 May 2019. English language was defined as a required criterium.
2.2. Selection Criteria
All original studies investigating the diagnosis of RA with X-ray, sonography, CT or MRI and describing false positive diagnoses of bone erosions and erosion-like changes published before 31 May 2019 were included. Exclusion criteria were animal studies, feasibility studies, other inflammatory diseases, clinical studies comparing therapeutic measurements in RA, studies comparing the sensitivity of imaging modalities without report of false positive diagnosed erosions or erosion-like lesions, surgical procedures or longitudinal studies without direct reference to this topic, case reports and conference papers. Additionally, all papers without full text availability were excluded from the analysis.
Data extraction was performed by using a standardized Excel (Microsoft Corporation, Redmont, WA, USA) data extraction form: first author, year of publication, country, study population, number of patients, imaging modality, joints evaluated, reported sensitivity of imaging modalities, reported false positive or false negative diagnosis of bone erosions, reported limitations in image interpretation with respect to anatomy, the differential diagnosis to other erosive diseases, artifacts and signal-to-noise reduction.
3. Results
The search with the defined terms resulted in a total of 1487 results. An additional number of 59 papers were added after reference-screening. The flow diagram of the literature review may be seen in Figure 1. Ultimately, only 25 papers reported specifically on false-positive results or erosion-like changes.
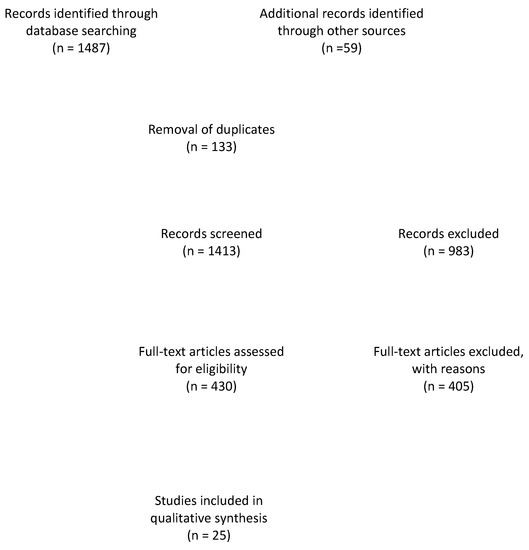
Figure 1.
Flow diagram of the literature review.
Based on the information gathered in the remaining papers, the false-positive results were subdivided into anatomic pseudoerosions, if the explanation for the false-positive diagnosis was described as a morphological phenomenon, and into artifact-related pseudoerosions, if the explanation for the false-positive diagnosis was related to the respective imaging technique.
3.1. Anatomic Pseudoerosions
Anatomic pseudoerosions, i.e., normal concavities of a bone with a potential for misinterpreting them as arthritis-related erosions, were described in twelve original papers and reviews and may be classified into four types according to their anatomic form and configuration (Table 1): (1) a groove or notch or its incomplete form, i.e., a jutty, (2) a sulcus as part of an osteofibrous channel, (3) a subcapital neck on long bones, or (4) a nutritional channel or a zonal roughness [3,11,41,51,52,53,54,55,56,57]. According to their shape, they may be grouped into (1) shallow or broad concavities and (2) subchondral cysts, if en-face displayed on an image and occasionally with a small opening to the joint space, or (3) channel-like structures (Figure 2a,c) [3,54,55]. The anatomic location of pseudoerosions is predominantly at the carpal bones, the MCP- and the MTP-joints. Almost always they are linked to a ligament insertion (Figure 2b), a mucosal fold fixation or the hood of a tendon sheath, and occur at the noncortical bone, also known calcified zones (i.e., borders of the subchondral and enthesial calcified bone with the adjacent underlying trabecular structures). The content of pseudoerosions is visible with US and MRI and may be normal or degenerated ligament tissue, or blood vessels [44,56] and the development of edematous changes [58]. With contrast media, a slight enhancement can be observed, however, in one publication rare cases of strong enhancement was documented [56].

Table 1.
Pseudoerosions.
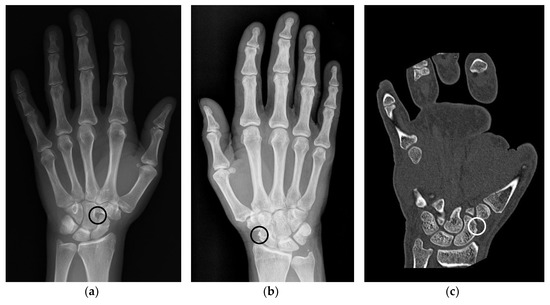
Figure 2.
Examples of anatomical pseudoerosions. (a) Example of a sulcus like pseudoerosion of the capitate bone (black circle) in a left hand of a 52 years old female patient. Referred for suspected scaphoid fracture, which was not verified. (b) Example of a pseudoerosion at the level of the scaphoid waist (black circle) in a right hand of a 66 years old female patient. Referred because of unspecific wrist pain, which afterwards subsided without treatment after one week. (c) Scaphoid rim simulating an erosion in a left hand of a 38 years old male patient (white circle). Referred because of presurgical planning after fracture of the fifth metacarpal and luxation of the fourth and third metacarpal.
3.2. Artifact-Related Pesudoerosions
Artifact-related pseudoerosions were mentioned in 18 original papers and reviews and may be caused due to (1) partial volume artifacts of cross-sectional images or other modality-specific artifacts (ultrasound diffraction or reflection, insufficient fat suppression with MRI), or (2) a low signal-to-noise ratio (Table 2) [1,5,52,57,58,59,60,61,62,63,64,65,66,67,68].

Table 2.
Imaging difficulties.
4. Discussion
From the viewpoint of imaging anatomy, a misinterpretation of erosions in RA may occur due to (1) anatomic pseudoerosions, or (2) artifact-related pseudoerosions as a result of an inadequate investigation technique. Pseudoerosions and erosions are commonly located at certain areas of the surface outline of the calcified bone, also known as calcified zones. These may therefore, besides cortical bone and trabecular bone, be regarded as a third type of organization of the bone matrix.
The term “calcified zones” (Figure 3) in this context is therefore proposed to describe the borders of the subchondral and enthesial calcified bone with the adjacent underlying trabecular structures. It may be extended for describing all parts of intraarticular bone apart from the cortex. With its overlying tissue of hyaline cartilage, synovium or capsule-ligamentous structures it forms anatomic units. The relationship between these zones and the adjacent tissues is so tight that the fibrous layers of tendon sheaths, bursae, periosteum or the cartilaginous zones of entheses or hyaline cartilage are in direct continuation with the subjacent bone, thus providing direct contact with synovial tissue. The concept of the subchondral zone was used by Dihlmann [71] to describe the mineralized zone of hyaline cartilage as part of the subchondral bone. It may be extended to describe a subligamentous, subtendinous or subbursal zone of the bone. Utilizing sub-millimeter spatial resolution CT, these calcified zones can be displayed. Differentiating the normal calcified zone from erosional changes, i.e., irregular margins and sclerotic reaction, is the main challenge in differentiating true erosions from pseudoerosions [72].
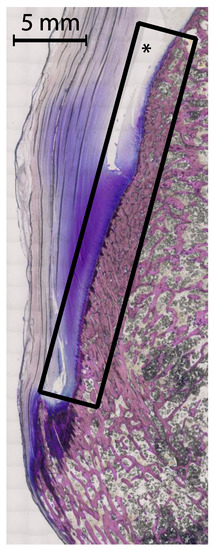
Figure 3.
Example of a calcified zone. Thin ground section of the calcaneal tuberosity, the calcaneal tendon and the calcaneal bursa—also a frequent location of bone erosions. The described calcified zone as subchondral and enthesial calcified bone with adjacent underlying trabecular structures including the overlying tissues is marked by the rectangle. The asterix marks the calcaneal bursa. A 5 mm scale is included, the tissue was stained with Giemsa.
Pseudoerosions have to be differentiated from other pathologies as ganglion cysts, crystal-induced arthropathies, tuberculosis or other infections, and from degenerative lesions in the form of erosions, subchondral (pseudo)cysts or beak-shaped osteophytes as there are so many similarities in location [38,60]. Intraosseous ganglion cysts are common and almost always have a continuity with a ligament which underwent mucous degeneration [73,74]. Especially in the elderly population, the more prevalent degenerative changes of the bone may be difficult to be differentiated from RA-related erosions [38,75]. However, in children interpretational problems may arise. There, normal concavities simulating erosions have been referred to as “bony depressions” at certain locations in the wrist [76,77,78]. Such pseudoerosions in children may be big, indicating that size is not a reliable feature for differentiating normal variants from true erosions.
4.1. Anatomic Pseudoerosions
An anatomic pseudoerosion can be defined as a normal concavity of a bone outlined by a smooth and thin calcified zone with the potential for a false-positive misinterpretation of an erosion. In this form, the term pseudoerosion is more precise than “notch” or “bony depression” and may be preferred as it contains a prognostic impact for the imaging assessment of arthritis. Such clinically oriented annotations, examples are the scaphoid waist and the metacarpal neck as typical sites for fractures, have been in use in traumatology and may be of help in the assessment of arthritis-related erosions, too (list of described pseudoerosions in Table 3, an overview of anatomical pseudoerosions in the hand may also be found in Figure 4).

Table 3.
List of pseudoerosions with anatomic description.
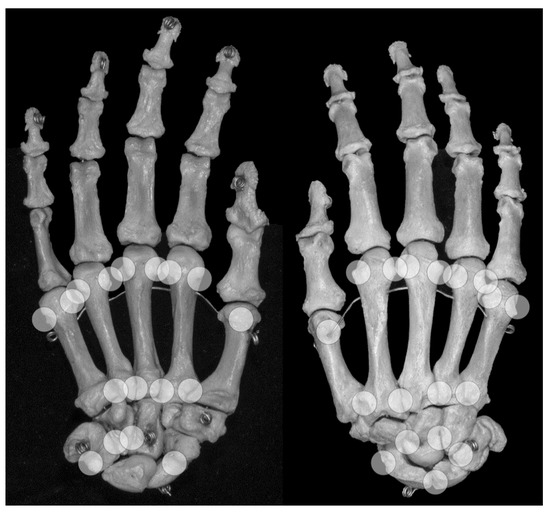
Figure 4.
Locations of anatomical pseudoerosions. Overview on possible locations of anatomical pseudoerosions as summarized in Table 3. Right skeletal hand, on the left view from palmar, on the right view from dorsal.
Grooves due to ligament or tendon insertions have a varying appearance as described in the enthesis concept by Benjamin and McGonagle [79]. Such prominent grooves can cause the appearance of a pseudoerosion (Figure 2a). A groove may occur in three forms: (1) at a non-apophyseal direct tendon or ligament attachment where the uncalcified components of the enthesis enters the bone, (2) at an apophysis with overhanging edges, or (3) at an incomplete apophysis, a jutty, at the indirect attachments of a tendon or ligament with a tangential transition into the periosteum. For example, pseudoerosions resulting from the first form are the metacarpal ligament insertions at the bases of the metacarpal bones [80]. At the dorsal aspect of the triquetral bone, such a pseudoerosion may be formed by the distal insertion of the radiotriquetral ligament along with other components of the dorsal radiocarpal ligament. On the capitate, on which several strong carpal ligaments have their insertion, and many other carpal bones, intercarpal ligaments may cause pseudoerosions [51]. Examples for the second form may be the non-spherical form of metacarpal and metatarsal heads, which can be explained by the collateral ligament complexes running laterally and medially with smoothly outlined shallow metacarpal grooves containing these structures. At the metacarpals, these grooves are bordered by little tubercles for the proximal attachment of the collateral ligaments (Figure 4) [81]. Moraes do Carmo et al. [54] identified three concavities in the first metacarpal head (intersesamoid, ulnar, and radial) and two in those of the fingers (ulnar and radial). They described dorsal depressions of the metacarpal heads due to the extensor digitorum tendons in one third of their anatomic specimens which correlated with observations with ultrasound made by Boutry et al. [82,83]. A similar study was done for defining pseudoerosions of the metatarsal heads by Torshizy et al. [55] who described anatomic variations in the normal osseous concavities of the lateral and medial aspects of each metatarsal head. Typical jutties, i.e., examples for the third form of grooves, are the small round or oval subcapsular notches at the proximal phalangeal bases [80,84]. At the Achilles tendon insertion, proximal to its jutty shallow irregularities beneath the calcaneal bursa may represent true erosions [85].
Osseous sulci are commonly roofed with a ligament, fascia or other fibrous tissue, thus forming an osteofibrous channel for a tendon within a synovial tendon sheath. A subcapital neck of the distal metacarpal and the metatarsal bones is a small metaphyseal narrowing that may cause a pseudoerosion on projection radiographs, ultrasound or MRI [86]. At the distal fifth metacarpal bone, due to its slight varus angulation this neck may be more prominent.
Nutritional channels may appear as pseudoerosion on MR if their orifice is displayed as a little T2-weighted hyperintense spot [51]. Their superficial orifice is often located at a roughness of the calcified zones which as a whole may simulate an erosion [11,34,87]. Some of these iuxtaarticular surface roughnesses may be specified as crests or ridges that correspond to attachment sites for redundant joint capsule [55]. Others, especially on carpal bones, may be due to indentations of innominate ligamentous attachments or synovial folds [51]. Such typical structures visible between the radial aspect of the scaphoid and the radial carpal collateral ligament may be called scapho-capsular ligaments (Figure 2C). Roughness of the calcified zones may be visible at various sites and should be differentiated from shallow extensive true erosions and from advanced cartilage degeneration [88].
4.2. Artefact-Related Pseudoerosions
Artefact-related pseudoerosions are defined as an interruption of the sharp outline of the calcified zones. Important causes are a low signal-to-noise ratio, a partial volume artefact, or in case of ultrasound irregular backscattering with artefacts on an incongruent or rough surface. A low signal-to-noise ratio could be caused by over-penetration of the X-ray beam through the bone or due to insufficient spatial or contrast resolution. This effect is more severe in cases with low calcium content in the calcified zones or the subjacent trabecular bone, previously referred to as subchondral osteoporosis or as pre-erosions, and may be enhanced by swelling of the overlying soft tissue. With ultrasound, diffraction or a complex backscattering of the waves on a curved or irregular surface may cause various pseudo-effects on the retrieved image [51,69,89].
Although X-ray is most commonly used in the diagnosis of RA it is CT which can be regarded as the best imaging modality for differentiating pseudoerosions from true erosions [53,62,63,90,91,92,93,94]. Several studies [34,95,96,97,98] describe a significant decrease of trabecular volume and number and an increased trabecular heterogeneity in patients with rheumatoid arthritis by using HRpqCT. This trabecular bone loss as the intramedullary component of bone erosions may contribute the largest part and may therefore be a reason for misinterpretation of erosions or pseudoerosions in radiographs as this imaging method is relatively insensitive to trabecular bone loss [60,99].
In addition, MRI and US are reported to be more sensitive than plain radiography [53,62,90,91,92,93,94], but this especially seems to be dependent on the location investigated [88,100]. In some cases, radiography may even be superior to MRI in detecting bony erosions despite its lack of three dimensionality [1,3,5,58,99,101,102]. Through its high spatial resolution it can differentiate smaller erosions which otherwise would present themselves as continuous on MRI [61,65].
Thus, it is important to recognize several parameters to achieve a decrease of cognitive diagnostic errors especially in early arthritis. These include slight variations in the respective projection technique and individual ligament laxity or postinflammatory scarring of ligaments. In addition, the roughness of a calcified zone, and the transitional changes between normal bone and true inflammatory erosions are until now not or only scarcely addressed. Even the projection of the joints, even if the relevant anatomic landmarks are displayed according to the standards, is highly variable. One has also to keep in mind that discrete forms of malalignment due to ulnar deviations or other forms of arthritic subluxation, ligament laxity with a slight rotation of bones, and variations in their arrangement may cause a more prominent appearance of a pseudoerosion [3,51,57,102].
4.3. Erosions-in-Pseudoerosions
Both anatomic and artefact-related pseudoerosions are located at sites with direct or indirect contact to inflammatory tissue in arthritis, and therefore, are at higher risk for destruction. Areas of the articular bone without any cartilage covering are more prone to erosive destructions by synovial tissue and effusion [3,32]. Hence, in an anatomically preformed concavity a true inflammatory erosion may develop. McQueen et al. [51] described these erosions-in-pseudoerosions (Figure 5) for the attachment sites of the intercarpal ligaments. It may be observed at the site of ligamentous attachments covered by synovial folds at the metacarpal or metatarsal heads or at the wrist. On the other hand, true erosions may be classified as normal variants. It remains unclear whether these are incidental findings or subclinical erosions [3,57,102].
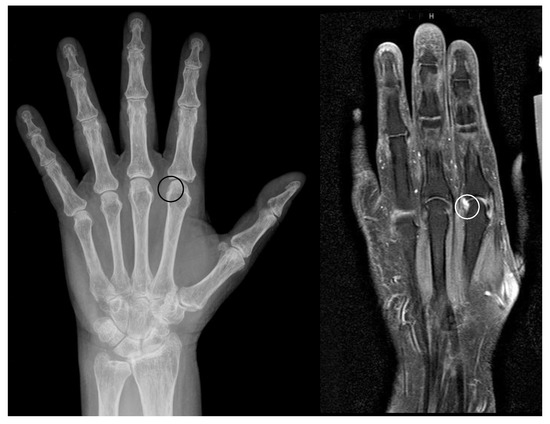
Figure 5.
Erosions within pseudoerosions. Example of the development of an erosion within a pseudoerosion (black/white circle) in a left hand of a 65 years old female patient with longstanding mild seropositive rheumatoid arthritis. Left: radiograph, right: MRI.
4.4. Limitations
A limitation of this study was that the defined search terms resulted in a large quantity of papers, which had to be screened. However, generally accepted terms for mimickers of true erosions do not exist, are described in various forms and additionally with more equivocal definitions than expected at the beginning of this project. Nonetheless, this wide search net allowed for the inclusion of all relevant sources describing the phenomenon of pseudoerosions and minimized the possibility of excluding the respective publications.
5. Conclusions
In conclusion, a pseudoerosion is more than just a negative definition of an erosion. It can be defined as a normal osseous concavity (anatomic pseudoerosion) and/or an artefactual interruption of the calcified zones (artefact-related pseudoerosion). It can be classified according to their configuration, shape, content, and can be directly anatomically named. “Calcified zone” is a term to describe the deep components of the subchondral, subligamentous and subtendinous bone and may be applied for all non-cancellous borders of a bone, thus representing a third type of the bone matrix beside the cortical and the trabecular bone. Anatomic pseudoerosions are almost always related to a ligament insertion or the osteo-fibrous channel of a tendon sheath, therefore, being of high risk for microdamage and the development of a “true” arthritic erosion. Understanding these peculiar aspects of the bony surface with relation to ligament insertions and osteofibrous channels may be of help in improving the assessment of erosions and for reducing over- and underdiagnosis of true erosions.
6. Take Home Message
- Pseudoerosions may be subclassified into anatomic (normal osseous cavity) and artefact-related (artefactual interruption of the calcified zone).
- The term “calcified zone” describes the deep components of the subchondral, subligamentous and subtendinous bone and may be applied for all non-cancellous borders of a bone.
- Pseudoerosions can be regarded as anatomic sites at risk for the development of “true” arthritic erosions.
Author Contributions
Conceptualization, L.H. and F.K.; methodology, L.H.; software, L.H.; validation, L.H., C.R. and F.K.; formal analysis, L.H. and F.K.; investigation, L.H.; resources, L.H.; data curation, L.H.; writing—original draft preparation, L.H. and F.K.; writing—review and editing, L.H., H.P., D.A., C.R. and F.K.; visualization, L.H.; supervision, F.K.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Albrecht, A.; Finzel, S.; Englbrecht, M.; Rech, J.; Hueber, A.; Schlechtweg, P.; Uder, M.; Schett, G. The structural basis of MRI bone erosions: An assessment by microCT. Ann. Rheum. Dis. 2013, 72, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Hetland, M.L.; Ejbjerg, B.; Horslev-Petersen, K.; Jacobsen, S.; Vestergaard, A.; Jurik, A.G.; Stengaard-Pedersen, K.; Junker, P.; Lottenburger, T.; Hansen, I.; et al. MRI bone oedema is the strongest predictor of subsequent radiographic progression in early rheumatoid arthritis. Results from a 2-year randomised controlled trial (CIMESTRA). Ann. Rheum. Dis. 2009, 68, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Martel, W.; Hayes, J.T.; Duff, I.F. The Pattern of Bone Erosion in the Hand and Wrist in Rheumatoid Arthritis. Radiology 1965, 84, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Buckland-Wright, J.C. Microfocal radiographic examination of erosions in the wrist and hand of patients with rheumatoid arthritis. Ann. Rheum. Dis. 1984, 43, 160–171. [Google Scholar] [CrossRef]
- Wakefield, R.J.; Gibbon, W.W.; Conaghan, P.G.; O’Connor, P.; McGonagle, D.; Pease, C.; Green, M.J.; Veale, D.J.; Isaacs, J.D.; Emery, P. The value of sonography in the detection of bone erosions in patients with rheumatoid arthritis: A comparison with conventional radiography. Arthritis Rheum. 2000, 43, 2762–2770. [Google Scholar] [CrossRef]
- Kellgren, J.H.; Lawrence, J.S. Radiological assessment of rheumatoid arthritis. Ann. Rheum. Dis. 1957, 16, 485–493. [Google Scholar] [CrossRef]
- Larsen, A.; Dale, K.; Eek, M. Radiographic evaluation of rheumatoid arthritis and related conditions by standard reference films. Acta Radiol. Diagn. 1977, 18, 481–491. [Google Scholar] [CrossRef]
- Larsen, A. Radiological grading of rheumatoid arthritis. An interobserver study. Scand. J. Rheumatol. 1973, 2, 136–138. [Google Scholar] [CrossRef]
- van der Heijde, D.M. Plain X-rays in rheumatoid arthritis: Overview of scoring methods, their reliability and applicability. Baillieres Clin. Rheumatol. 1996, 10, 435–453. [Google Scholar] [CrossRef]
- van der Heijde, D.; Dankert, T.; Nieman, F.; Rau, R.; Boers, M. Reliability and sensitivity to change of a simplification of the Sharp/van der Heijde radiological assessment in rheumatoid arthritis. Rheumatology 1999, 38, 941–947. [Google Scholar] [CrossRef]
- Barnabe, C.; Toepfer, D.; Marotte, H.; Hauge, E.M.; Scharmga, A.; Kocijan, R.; Kraus, S.; Boutroy, S.; Schett, G.; Keller, K.K.; et al. Definition for Rheumatoid Arthritis Erosions Imaged with High Resolution Peripheral Quantitative Computed Tomography and Interreader Reliability for Detection and Measurement. J. Rheumatol. 2016, 43, 1935–1940. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim-Nasser, N.; Marotte, H.; Valery, A.; Salliot, C.; Toumi, H.; Lespessailles, E. Precision and sources of variability in the assessment of rheumatoid arthritis erosions by HRpQCT. Jt. Bone Spine 2018, 85, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2010, 69, 1580–1588. [Google Scholar] [CrossRef] [PubMed]
- Funovits, J.; Aletaha, D.; Bykerk, V.; Combe, B.; Dougados, M.; Emery, P.; Felson, D.; Hawker, G.; Hazes, J.M.; Huizinga, T.; et al. The 2010 American College of Rheumatology/European League Against Rheumatism classification criteria for rheumatoid arthritis: Methodological report phase I. Ann. Rheum. Dis. 2010, 69, 1589–1595. [Google Scholar] [CrossRef]
- van der Heijde, D.; van der Helm-van Mil, A.H.; Aletaha, D.; Bingham, C.O.; Burmester, G.R.; Dougados, M.; Emery, P.; Felson, D.; Knevel, R.; Kvien, T.K. EULAR definition of erosive disease in light of the 2010 ACR/EULAR rheumatoid arthritis classification criteria. Ann. Rheum. Dis. 2013, 72, 479–481. [Google Scholar] [CrossRef]
- Siddle, H.J.; Hensor, E.M.; Hodgson, R.J.; Grainger, A.J.; Redmond, A.C.; Wakefield, R.J.; Helliwell, P.S. Anatomical location of erosions at the metatarsophalangeal joints in patients with rheumatoid arthritis. Rheumatology 2014, 53, 932–936. [Google Scholar] [CrossRef]
- Alcalde, M.; D’Agostino, M.A.; Bruyn, G.A.; Moller, I.; Iagnocco, A.; Wakefield, R.J.; Naredo, E.; Force, O.U.T. A systematic literature review of US definitions, scoring systems and validity according to the OMERACT filter for tendon lesion in RA and other inflammatory joint diseases. Rheumatology 2012, 51, 1246–1260. [Google Scholar] [CrossRef]
- Ostergaard, M.; Peterfy, C.; Conaghan, P.; McQueen, F.; Bird, P.; Ejbjerg, B.; Shnier, R.; O’Connor, P.; Klarlund, M.; Emery, P.; et al. OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging Studies. Core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system. J. Rheumatol. 2003, 30, 1385–1386. [Google Scholar]
- Ostergaard, M.; Moller Dohn, U.; Duer-Jensen, A.; Hetland, M.L.; Horslev-Petersen, K.; Stengaard-Pedersen, K.; Junker, P.; Podenphant, J.; Ejbjerg, B. Patterns of magnetic resonance imaging bone erosion in rheumatoid arthritis--which bones are most frequently involved and show the most change? J. Rheumatol. 2011, 38, 2014–2017. [Google Scholar] [CrossRef]
- Hammer, H.B.; Haavardsholm, E.A.; Boyesen, P.; Kvien, T.K. Bone erosions at the distal ulna detected by ultrasonography are associated with structural damage assessed by conventional radiography and MRI: A study of patients with recent onset rheumatoid arthritis. Rheumatology 2009, 48, 1530–1532. [Google Scholar] [CrossRef][Green Version]
- Resnick, D.; Gmelich, J.T. Bone fragmentation in the rheumatoid wrist: Radiographic and pathologic considerations. Radiology 1975, 114, 315–321. [Google Scholar] [CrossRef]
- Boutry, N.; Morel, M.; Flipo, R.M.; Demondion, X.; Cotten, A. Early rheumatoid arthritis: A review of MRI and sonographic findings. AJR Am. J. Roentgenol. 2007, 189, 1502–1509. [Google Scholar] [CrossRef]
- Leak, R.S.; Rayan, G.M.; Arthur, R.E. Longitudinal radiographic analysis of rheumatoid arthritis in the hand and wrist. J. Hand Surg. Am. Vol. 2003, 28, 427–434. [Google Scholar] [CrossRef]
- Lee, K.A.; Min, S.H.; Kim, T.H.; Lee, S.H.; Kim, H.R. Magnetic resonance imaging-assessed synovial and bone changes in hand and wrist joints of rheumatoid arthritis patients. Korean J. Intern. Med. 2019, 34, 651–659. [Google Scholar] [CrossRef]
- Kitamura, T.; Murase, T.; Hashimoto, J.; Tomita, T.; Arimitsu, S.; Yoshikawa, H.; Sugamoto, K. Radiographic study on the pattern of wrist joint destruction in rheumatoid arthritis. Clin. Rheumatol. 2011, 30, 353–359. [Google Scholar] [CrossRef]
- Taleisnik, J. Rheumatoid synovitis of the volar compartment of the wrist joint: Its radiological signs and its contribution to wrist and hand deformity. J. Hand Surg. Am. Vol. 1979, 4, 526–535. [Google Scholar] [CrossRef]
- Karasick, D.; Schweitzer, M.E.; O’Hara, B.J. Distal fibular notch: A frequent manifestation of the rheumatoid ankle. Skelet. Radiol. 1997, 26, 529–532. [Google Scholar]
- Baan, H.; Bezooijen, R.; Avenarius, J.K.; Dubbeldam, R.; Drossaers-Bakker, W.K.; van de Laar, M.A. Magnetic resonance imaging of the rheumatic foot according to the RAMRIS system is reliable. J. Rheumatol. 2011, 38, 1003–1008. [Google Scholar] [CrossRef]
- Lee, D.M.; Weinblatt, M.E. Rheumatoid arthritis. Lancet 2001, 358, 903–911. [Google Scholar] [CrossRef]
- Tan, A.L.; Tanner, S.F.; Conaghan, P.G.; Radjenovic, A.; O’Connor, P.; Brown, A.K.; Emery, P.; McGonagle, D. Role of metacarpophalangeal joint anatomic factors in the distribution of synovitis and bone erosion in early rheumatoid arthritis. Arthritis Rheum. 2003, 48, 1214–1222. [Google Scholar] [CrossRef]
- Schett, G.; Gravallese, E. Bone erosion in rheumatoid arthritis: Mechanisms, diagnosis and treatment. Nat. Rev. Rheumatol. 2012, 8, 656–664. [Google Scholar] [CrossRef]
- Werner, D.; Simon, D.; Englbrecht, M.; Stemmler, F.; Simon, C.; Berlin, A.; Haschka, J.; Renner, N.; Buder, T.; Engelke, K.; et al. Early Changes of the Cortical Micro-Channel System in the Bare Area of the Joints of Patients with Rheumatoid Arthritis. Arthritis Rheumatol. 2017, 69, 1580–1587. [Google Scholar] [CrossRef]
- McQueen, F.; Clarke, A.; McHaffie, A.; Reeves, Q.; Williams, M.; Robinson, E.; Dong, J.; Chand, A.; Mulders, D.; Dalbeth, N. Assessment of cartilage loss at the wrist in rheumatoid arthritis using a new MRI scoring system. Ann. Rheum. Dis. 2010, 69, 1971–1975. [Google Scholar] [CrossRef]
- Peters, M.; van Tubergen, A.; Scharmga, A.; Driessen, A.; van Rietbergen, B.; Loeffen, D.; Weijers, R.; Geusens, P.; van den Bergh, J. Assessment of Cortical Interruptions in the Finger Joints of Patients with Rheumatoid Arthritis Using HR-pQCT, Radiography, and MRI. J. Bone Miner. Res. 2018, 33, 1676–1685. [Google Scholar] [CrossRef]
- Conaghan, P.G.; O’Connor, P.; McGonagle, D.; Astin, P.; Wakefield, R.J.; Gibbon, W.W.; Quinn, M.; Karim, Z.; Green, M.J.; Proudman, S.; et al. Elucidation of the relationship between synovitis and bone damage: A randomized magnetic resonance imaging study of individual joints in patients with early rheumatoid arthritis. Arthritis Rheum. 2003, 48, 64–71. [Google Scholar] [CrossRef]
- Schleich, C.; Muller-Lutz, A.; Sewerin, P.; Ostendorf, B.; Buchbender, C.; Schneider, M.; Antoch, G.; Miese, F. Intra-individual assessment of inflammatory severity and cartilage composition of finger joints in rheumatoid arthritis. Skelet. Radiol. 2015, 44, 513–518. [Google Scholar] [CrossRef]
- Favalli, E.G.; Becciolini, A.; Biggioggero, M. Structural integrity versus radiographic progression in rheumatoid arthritis. RMD Open 2015, 1, e000064. [Google Scholar] [CrossRef]
- Boeters, D.M.; Nieuwenhuis, W.P.; van Steenbergen, H.W.; Reijnierse, M.; Landewe, R.B.M.; van der Helm-van Mil, A.H.M. Are MRI-detected erosions specific for RA? A large explorative cross-sectional study. Ann. Rheum. Dis. 2018, 77, 861–868. [Google Scholar] [CrossRef]
- Felloni, P.; Larkman, N.; Dunca, R.; Cotton, A. Inflammatory Arthritides: Imaging Pitfalls. In Pitfalls in Musculoskeletal Radiology; Peh, W., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 697–712. [Google Scholar]
- Dihlmann, W.; Bandick, J. Die Gelenksilhouette: Das Informationspotential der Röntgenstrahlen; Springer: Berlin/Heidelberg, Germany, 1995. [Google Scholar]
- Wawer, R.; Budzik, J.F.; Demondion, X.; Forzy, G.; Cotten, A. Carpal pseudoerosions: A plain X-ray interpretation pitfall. Skelet. Radiol. 2014, 43, 1377–1385. [Google Scholar] [CrossRef]
- Sharp, J.T.; Wolfe, F.; Lassere, M.; Boers, M.; Van Der Heijde, D.; Larsen, A.; Paulus, H.; Rau, R.; Strand, V. Variability of precision in scoring radiographic abnormalities in rheumatoid arthritis by experienced readers. J. Rheumatol. 2004, 31, 1062–1072. [Google Scholar]
- McQueen, F.M.; Benton, N.; Perry, D.; Crabbe, J.; Robinson, E.; Yeoman, S.; McLean, L.; Stewart, N. Bone edema scored on magnetic resonance imaging scans of the dominant carpus at presentation predicts radiographic joint damage of the hands and feet six years later in patients with rheumatoid arthritis. Arthritis Rheum. 2003, 48, 1814–1827. [Google Scholar] [CrossRef]
- Ejbjerg, B.; Narvestad, E.; Rostrup, E.; Szkudlarek, M.; Jacobsen, S.; Thomsen, H.S.; Ostergaard, M. Magnetic resonance imaging of wrist and finger joints in healthy subjects occasionally shows changes resembling erosions and synovitis as seen in rheumatoid arthritis. Arthritis Rheum. 2004, 50, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Olech, E.; Crues, J.V.; Yocum, D.E.; Merrill, J.T. Bone marrow edema is the most specific finding for rheumatoid arthritis (RA) on noncontrast magnetic resonance imaging of the hands and wrists: A comparison of patients with RA and healthy controls. J. Rheumatol. 2010, 37, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, B.M. Significance of “Erosion-like Lesions” in “Healthy Controls”. J. Rheumatol. 2010, 37, 1964. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Finzel, S.; Ohrndorf, S.; Englbrecht, M.; Stach, C.; Messerschmidt, J.; Schett, M.; Backhaus, G. A detailed comparative study of high-resolution ultrasound and micro-computed tomography for detection of arthritic bone erosions. Arthritis Rheum. 2011, 63, 1231–1236. [Google Scholar] [CrossRef]
- Langs, G.; Peloschek, P.; Bischof, H.; Kainberger, F. Model-based erosion spotting and visualization in rheumatoid arthritis. Acad. Radiol. 2007, 14, 1179–1188. [Google Scholar] [CrossRef]
- Yang, H.; Rivoire, J.; Hoppe, M.; Srikhum, W.; Imboden, J.; Link, T.M.; Li, X. Computer-aided and manual quantifications of MRI synovitis, bone marrow edema-like lesions, erosion and cartilage loss in rheumatoid arthritis of the wrist. Skelet. Radiol. 2015, 44, 539–547. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1-34. [Google Scholar] [CrossRef]
- McQueen, F.; Ostergaard, M.; Peterfy, C.; Lassere, M.; Ejbjerg, B.; Bird, P.; O’Connor, P.; Genant, H.; Shnier, R.; Emery, P.; et al. Pitfalls in scoring MR images of rheumatoid arthritis wrist and metacarpophalangeal joints. Ann. Rheum. Dis. 2005, 64, i48–i55. [Google Scholar] [CrossRef]
- Peluso, G.; Bosello, S.L.; Gremese, E.; Mirone, L.; Di Gregorio, F.; Di Molfetta, V.; Pirronti, T.; Ferraccioli, G. Detection of bone erosions in early rheumatoid arthritis: 3D ultrasonography versus computed tomography. Clin. Rheumatol. 2015, 34, 1181–1186. [Google Scholar] [CrossRef]
- Dohn, U.M.; Ejbjerg, B.J.; Court-Payen, M.; Hasselquist, M.; Narvestad, E.; Szkudlarek, M.; Moller, J.M.; Thomsen, H.S.; Ostergaard, M. Are bone erosions detected by magnetic resonance imaging and ultrasonography true erosions? A comparison with computed tomography in rheumatoid arthritis metacarpophalangeal joints. Arthritis Res. Ther. 2006, 8, R110. [Google Scholar] [CrossRef]
- Canella Moraes Carmo, C.; Cruz, G.P.; Trudell, D.; Hughes, T.; Chung, C.; Resnick, D. Anatomical features of metacarpal heads that simulate bone erosions: Cadaveric study using computed tomography scanning and sectional radiography. J. Comput. Assist. Tomogr. 2009, 33, 573–578. [Google Scholar] [CrossRef]
- Torshizy, H.; Hughes, T.H.; Trudell, D.; Resnick, D. Anatomic features of metatarsal heads that simulate erosive disease: Cadaveric study using CT, radiography, and dissection with special emphasis on cross-sectional characterization of osseous anatomy. AJR Am. J. Roentgenol. 2008, 190, W175–W181. [Google Scholar] [CrossRef]
- Robertson, P.L.; Page, P.J.; McColl, G.J. Inflammatory arthritis-like and other MR findings in wrists of asymptomatic subjects. Skelet. Radiol. 2006, 35, 754–764. [Google Scholar] [CrossRef]
- Dohn, U.M.; Terslev, L.; Szkudlarek, M.; Hansen, M.S.; Hetland, M.L.; Hansen, A.; Madsen, O.R.; Hasselquist, M.; Moller, J.; Ostergaard, M. Detection, scoring and volume assessment of bone erosions by ultrasonography in rheumatoid arthritis: Comparison with CT. Ann. Rheum. Dis. 2013, 72, 530–534. [Google Scholar] [CrossRef]
- Alasaarela, E.; Suramo, I.; Tervonen, O.; Lahde, S.; Takalo, R.; Hakala, M. Evaluation of humeral head erosions in rheumatoid arthritis: A comparison of ultrasonography, magnetic resonance imaging, computed tomography and plain radiography. Br. J. Rheumatol. 1998, 37, 1152–1156. [Google Scholar] [CrossRef]
- Foley-Nolan, D.; Stack, J.P.; Ryan, M.; Redmond, U.; Barry, C.; Ennis, J.; Coughlan, R.J. Magnetic resonance imaging in the assessment of rheumatoid arthritis—A comparison with plain film radiographs. Br. J. Rheumatol. 1991, 30, 101–106. [Google Scholar] [CrossRef]
- Cimmino, M.A.; Bountis, C.; Silvestri, E.; Garlaschi, G.; Accardo, S. An appraisal of magnetic resonance imaging of the wrist in rheumatoid arthritis. Semin. Arthritis Rheum. 2000, 30, 180–195. [Google Scholar] [CrossRef]
- Forslind, K.; Johanson, A.; Larsson, E.M.; Svensson, B. Magnetic resonance imaging of the fifth metatarsophalangeal joint compared with conventional radiography in patients with early rheumatoid arthritis. Scand. J. Rheumatol. 2003, 32, 131–137. [Google Scholar] [CrossRef]
- Amin, M.F.; Ismail, F.M.; el Shereef, R.R. The role of ultrasonography in early detection and monitoring of shoulder erosions, and disease activity in rheumatoid arthritis patients; comparison with MRI examination. Acad. Radiol. 2012, 19, 693–700. [Google Scholar] [CrossRef]
- Aurell, Y.; Andersson, M.; Forslind, K. Cone-beam computed tomography, a new low-dose three-dimensional imaging technique for assessment of bone erosions in rheumatoid arthritis: Reliability assessment and comparison with conventional radiography—A BARFOT study. Scand. J. Rheumatol. 2018, 47, 173–177. [Google Scholar] [CrossRef]
- Ejbjerg, B.J.; Vestergaard, A.; Jacobsen, S.; Thomsen, H.; Ostergaard, M. Conventional radiography requires a MRI-estimated bone volume loss of 20% to 30% to allow certain detection of bone erosions in rheumatoid arthritis metacarpophalangeal joints. Arthritis Res. Ther. 2006, 8, R59. [Google Scholar] [CrossRef]
- Dohn, U.M.; Ejbjerg, B.J.; Hasselquist, M.; Narvestad, E.; Moller, J.; Thomsen, H.S.; Ostergaard, M. Detection of bone erosions in rheumatoid arthritis wrist joints with magnetic resonance imaging, computed tomography and radiography. Arthritis Res. Ther. 2008, 10, R25. [Google Scholar] [CrossRef]
- Kleyer, A.; Krieter, M.; Oliveira, I.; Faustini, F.; Simon, D.; Kaemmerer, N.; Cavalcante, A.; Tabosa, T.; Rech, J.; Hueber, A.; et al. High prevalence of tenosynovial inflammation before onset of rheumatoid arthritis and its link to progression to RA-A combined MRI/CT study. Semin. Arthritis Rheum. 2016, 46, 143–150. [Google Scholar] [CrossRef]
- Ulas, S.T.; Diekhoff, T.; Hermann, K.G.A.; Poddubnyy, D.; Hamm, B.; Makowski, M.R. Susceptibility-weighted MR imaging to improve the specificity of erosion detection: A prospective feasibility study in hand arthritis. Skelet. Radiol. 2019, 48, 721–728. [Google Scholar] [CrossRef]
- Emond, P.D.; Inglis, D.; Choi, A.; Tricta, J.; Adachi, J.D.; Gordon, C.L. Volume measurement of bone erosions in magnetic resonance images of patients with rheumatoid arthritis. Magn. Reson. Med. 2012, 67, 814–823. [Google Scholar] [CrossRef]
- McQueen, F.M.; Stewart, N.; Crabbe, J.; Robinson, E.; Yeoman, S.; Tan, P.L.; McLean, L. Magnetic resonance imaging of the wrist in early rheumatoid arthritis reveals a high prevalence of erosions at four months after symptom onset. Ann. Rheum. Dis. 1998, 57, 350–356. [Google Scholar] [CrossRef]
- McQueen, F.M.; Benton, N.; Crabbe, J.; Robinson, E.; Yeoman, S.; McLean, L.; Stewart, N. What is the fate of erosions in early rheumatoid arthritis? Tracking individual lesions using X rays and magnetic resonance imaging over the first two years of disease. Ann. Rheum. Dis. 2001, 60, 859–868. [Google Scholar]
- Dihlmann, W. A early sign of arthritis. The loss of subchondral marginal lamellae. Z. Rheumaforsch. 1968, 27, 129–132. [Google Scholar]
- Regensburger, A.; Rech, J.; Englbrecht, M.; Finzel, S.; Kraus, S.; Hecht, K.; Kleyer, A.; Haschka, J.; Hueber, A.J.; Cavallaro, A.; et al. A comparative analysis of magnetic resonance imaging and high-resolution peripheral quantitative computed tomography of the hand for the detection of erosion repair in rheumatoid arthritis. Rheumatology 2015, 54, 1573–1581. [Google Scholar] [CrossRef][Green Version]
- Schrank, C.; Meirer, R.; Stabler, A.; Nerlich, A.; Reiser, M.; Putz, R. Morphology and topography of intraosseous ganglion cysts in the carpus: An anatomic, histopathologic, and magnetic resonance imaging correlation study. J. Hand Surg. Am. 2003, 28, 52–61. [Google Scholar] [CrossRef]
- Paparo, F.; Fabbro, E.; Piccazzo, R.; Revelli, M.; Ferrero, G.; Muda, A.; Cimmino, M.A.; Garlaschi, G. Multimodality imaging of intraosseous ganglia of the wrist and their differential diagnosis. Radiol. Med. 2012, 117, 1355–1373. [Google Scholar] [CrossRef]
- Mangnus, L.; van Steenbergen, H.W.; Lindqvist, E.; Brouwer, E.; Reijnierse, M.; Huizinga, T.W.; Gregersen, P.K.; Berglin, E.; Rantapaa-Dahlqvist, S.; van der Heijde, D.; et al. Studies on ageing and the severity of radiographic joint damage in rheumatoid arthritis. Arthritis Res. Ther. 2015, 17, 222. [Google Scholar] [CrossRef]
- Avenarius, D.M.; Ording Muller, L.S.; Eldevik, P.; Owens, C.M.; Rosendahl, K. The paediatric wrist revisited—Findings of bony depressions in healthy children on radiographs compared to MRI. Pediatr. Radiol. 2012, 42, 791–798. [Google Scholar] [CrossRef]
- Boavida, P.; Hargunani, R.; Owens, C.M.; Rosendahl, K. Magnetic resonance imaging and radiographic assessment of carpal depressions in children with juvenile idiopathic arthritis: Normal variants or erosions? J. Rheumatol. 2012, 39, 645–650. [Google Scholar] [CrossRef]
- Boavida, P.; Lambot-Juhan, K.; Muller, L.S.; Damasio, B.; de Horatio, L.T.; Malattia, C.; Owens, C.M.; Rosendahl, K. Carpal erosions in children with juvenile idiopathic arthritis: Repeatability of a newly devised MR-scoring system. Pediatr. Radiol. 2015, 45, 1972–1980. [Google Scholar] [CrossRef]
- Benjamin, M.; McGonagle, D. The anatomical basis for disease localisation in seronegative spondyloarthropathy at entheses and related sites. J. Anat. 2001, 199, 503–526. [Google Scholar] [CrossRef]
- Platzer, W. Taschenatlas Anatomie 01. Bewegungsapparat; Thieme Georg Verlag: New York, NY, USA, 2009. [Google Scholar]
- Standring, S. Gray’s Anatomy: The Anatomical Basis of Clinical Practice; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Boutry, N.; Larde, A.; Demondion, X.; Cortet, B.; Cotten, H.; Cotten, A. Metacarpophalangeal joints at US in asymptomatic volunteers and cadaveric specimens. Radiology 2004, 232, 716–724. [Google Scholar] [CrossRef]
- Fanghänel, J.; Pera, F.; Anderhuber, F.; Nitsch, R. Waldeyer—Anatomie des Menschen, 18th ed.; Walter de Gruyter: Berlin, Germany, 2009. [Google Scholar]
- Manaster, B.J.; Crim, J.R. Imaging Anatomy: Musculoskeletal; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Eshed, I.; Bollow, M.; McGonagle, D.G.; Tan, A.L.; Althoff, C.E.; Asbach, P.; Hermann, K.G. MRI of enthesitis of the appendicular skeleton in spondyloarthritis. Ann. Rheum. Dis. 2007, 66, 1553–1559. [Google Scholar] [CrossRef]
- Boutry, N.; Hachulla, E.; Flipo, R.M.; Cortet, B.; Cotten, A. MR imaging findings in hands in early rheumatoid arthritis: Comparison with those in systemic lupus erythematosus and primary Sjogren syndrome. Radiology 2005, 236, 593–600. [Google Scholar] [CrossRef]
- Boutroy, S.; Chapurlat, R.; Vanden-Bossche, A.; Locrelle, H.; Thomas, T.; Marotte, H. Erosion or vascular channel? Arthritis Rheum. 2015, 67, 2956. [Google Scholar] [CrossRef]
- Hoving, J.L.; Buchbinder, R.; Hall, S.; Lawler, G.; Coombs, P.; McNealy, S.; Bird, P.; Connell, D. A comparison of magnetic resonance imaging, sonography, and radiography of the hand in patients with early rheumatoid arthritis. J. Rheumatol. 2004, 31, 663–675. [Google Scholar]
- Wang, B.; Overgaard, S.; Chemnitz, J.; Ding, M. Cancellous and Cortical Bone Microarchitectures of Femoral Neck in Rheumatoid Arthritis and Osteoarthritis Compared with Donor Controls. Calcif. Tissue Int. 2016, 98, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Perry, D.; Stewart, N.; Benton, N.; Robinson, E.; Yeoman, S.; Crabbe, J.; McQueen, F. Detection of erosions in the rheumatoid hand; a comparative study of multidetector computerized tomography versus magnetic resonance scanning. J. Rheumatol. 2005, 32, 256–267. [Google Scholar] [PubMed]
- Dohn, U.M.; Ejbjerg, B.J.; Hasselquist, M.; Narvestad, E.; Court-Payen, M.; Szkudlarek, M.; Moller, J.; Thomsen, H.S.; Ostergaard, M. Rheumatoid arthritis bone erosion volumes on CT and MRI: Reliability and correlations with erosion scores on CT, MRI and radiography. Ann. Rheum. Dis. 2007, 66, 1388–1392. [Google Scholar] [CrossRef] [PubMed]
- Hermann, K.G.; Backhaus, M.; Schneider, U.; Labs, K.; Loreck, D.; Zuhlsdorf, S.; Schink, T.; Fischer, T.; Hamm, B.; Bollow, M. Rheumatoid arthritis of the shoulder joint: Comparison of conventional radiography, ultrasound, and dynamic contrast-enhanced magnetic resonance imaging. Arthritis Rheum. 2003, 48, 3338–3349. [Google Scholar] [CrossRef]
- Hodler, J.; Terrier, B.; von Schulthess, G.K.; Fuchs, W.A. MRI and sonography of the shoulder. Clin. Radiol. 1991, 43, 323–327. [Google Scholar] [CrossRef]
- Klarlund, M.; Ostergaard, M.; Jensen, K.E.; Madsen, J.L.; Skjodt, H.; Lorenzen, I. Magnetic resonance imaging, radiography, and scintigraphy of the finger joints: One year follow up of patients with early arthritis. The TIRA Group. Ann. Rheum. Dis. 2000, 59, 521–528. [Google Scholar] [CrossRef]
- Yang, H.; Yu, A.; Burghardt, A.J.; Virayavanich, W.; Link, T.M.; Imboden, J.B.; Li, X. Quantitative characterization of metacarpal and radial bone in rheumatoid arthritis using high resolution-peripheral quantitative computed tomography. Int. J. Rheum. Dis. 2017, 20, 353–362. [Google Scholar] [CrossRef]
- Fouque-Aubert, A.; Boutroy, S.; Marotte, H.; Vilayphiou, N.; Bacchetta, J.; Miossec, P.; Delmas, P.D.; Chapurlat, R.D. Assessment of hand bone loss in rheumatoid arthritis by high-resolution peripheral quantitative CT. Ann. Rheum. Dis. 2010, 69, 1671–1676. [Google Scholar] [CrossRef]
- Feehan, L.; Buie, H.; Li, L.; McKay, H. A customized protocol to assess bone quality in the metacarpal head, metacarpal shaft and distal radius: A high resolution peripheral quantitative computed tomography precision study. BMC Musculoskelet. Disord. 2013, 14, 367. [Google Scholar] [CrossRef] [PubMed]
- Kocijan, R.; Finzel, S.; Englbrecht, M.; Engelke, K.; Rech, J.; Schett, G. Decreased quantity and quality of the periarticular and nonperiarticular bone in patients with rheumatoid arthritis: A cross-sectional HR-pQCT study. J. Bone Miner. Res. 2014, 29, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Guermazi, A.; Taouli, B.; Lynch, J.A.; Peterfy, C.G. Imaging of bone erosion in rheumatoid arthritis. Semin. Musculoskelet. Radiol. 2004, 8, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Zayat, A.S.; Ellegaard, K.; Conaghan, P.G.; Terslev, L.; Hensor, E.M.; Freeston, J.E.; Emery, P.; Wakefield, R.J. The specificity of ultrasound-detected bone erosions for rheumatoid arthritis. Ann. Rheum. Dis. 2015, 74, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Buckland-Wright, J.C. X-ray assessment of activity in rheumatoid disease. Br. J. Rheumatol. 1983, 22, 3–10. [Google Scholar] [CrossRef]
- Canella, C.; Philippe, P.; Pansini, V.; Salleron, J.; Flipo, R.M.; Cotten, A. Use of tomosynthesis for erosion evaluation in rheumatoid arthritic hands and wrists. Radiology 2011, 258, 199–205. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).